« Prev Next »

Just like you, plants are multicellular organisms that contain a variety of specialized cell types that work together to perform a variety of complex tasks. For example, flowers consist of several specialized structures, all made of different specialized cell types, that function together and allow the plant to reproduce. But reproduction cannot take place without the functions of other specialized cells. The specialized cells in the leaves harvest the energy of light and convert it into chemical energy that can be used for basic metabolism. Both leaves and flowers require water and nutrients that are absorbed by specialized cells in the roots. The water and nutrients absorbed by the roots are transported to the leaves and flowers by specialized cells in the stem.
Both plants and animals must produce different and specialized cell types in order for the organism to function as a whole. But how are the cells in plants and animals instructed to become specialized in function? What instructions do the cells receive and are the instructions the same in both plants and animals? What happens if the instructions are wrong?
How Do Cells Become Specialized?
At the DNA level, the vast majority of cells within a multicellular organism (plant or animal) are the same. Cells contain copies of every gene needed during the organism's lifetime. Genes are stored in the DNA which is found in the nucleus of plant and animal cells. The genes are transcribed into RNA in the nucleus and the RNAs are then transported to the cytoplasm where they are translated into proteins. When the information stored in a gene is converted into functional protein, the gene is said to be expressed. Not all genes are expressed in every cell. In other words, at the RNA and protein levels, not all cells are created equal. Instead, each specialized cell has a specific set of genes that are expressed. Controlling which genes are expressed in a cell determines what type of specialized cell it will become.
The regulation of gene expression at the transcriptional level has traditionally received much attention. Genes contain DNA sequences in a region known as the promoter. The promoter sequences are bound by proteins that specify when and where a gene will be transcribed into RNA. On top of this, how tightly the DNA is packaged around the structures called nucleosomes can also influence whether or not a gene is transcribed. Tightly wrapped DNA is generally not transcribed while loosely wrapped DNA is transcribed. Thus, at the transcriptional level both the binding of proteins to the promoter region and changes in DNA packaging influence gene expression.
Gene expression can also be regulated post-transcriptionally. That is, some mRNAs are transcribed from a gene but the mRNA is not translated into a protein. Although proteins are assembled from the information stored in the DNA and carried to the cytoplasm by the mRNA, only the proteins themselves, not the DNA or mRNA, actively participate in cellular function. Therefore, if no protein is made, then the information stored in a gene does not function and the gene is not expressed. There are several mechanisms to control gene expression post-transcriptionally. One of these mechanisms involves a group of small RNAs known as microRNAs (miRNAs).
How Do miRNAs Instruct Cell Specialization?
MicroRNAs are small 21–24 nucleotide RNAs that are transcribed from a gene, but the transcript is never translated into a protein. Instead the long RNA that is transcribed from the gene encoding the miRNA (the pri-miRNA) base pairs with itself, creating a stem-loop double-stranded RNA. The pri-miRNA is then cut by an enzyme into a double-stranded 21–24 nucleotide RNA referred to as the miRNA/miRNA*. (Since it is double-stranded, miRNA refers to the strand that will become the miRNA and miRNA* refers to the strand that is complementary to the miRNA.) Each end of the miRNA/miRNA* is methylated to protect it from degradation before it is transported out of the nucleus. In the cytoplasm, the miRNA strand of the miRNA/miRNA* duplex is incorporated into a protein complex while the other half of the duplex (miRNA*) is degraded. This protein/miRNA complex, known as RISC (RNA-induced silencing complex), binds to mRNAs in the cytoplasm that base pair with the miRNA in RISC. The binding of the RISC to the target mRNA prevents translation of the mRNA either by directly blocking translation (translational inhibition) or by cleaving the mRNA before it is translated (cleavage) (Figure 1).
Since their initial discovery, miRNAs have been identified in many plant and animal species. MicroRNAs have been extensively studied in Arabidopsis thaliana, a flowering plant that is a member of the mustard family (for reviews see Garcia 2008; Voinnet 2009; Mallory & Bouche 2008). In Arabidopsis, there are over 160 genes that encode miRNAs (Arabidopsis Small RNA Project). Cleaving of the pri-miRNAs actually requires the concerted action of several proteins including: DICER-LIKE1 (DCL1), HYPONASTIC LEAVES1 (HYL1) and SERRATE (SE). A protein known as HUA ENHANCER1 (HEN1) adds the methyl groups to the miRNA/miRNA* to protect it from degradation. The export of the miRNA/miRNA* from the nucleus requires a protein known as HASTY (HST). Once in the cytoplasm, a protein known as ARGONAUTE1 (AGO1), a member of RISC, binds to the miRNA. Once an mRNA base pairs with the miRNA in RISC, the mRNA is either cleaved by AGO1 or translationally repressed.
What If miRNAs Fail to Regulate Gene Expression?
MicroRNAs and their associated proteins were originally identified by researchers trying to uncover the molecular basis of several developmental abnormalities. The very first two miRNAs discovered (lin-4 and let-7; Wightman et al. 1993; Reinhart et al. 2002) were identified because mutations in the genes encoding these miRNAs result in defects in the larval-to-adult phase change of C. elegans. Since the discovery of lin-4 and let-7, the molecular basis of other developmental defects, in both plants and animals, has been traced to mutations within genes that encode miRNAs or their associated proteins. In Arabidopsis, DCL1 (Golden et al. 2002) and HEN1 (Chen et al. 2002) were identified by characterizing plants with flower and/or embryo defects. Analyses of plants with leaf abnormalities led to the identification of HYL1 (Yu et al. 2004), SE (Grigg et al. 2005) and AGO1 (Bohmert et al. 1998). The fact that miRNAs and their associated proteins have been found to be the underlying cause of many developmental abnormalities shows how critical miRNAs are for proper development.
What Kind of mRNAs Do miRNA Target?
There are several mRNAs known to be targeted for destruction by RISC/miRNA binding. In Arabidopsis, among the most extensively studied miRNA targets are the HD-ZIPIII class transcription factors. There are five HD-ZIPIII transcription factors in Arabidopsis. Some of these transcription factors instruct the cells on the top surface of the leaf, or the adaxial surface, to make cells that specialize in photosynthesis. The other HD-ZIPIIIs instruct the cells on the bottom of the leaf, or abaxial surface, to make cells that specialize in gas exchange. Thus the top and the bottom of a leaf have different cells with specialized functions because miRNAs post-transcriptionally regulate which HD-ZIPIII transcription factors are expressed on the adaxial or abaxial side of the leaf. For example, the HD-ZIPIII transcription factor known as PHABULOSA (PHB) is normally expressed on the adaxial side of the leaf. On the abaxial side of the leaf PHB is not expressed because the PHB transcript is repressed by a miRNA. If PHB is not repressed by a miRNA on the abaxial side of the leaf, the abaxial side of the leaf develops adaxial characteristics (Kidner & Martienssen 2004). Since the HD-ZIPIIIs are transcription factors, they function to transcriptionally regulate genes that instruct the leaf cells to become specialized. The regulation of the HD-ZIPIIIs by miRNAs demonstrates how miRNAs initiate a cascade of gene regulation events where a single short RNA sequence controls the expression of multiple genes which establishes the differential gene expression that results in specialized cell types.
An Evolutionary Lens on miRNAs
Many of the miRNAs involved in regulating basic developmental processes, such as those described above, can be identified in multiple plant species, indicating that these miRNAs are evolutionarily ancient. These conserved miRNAs are often present in multiple copies and are highly expressed, which has aided in their isolation and characterization. Recently scientists identified a new group of miRNAs that appear to be evolutionarily younger than those previously characterized. These miRNAs have been overlooked by traditional technologies because they are less conserved between plant species, typically single-copy, and expressed at a lower level. The targets of this new class of miRNAs remain to be verified but initial predictions suggest that the targets of these miRNAs are involved in a wider variety of plant functions than the highly expressed, conserved miRNAs.
Mechanisms of miRNAs Are Similar in Plants and Animals
As described above, cleavage and translational inhibition are the two mechanisms by which miRNAs repress their mRNA target. The degree of base pairing (complementarity) between the miRNA and the target mRNA determines the mechanism by which the target mRNA is repressed. Traditionally it was thought that cleavage occurred in plants, and translational repression occurred in animals. This assumption was based on the fact that in plants, most identified miRNAs perfectly (or nearly perfectly) base pair with their mRNA targets while in animals, mismatches are more common between the miRNA and mRNA target. It has since been shown that most plant miRNAs use a combination of cleavage and translational repression (regardless of complementarity) to regulate the target mRNA (Brodersen et al. 2008). It's not yet clear if these two mechanisms can simultaneously function to regulate the target mRNA within a single cell, nor is it clear what factors determine if a target is cleaved or translationally repressed.
Summary
Since they were first discovered in animals, miRNAs have been shown to be powerful regulators of gene expression in many different organisms. These small RNAs base pair with and repress their target mRNAs by cleaving the target mRNA or preventing its translation. Many studies have examined the role of miRNA-directed gene regulation during development; however, it's becoming clear that the regulation of gene expression by miRNAs is not limited to developmental processes. Despite a flurry of research activity, many questions still remain about miRNA directed gene regulation. Future work will likely identify even more miRNAs and their mRNAs targets. In addition, scientists still wonder, what determines whether a target mRNA is destroyed by cleavage or translationally repressed?
References and Recommended Reading
Bohmert, K.et al. AGO1 defines a novel locus of Arabidopsis controlling leaf development. The EMBO Journal 17, 170–180 (1998) doi:10.1093/emboj/17.1.170.
Brodersen, P. et al. Widespread translational inhibition by plant miRNAs and siRNAs.Science 320, 1185–1190 (2008) doi: 10.1126/science.1159151.
Chen, X. et al. HEN1 functions pleiotropically in Arabidopsis development and acts in C function in the flower. Development 129, 1085–1094 (2002).
Garcia, D. A miRacle in plant development: Role of microRNAs in cell differentiation and patterning. Seminars in Cell & Developmental Biology
19, 586–595 (2008).
Golden, T. A. et al. Short integuments1/suspensor1/carpel factory, a dicer homolog, is a maternal effect gene required for embryo. Plant Physiology 130, 808–822 (2002).
Grigg, S. P. et al. SERRATE coordinates shoot meristem function and leaf axial patterning in Arabidopsis. Nature 437, 1022–1026 (2005) doi:10.1038/nature04052.
Kidner, C. A. & Martienssen, R. A. Spatially restricted microRNA directs leaf polarity through ARGONAUTE1. Nature 428, 81–84 (2004) doi:10.1038/nature02366.
Lee, R.C., Feinbaum, R. L., & Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75, 843–854 (1993).
Mallory, A. & Bouche, N. MicroRNA-directed regulation: to cleave or not to cleave. Trends in Plant Science 13, 359–367 (2008).
Reinhart, B.J. et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 403, 901–906 (2000) doi:10.1038/35002607.
Voinnet, O. Origin, biogenesis, and activity of plant microRNAs. Cell 136, 669–687 (2009).
Wightman, B., Ha, I. & Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75, 855–862 (1993).
Yu, L. et al. HYL1 gene maintains venation and polarity of leaves. Planta 221, 231–242 (2004) doi:10.1007/s00425-004-1439-7.



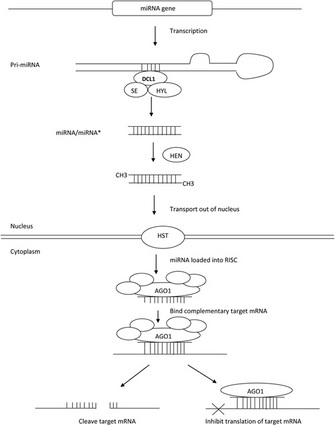
 Figure 1: An overview of miRNA biogenesis and function in Arabidopsis
Figure 1: An overview of miRNA biogenesis and function in Arabidopsis