« Prev Next »
All multicellular organisms begin life as a single cell, the zygote, created by the union of two germ cells, an oocyte and a sperm. Although scientists have understood for decades that the quantity of DNA an individual inherits from each parent is equivalent, a 1984 discovery by McGrath and Solter revealed that these genes are used differently depending on whether the genes are inherited from the mother or the father (McGrath & Solter 1984). These two scientists used the technique of nuclear transplantation — when the nucleus of one cell is removed and placed into another cell — and asked two questions: Can mouse embryos derived from the nuclei of two oocytes create a viable embryo? What about nuclei from two sperm? They found that these uniparental embryos were not viable and concluded that both maternal and paternal nuclear genomes are necessary for proper embryonic development. But why?
Early Clues That Maternal and Paternal Genes Are Not Equivalent
Differences between the maternal and paternal genomes were even apparent, at some level, to horse breeders in ancient times: they observed that when a female horse is mated to a male donkey, the resulting offspring is a mule. On the other hand, when a male horse is mated to a female donkey, the offspring is a hinny — an incorrigible animal with temperament and physical traits that are much less desirable than those of a mule.
In the mating of a horse and a donkey, it seems to matter which parent was the horse (male or female), and which was the donkey. But why would this matter?
Not until the late 1980s did scientists begin making discoveries that helped explain these and other related observations. Using two different restriction enzymes that recognize the same DNA sequence but that respond differently if the cytosine in the recognition sequence is methylated or not (called isoschizomers), scientists learned that the nuclear genes of sperm and oocyte are modified differently and carry different tags on their DNA, called epigenetic marks. But what, exactly, are these marks that are attached to DNA? Epigenetic marks are heritable non-base-pair changes such as DNA methylation, histone modifications, and higher-order chromatin organization events that affect how genes get expressed.
Scientists give a special name to oocyte and sperm genes from the same species that contain different epigenetic marks, which cause them to be differentially expressed in the zygote and subsequent developing embryo; they are called imprinted genes. It turns out that some genes, a few hundred at most, are imprinted differently in the sperm than they are in the egg, and this difference explains why nuclei from two oocytes or two sperm are unable produce a viable embryo. For this reason, we can explain the donkey versus the hinny phenomenon: Imprinted genes in oocytes of female horses are different from those in sperm of male horses, and the same is true for donkeys.
The Cycle of Imprinting in a Series of Generations
Now that we know that some oocyte and sperm genes are imprinted differently, this knowledge raises a very interesting and important question: How do these differences get established in the first place? Because germ cell genes, but not somatic cell genes, are passed on to the next generation, imprinted marks present in the zygote must be erased and then reset at some point during germ cell development. The gametes produced by the germ cells of the developing embryo need to be imprinted with the proper marks according to the sex of the developing embryo so that their genes will be programmed properly as "on" or "off" in the next generation. However, in the somatic cells of the developing embryo, these same imprinted marks must be retained because correct expression of the imprinted genes is essential for normal somatic development. How do germ cells become epigenetically different from somatic cells, and how do germ cells navigate the process of erasing and then resetting their imprinted marks?
Scientists have focused on the mouse as a model system for studying the dynamic nature of epigenetic marks during germ cell establishment because (unlike humans) mice are very amenable to genetic manipulations. Also important is that imprinting patterns in mice are very similar to those in humans, so scientists can relate their discoveries in mice to human germ cell development. How does germ cell development in mice work? What are the epigenetic changes that occur in mice germ cells throughout their maturation? Studies in mice help us generate a picture of exactly how oocyte and sperm become properly programmed at the epigenetic level.
The Creation of Primordial Germ Cells
To understand how germ cell genes become imprinted, we must first learn how germ cells develop in an embryo. Germ cells are first created in a 6.5-day embryo, and at this stage they are called primordial germ cells (PGCs). How are PGCs specified? A critical experiment that helped answer that question was performed by Lawson and coworkers who made the observation that embryos lacking both copies of Bmp4, a gene that makes a signaling molecule BMP4, never developed any PGCs. They went on to show that early in the developmental program of mice, certain embryonic cells signal their neighbors, via BMP4, and cause the cells that receive the BMP4 signal to become PGCs (Lawson et al. 1999). Those experiments were strong evidence that BMP4 has a strong influence over whether a cell will become a germ cell.
Several years later, another group of scientists determined that this BMP4 signaling event induced the PGCs to express a protein called BLIMP1 (Chang & Calame 2002). Ohinata and colleagues then asked: What would happen to PGCs in mice with only one copy of the Blimp1 gene? They observed that mice heterozygous for Blimp1 produced approximately one-half the number of germ cells compared with animals with two wild type copies of the gene (Ohinata et al. 2005; Vincent et al. 2005). They concluded that BLIMP1 is therefore critical to the complete formation of a germ cell lineage.
Although scientists do not completely understand how BLIMP1 works, they have some ideas of how it does what it does to specify germ cells. One major clue comes from the observation that BLIMP1 suppresses HOX gene expression. This interaction between BLIMP1 and HOX genes is significant because certain HOX genes activate somatic cell differentiation (Saitou et al. 2002). However, at day 6.5 of development, PGCs seem to be epigenetically similar to their somatic cell counterparts in the developing embryo. Not until they start to move around do they become distinct.
Epigenetic Changes in Migrating PGCs of the Developing Embryo
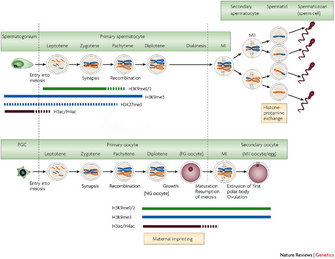
Scientists have observed that in the migrating PGCs, compared with the somatic cells around them, the levels of methylated cytosine are reduced and certain types of methylated histones are increased (Figure 2) (Sasaki & Matsui 2008; Hajkova et al. 2010). These epigenetic marks are one of the first ways that PGCs become epigenetically different from other cells, and the marks are usually repressive, meaning that they suppress gene expression. However, scientists still do not know how these methylation differences might be important for PGC function.
Scientists do know that subsequent changes in a different type of epigenetic mark, the Xist RNA, are very important for PGC biology. These changes are female-specific, meaning that they happen only in female embryos, and only on X chromosomes. Remember that female mice have two X chromosomes, whereas male mice have only one. In embryos with two X chromosomes, Xist RNA coats one of the them so as to keep that chromosome transcriptionally repressed. This coating action equalizes the expression of genes located on the X chromosome in males and females. Teams of researchers led by de Napoles and Sugimoto found that, specifically during migration, the PGCs of female embryos undergo a reduction in Xist RNA levels. This reduction of Xist RNA only occurs in the PGCs, not in somatic cells (de Napoles et al. 2007; Sugimoto & Abe 2007). PGC-specific reduction of Xist RNA is critical because, unlike somatic cells, germ cells must maintain two active X chromosomes for subsequent gamete formation. During the process of meiosis, the homologous X chromosomes exchange DNA, and this process requires two "equal" X chromosomes.
Epigenetic Changes at the Gonad Development Stage
What happens after migration ends and the PGCs arrive at the gonad? In 2002, Lee and colleagues discovered that at this developmental stage, a second wave of DNA demethylation occurs and erases the methylation marks of imprinted genes in the PGC genomes, thereby wiping their slates clean (Lee et al. 2002). As discussed above, because genes are imprinted differently depending on whether they are of maternal or paternal origin, such erasure is essential to ensure that the epigenetic marks in the PGCs can be reset and reflect the sex of the developing embryo. Specifically, the PGCs of developing females receive one type of imprint, whereas the PGCs of developing males receive another.
Lee and colleagues also observed that imprinted genes of somatic cells are not demethylated. This result makes sense because somatic cells need to retain their maternal and paternal imprints, an action that ensures proper gene expression in the developing embryo.
After this erasing of methylation marks, how do new gene imprints become properly reestablished in the PGC genomes? This is still largely a mystery, but early in the first decade of the twenty-first century, scientists discovered that marks are not reset until after sex determination has started within the embryo; imprints need to be either maternal or paternal in nature, depending on the sex of the developing embryo and, as one might expect, proper resetting requires at least several different enzymes, known collectively as DNA methyltransferases. These enzymes add methyl groups to the certain cytosine nucleotides. The specific methyltransferases will only act on certain gene loci, and some are specific to whether the imprint is maternal or paternal.
Interestingly, the timing of imprint reestablishment is also sex-dependent. This discovery was made in two different research groups, around the same time. In 2001, Bourc'his and colleagues determined that, in females, imprinting occurs postembryonically, well after meiosis I is completed (Bourc'his et al. 2001). Separately, only a year earlier, Udea and colleagues found that, in males, imprinting begins shortly after sex determination in the developing embryo, well before meiosis begins (Ueda et. al. 2000). Why would the timing of imprint reestablishment be different in males and females? There are no answers to this question yet. These discoveries about timing differences are only two examples on an ever-growing list of how epigenetic marks are generated differently in the two sexes.
Completing the Cycle: Epigenetic Control During Gametogenesis
Up until now the discussion has focused on how the establishment and maintenance of epigenetic marks differ for PGCs versus somatic cells and in male germ cells versus female germ cells. What about gametogenesis? Are epigenetic marks also important for the very process that generates oocytes and sperm?
As a complement to this work with methyltransferases, Akiyama and colleagues determined in 2006 that another histone modification, histone acetylation, is also important for proper chromosome segregation within developing gametes (Akiyama et al. 2006). When these scientists chemically inhibited histone deacetylation (an experimental trick to retain the acetylation when it would normally be removed), they found that oocytes were fertilized normally, but the number of live births from the treated oocytes was substantially reduced. Somehow, the level of acetylation influenced how many successful embryos were formed. They then asked: What makes these embryos produced from the chemically treated oocytes fail at such high rates? When they looked at the embryos, they discovered that more than 50 percent of them were aneuploid; that is, they had the wrong number of chromosomes. Therefore, there was likely some error in their chromosome duplication or segregation.
To understand further, Akiyama et al. then examined histone acetylation levels in oocytes of mice of different ages to discern if the age of the mother affected histone acetylation levels, and they found significantly higher levels of histone acetylation in oocytes from older mice. Interestingly, scientists have known for some time that the rate of aneuploidy in oocytes of both mice and humans increases with maternal age. For instance, the rate of trisomy 21 (Down syndrome) in humans, wherein there is an extra copy of chromosome 21, increases dramatically with the age of the mother. With these studies in mice, Akiyama and colleagues have demonstrated a plausible link between the retention of acetylated histone marks in oocytes of older mothers and nondisjunction at metaphase of meiosis II. These findings underscore how important histone deacetylases are for chromosome segregation during gamete formation (Akiyama et al. 2006). In the above examples, certain epigenetic marks are essential for proper progression of a cell through meiosis, the fundamentally important process that produces germ cells, and distinguishes them from all other cells in the body.
Summary
Scientists have learned much about the formation of germ cells and how epigenetic marks in these cells are erased and reset to ensure normal development in subsequent generations. Clearly, the instructions for germ cell specification, maturation, and function involve much more than the simple order of bases in the genomes of these cells. Proper DNA methylation, histone methylation, and histone acetylation are all essential for the production of mature, functional gametes that make it possible for the cycle of life to perpetuate generations of animal species. But the most difficult questions still remain unanswered.
For instance, what triggers the erasure of imprinted marks in germ cells, and how is such erasure prevented in somatic cells? And, once DNA methyl marks are erased in the PGCs, how are the correct cytosines methylated later while keeping track of one pattern for male gametes and a very different one for female gametes? How is it that histones of only certain nucleosomes are recognized for modification during germ cell development? And how do epigenetic marks help control proper chromosome segregation during meiosis? Given the pace at which investigators have made recent progress, it seems likely that even these difficult puzzles will be solved quite soon, giving us a much more complete picture of how germ cells are programmed correctly to carry out their many incredible tasks.
References and Recommended Reading
Akiyama, T. et al. Inadequate histone deacetylation during oocyte meiosis causes aneuploidy and embryo death in mice. Proceedings of the National Academy of Sciences 103, 7339–7344 (2006).
Bourc'his, D. et. al. Dnmt3L and the establishment of maternal genomic imprints. Science 294, 2536–2539 (2001).
Chang, D. & Calame, K. The dynamic expression pattern of B lymphocyte induced maturation protein-1 (Blimp-1) during mouse embryonic development. Mechanisms of Development 117, 305–309 (2002).
de Napoles, M. et al. Early loss of Xist RNA expression and inactive X chromosome associated chromatin modification in developing primordial germ cells. PLoS ONE 2, e860 doi:10.1371/journal.pone.0000860.
Hajkova, P. et al. Genome-wide reprogramming in the mouse germ line entails the base excision repair pathway. Science 329, 78–82 (2010) doi:10.1126/science.1187945.
Hayashi, K. et al. A histone H3 methyltransferase controls epigenetic events required for meiotic prophase. Nature 438, 374–378 (2005) doi:10.1038/nature04112.
Lawson, K. A. et. al. BMP4 is required for the generation of primordial germ cells in the mouse embryo. Genes and Development 13: 424–436 (1999).
Lee, J. et. al. Erasing genomic imprinting memory in mouse clone embryos produced from day 11.5 primordial germ cells. Development 129, 1807–1817 (2002).
McGrath, J. & Solter, D. Completion of mouse embryogenesis requires both the maternal and paternal genomes. Cell 37, 179–183 (1984).
Ohinata, Y. et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature 436, 207–213 (2005) doi:10.1038/nature03813.
Saitou, M. et al. A molecular programme for the specification of germ cell fate in mice. Nature 418, 293–300 (2002) doi:10.1038/nature00927.
Sasaki, H. & Matsui, Y. Epigenetic events in mammalian germ-cell development: Reprogramming and beyond. Nature Reviews Genetics 9, 129–40 (2008) doi:10.1038/nrg2295.
Sugimoto, M. & Abe, K. X. Chromosome reactivation initiates in nascent primordial germ cells in mice. PLoS Genetics 3, 1309–1317 (2007) doi:10.1371/journal.pgen.0030116.st002.
Tachibana, M. et al. Functional dynamics of H3K9 methylation during meiotic prophase progression. Embo Journal 26, 3346–3359 (2007).
Ueda, T. et al. The paternal methylation imprint of the mouse H19 locus is acquired in the gonocyte stage during foetal testis development. Genes Cells 5, 649–659 (2000).
Vincent, S. D. et al. The zinc finger transcriptional repressor Blimp1/Prdm1 is dispensable for early axis formation but is required for specification of primordial germ cells in the mouse. Development 132, 1315–1325 (2005) doi:10.1242/dev.01711.



 Figure 1
Figure 1