« Prev Next »
Changes in gene expression can be mediated by a variety of signals emanating from the environment. One such signal is the amount of oxygen in the atmosphere. Aerobic animals require oxygen in order to survive. In the short term, however, these organisms can adapt to conditions of low oxygen (hypoxia) through a variety of gene expression changes mediated primarily by a heterodimeric transcription factor called hypoxia-inducible factor (HIF). HIF has two protein parts: HIF1β and HIF1α. HIF1β is constitutively expressed. This stable expression in one part of the HIF complex is countered by the unstable HIF1α, whose expression changes depending on the oxygen concentration sensed by an individual cell. The HIF1β subunit is the component that binds to DNA at specific consensus sequences called hypoxia response elements (HREs), but transcriptional activation only occurs when HIF1α is bound together with HIF1β at these sites.
While some control of gene expression is mediated through control of whether a gene is transcribed, the effects of gene expression can also be controlled after translation has occurred. For example, once a protein is produced, a cell has ways of increasing how rapidly that protein is degraded. This is true for a variety of proteins, including HIF1α.
"Tagging" HIF1α for Degradation
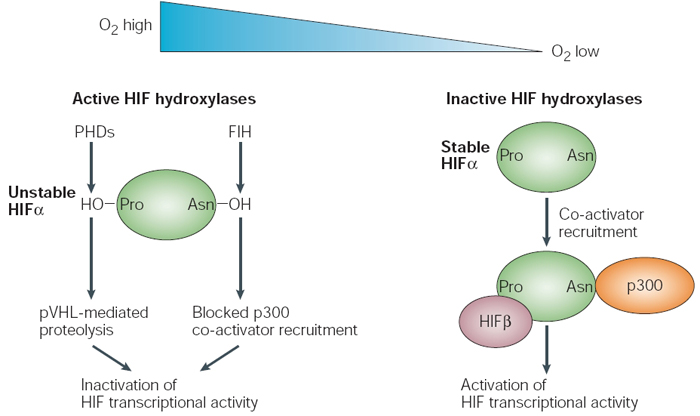
Within HIF1α, there are conserved proline residues (in the ODD domains) and asparagine residues (in the CAD domain) that undergo hydroxylation in the presence of oxygen. Hydroxylation is a biochemical process whereby hydroxyl groups are attached to important places in these domains under normoxic conditions. Specifically, enzymes called prolyl hydroxylases (PHDs) add hydroxide to the proline residues, while a protein called FIH (factor-inhibiting HIF) adds hydroxide to the asparagine residue. The resulting amino acid hydroxylation causes a change in the structure of HIF1α, and this change gives the protein an increased affinity for binding with another cellular protein called the von Hippel-Lindau tumor suppressor (pVHL). The pVHL is a protein involved in a chemical process that covalently modifies other proteins with a chemical called ubiquitin, which marks these proteins for degradation by proteasomes. However, when oxygen is not present in adequate amounts, hydroxylation cannot occur, which means that HIF1α can be stably expressed. As a result, this protein can bind to HIF1β and facilitate gene transcription of many HIF target genes (Figure 1; Schofield & Ratcliffe, 2004).
Hypoxia as a Signal?
Some organisms, like the roundworm C. elegans, can develop normally without functional HIF. Under normal oxygen conditions, these organisms develop alongside their wild-type counterparts. However, when stressed with low oxygen conditions, the HIF-deficient animals are highly sensitive, suggesting that their cells are not able to adapt to the hypoxic environment. This leads to high rates of death, as illustrated in Table 1 (Jiang et al., 2001).
| % Oxygen | Wild-Type Worms | HIF1α Mutant Worms | ||
| % Embryonic Lethality | Total Animals | % Embryonic Lethality | Total Animals | |
| 21 (normal) | 1.3 | 475 | 0.2 | 501 |
| 1 | 3.6 | 503 | 66.3 | 596 |
| Table 1: HIF1α mutant worms exhibit reduced viability under hypoxic conditions (Table adapted from Jiang et al. 2001) | ||||
In other organisms, including mice, it seems that hypoxia
might play a more important role in development. For example, scientists have
been able to remove the normal HIF1α gene in mice, resulting in a HIF1α knockout. Early in development,
the knockout embryos appear to develop almost normally, but they soon show
clear signs of developmental abnormalities and eventually die before birth. There are clear differences during the early embryonic days of development, most notably the size of the embryo. After initial periods of growth, the embryo stalls, and from day 8 to day 9 the size shrinks by almost 50%. Close inspection of the tissue reveals that one of the clearest defects in HIF1α knockout mice is severe reduction in the vascularization of many tissues. This is not surprising considering that it is well-documented that hypoxic conditions trigger the growth of vessels; moreover, one of the major HIF target genes is vascular endothelial growth factor (VEGF), which creates a protein that stimulates angiogenesis.
HIF Target Genes
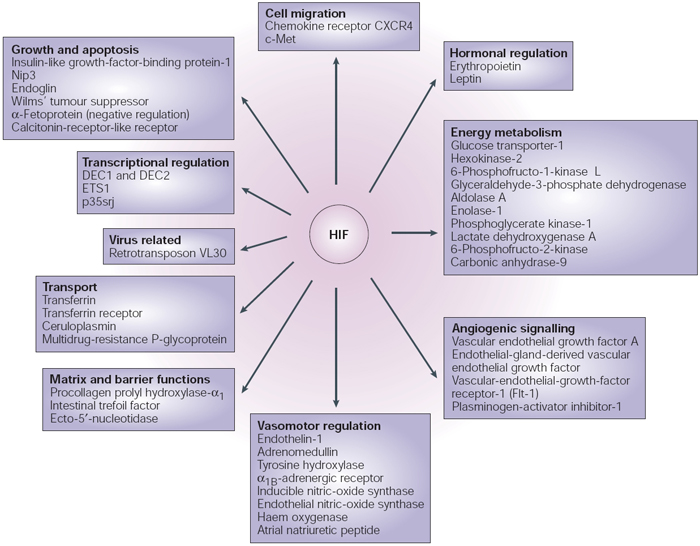
Multiple studies have attempted to identify the downstream targets of HIF. Investigators have often found differential responses depending on the conditions and cell types used. This heterogeneity of gene regulation responses reflects the heterogeneity of cellular requirements. While some cells, like those in the bronchial epithelium, are exposed almost constantly to atmospheric levels of oxygen, other cells, such as those in cartilage with few blood vessels, exist in low oxygen conditions; thus, the cells in both tissues are best adjusted to thrive in their unique environments.
In one study that attempted to tease out this variation by examining specific cell types under hypoxic and normoxic conditions, scientists were able to identify a potential "signature" of hypoxic gene expression (Chi et al., 2006). Using DNA microarrays, these investigators examined changes in gene expression at a global level in different cell types. In looking for a signature of hypoxia, the researchers compared expression changes among all the different cell types in different oxygen concentrations, and they then had a computer program search the data for similarities (and differences). In total, the research team looked at almost 2.5 million measurements of gene expression, and they noted an expression signature whenever the same gene appeared to be controlled in the same way under the same conditions in multiple cell types.
After examining thousands of different genes for changes in expression, the scientists were indeed able to identify the patterns depicted in Figure 2. (In addition to describing these signatures, the researchers also noted that each individual cell and/or tissue type had certain genes specific to its own response to hypoxia.) Many of the genes that showed up in the signatures were those that had previously been shown to be HIF targets, including various genes involved in glucose metabolism, angiogenesis, cell division, and cell death. Many of the genes repressed by hypoxic conditions were involved in cell proliferation, suggesting that, under adverse conditions, cells shift from a growth mode to a mode that attempts to conserve energy.
Hypoxia and Disease
One reason why C. elegans might be able to develop properly without a HIF1α gene is because this organism is so small that a truly hypoxic environment is never created during development. Oxygen can reach all of the worm's cells directly from the environment; therefore, there is never a need for the cells to "turn on" HIF target genes. This might also be why larger organisms, like mice, exhibit an increased need for HIF activity during development.
This is also why HIF plays such an important role in cancer development. As a solid tumor grows in size, the central cells of that mass have less and less access to oxygen via diffusion. Therefore, the central cells begin to experience hypoxia, stabilize HIF1α, and turn on downstream genes, including VEGF. The VEGF protein thus not only plays an important role in vascularizing the embryo (thereby ensuring that oxygen is available to growing tissues), but it also plays an important role in recruiting blood vessels to growing tumors.
When Chi et al. (2006) looked at different cell types, they concentrated on looking for commonalities between epithelial cells—the type of cells that are involved in carcinomas. When they compared the genes in the hypoxia-response signature of normal cells to that of different cancer cells, they found that they were able to classify different tumors by their hypoxia response. For example, clear-cell renal cell carcinoma had a high hypoxia response, while all other kidney cancers had a low response. In addition, when the research team compared different types of breast cancer, patients with tumors that had a high hypoxia response also had lower survival rates.
Transcriptional Regulation Using Posttranslational Modifications and Stability
There are several hundred genes regulated by hypoxia, and much of that regulation occurs through HIF (Figure 3). HIF1α protein instability and ultimate degradation of the protein under normoxic conditions is just one example of the regulation of gene products that occurs after transcription has been completed. This kind of regulation of a transcription factor controls the expression of numerous other important genes that have impacts on both development and disease.
References and Recommended Reading
Chi,
J. T.,
et al. Gene expression programs in
response to hypoxia: Cell type specificity and prognostic significance in human
cancers. PLoS Medicine
3, e47 (2006) (link to article)
Giaccia, A. J., et al. The biology of hypoxia: The role of oxygen sensing in development, normal function, and disease. Genes and Development 18, 2183–2194 (2004) (link to article)
Jiang, H., et al. The Caenorhabditis elegans hif-1 gene encodes a bHLH-PAS protein that is required for adaptation to hypoxia. Proceedings of the National Academy of Sciences 98, 7916–7921 (2001)
Ryan, H. E., et al. HIF-1α is required for solid tumor formation and embryonic vascularization. EMBO Journal 17, 3005–3015 (1998)
Schofield, C. J., & Ratcliffe, P. J. Oxygen sensing by HIF hydroxylases. Nature Reviews Molecular Cell Biology 5, 343–354 (2004) (link to article)



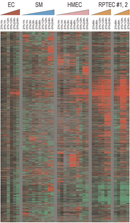
 Figure 2
Figure 2