« Prev Next »
Spinal muscular atrophy (SMA) is an autosomal recessive disorder characterized by degeneration of motor neurons, thereby leading to progressive muscle weakness and atrophy. SMA is caused by the deletion of, or a mutation in, the survival motor neuron 1 (SMN1) gene on chromosome 5. The SMN locus on chromosome 5 is fairly complex and includes multiple copies of the SMN gene. In fact, the number of gene copies, as well as the number and types of mutations present in these copies, act together to influence the disease phenotype. SMA affects between 1 in 6,000 and 1 in 10,000 babies, and approximately 1 out of every 80 individuals is a carrier of the disease mutation in the SMN1 gene. These statistics mean that SMA is one of the more common human genetic disorders.
The SMA phenotype is widely variable, with the severity of symptoms influenced by several genetic factors. In fact, siblings who have inherited the same SMN mutations can have very different phenotypes, possibly due to gene interaction. Diagnosis of SMA is made based upon physical symptoms that include poor muscle tone in the limbs and trunk, feeble movements of the arms and legs, swallowing difficulties, a weak sucking reflex, and impaired breathing. Type I SMA is the most severe form of this disease, and it is typically diagnosed within a few months of birth. The prognosis is poor for children with type I SMA, and most die within two years. Type II SMA has an onset between six and 18 months. Affected children are usually able to sit without support if placed in position, and some may be able to stand or walk with help. Those children with a later age of onset tend to have a better prognosis, with some having a normal life expectancy. Type III SMA has an even later age of onset (toddlerhood to adolescence). Children with this form of the disease can stand alone and walk, but they may have difficulty getting up from a sitting position. Finally, many authorities also recognize an adult-onset type IV SMA, which has even milder symptoms. While the classification of SMA into these subtypes is helpful to patients and their doctors, experts actually consider the disease to exist on a continuum, with indistinct separations between the types. This further emphasizes the genetically complex nature of SMA.
Families that are affected by SMA will be faced with many decisions regarding the health of affected children and the well-being of other family members. Some of these families' decisions may elicit criticism from the general public, but choices regarding medical care and familial genetics can only be made by those people whose lives are affected.
Sharing Genetic Information
To better understand the ethical difficulties associated with a condition like SMA, suppose that a couple who has one child with SMA decides to undergo genetic testing, and both parents are found to be carriers of mutations causing SMA. Based upon this information, it is almost certain that other family members are also carriers of these mutations. A genetic counselor could help this couple develop strategies to accurately inform their family members of their possible risk of SMA without being alarming. For instance, siblings of this couple, especially if they are of reproductive age, might want to know whether they are carrying the mutation that causes this possibly fatal genetic disorder. But what if the couple is unwilling to share this information with their family members? Should the counselor be expected to break confidentiality to inform the at-risk family members of their genetic risk?
Family Planning
Next, imagine that a couple with one child who has SMA would like to have additional children. In this case, the genetic counselor will need to make sure that the couple understands the genetic basis of SMA and its inheritance pattern so that they may make informed choices about family planning. Because each parent is a carrier of a mutation causing SMA, this couple's risk for having a child with SMA is 25% for each pregnancy. Thus, one option for this couple is to do nothing at all and take their chances on having another child with SMA. A family whose child is not severely affected might be more willing to choose this route. A genetic counselor should remind them, however, that the disease phenotype is not predictable, and even siblings with the same SMN mutations can have very different outcomes.
Another option available to this couple is in vitro fertilization (IVF) with preimplantation genetic diagnosis (PGD). In this case, only those embryos without SMA are transferred back to the mother's uterus. This is obviously an expensive course of action. The couple might therefore instead choose to test an ongoing pregnancy by way of chorionic villus sampling (CVS) and then decide whether to terminate. CVS is similar to amniocentesis in that a sample of fetal tissue is removed from the mother for testing. Specifically, in the CVS procedure, a sample of the placenta is removed via catheter at 10-12 weeks of gestation. This is much earlier in the pregnancy than when amniocentesis (a sampling of the amniotic fluid surrounding the fetus) is typically performed. Earlier diagnosis with CVS allows a couple to terminate a pregnancy before fetal movements are felt. Of course, for some couples, this is not an option. These couples might still choose prenatal testing, however, to have time to prepare for an affected baby and to set up early intervention breathing and physical therapies for the newborn. It is the genetic counselor's job to inform a couple of all of their choices, along with the associated risks and benefits, to enable the couple to make the family planning decision that is right for them.
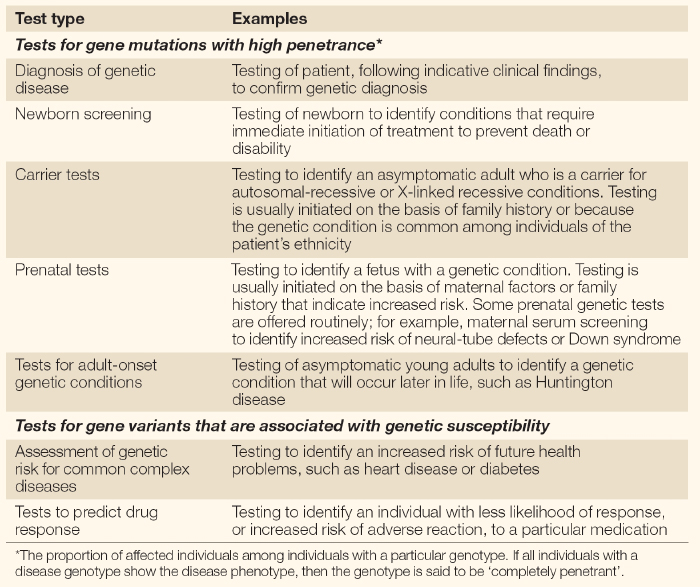
Carrier Testing in Healthy Children
Now, say that a couple with three children, the youngest of whom has SMA, wants to know whether their older children are carriers of the disease mutation. The mother is shocked when the children's doctor refuses to order the DNA test to determine the older children's carrier status. The doctor explains to the mother that the children have no symptoms of SMA, so there is no clinical reason to perform this test. Furthermore, the doctor feels that deciding to be tested for the SMA mutation is a choice the children should make for themselves when they are adults. Their mother, on the other hand, simply wants to be sure her children are able to make informed decisions about their reproductive health. But who has the right to this vital information?
Newborn Screening
Genetic testing for SMA is available, but should this disorder be added to the newborn screening panel? Some parents of children with SMA feel that diagnosis at birth would be helpful (Meldrum et al., 2007). They argue that with newborn screening, families can begin intervention strategies right away, rather than waiting until symptoms manifest and a diagnosis is made. Opponents argue that there is not enough benefit gained from early diagnosis to justify the cost of testing every newborn. In short, there are simply no effective treatments for SMA to prevent or delay symptoms.
State guidelines governing the inclusion of a disease on the newborn screening panel suggest that if the cost of testing every infant is not outweighed by the benefit of early detection, then the test should not be added to the panel. Unfortunately for SMA families, this critical information is therefore not available to most parents until later on. Perhaps if a treatment became available that could delay or prevent the onset of symptoms, adding SMA to a newborn screening panel would seem more justified. An outspoken advocacy group, however, can greatly influence policy decisions. Is this fair? Is there ever one debilitating disease that is more deserving of attention than others? Is there a fair way to prioritize which diseases are added to newborn screening panels?
Access to Long-Term Care
Many families who receive a diagnosis of SMA become overwhelmed by the costs of caring for their affected child. For example, children with a moderate phenotype (type II SMA) typically need mobility assistance at some point in their lives. Say that a family wants to provide their child who has SMA with a self-maneuvering wheelchair to give her more independence, but they cannot afford one. Should resources be provided for such families? Similarly, children with a severe phenotype (type I SMA) have trouble breathing and swallowing and require interventions to overcome these problems. Should families without the resources to pay for specialized medical equipment have to settle for palliative care for their children?
Of course, cost is not the only issue to consider when thinking about access to care. Even in cases in which care expenses might be covered through a variety of programs, individuals need to be aware that such programs exist. Parents who do not speak English, for example, might encounter difficulty in learning about and managing different treatment programs. Others might find it difficult to balance the requirements of work with medical visits. Planning to care for a loved one with a disorder that lasts a lifetime should thus involve help from long-term care professionals.
Stem Cell Research
Stem cells hold great medical potential. These cells occur naturally in embryonic tissue and the placenta, and they can differentiate into any type of tissue in the body. In the case of SMA, for instance, stem cells might be used to regenerate damaged motor neurons. For many SMA families, sacrificing human embryos in the name of research to cure their children is justifiable; for other families, it is unconscionable. Stem cells derived from human embryos and placental tissue are coveted for many areas of research, and they are viewed as a possible treatment for Parkinson's and Alzheimer's diseases, spinal cord injury, stroke, burns, heart disease, diabetes, osteoarthritis, and rheumatoid arthritis. Interestingly, in June 2007, scientists reported that by turning on the expression of certain genes, adult mouse cells could be reverted to an embryonic state (Okita et al., 2007; Wernig et al., 2007; Maherali et al., 2007). If an adult source of stem cells can be harvested in this way, the debate over use of embryonic stem cells will end. Until then, we can expect continued gridlock between those who support research using human stem cells and those who believe that experimenting with human fetal tissue is unethical and immoral.
SMA is just one of many medical conditions providing a context for these discussions of ethical dilemmas. Just as approaches to treating disease will change with progressing technology, our responses to these dilemmas will evolve alongside society's shifting viewpoints. Thus, in the future we should expect new topics for ethical discussions of genetic disease.
References and Recommended Reading
Burke, W., et al. Ensuring the appropriate use of genetic tests. Nature Reviews Genetics 5, 955–959 (2004) doi: 10.1038/nrg1495 (link to article)
Maherali, N., et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell 1, 55–70 (2007) doi:10.1016/j.stem.2007.05.014
Meldrum, C., et al. Spinal muscular atrophy genetic counseling access and genetic knowledge: Parents' perspectives. Journal of Child Neurology 22, 1019–1026 (2007) doi:10.1177/0883073807305672
Okita, K., et al. Generation of germline-competent induced pluripotent stem cells. Nature 448, 313–317 (2007) doi:10.1038/nature05934 (link to article)
Wernig, M., et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448, 318–324 (2007) doi:10.1038/nature05944 (link to article)






























