« Prev Next »
Historically, advances in science are typically made before society can conduct meaningful ethical and moral discussions about these advances. However, such discussions are crucial to setting policies that balance individual and public protections with the use of technology in medicine.
The in vitro fertilization (IVF) "miracle" of the late 1970s occurred five years before the polymerase chain reaction revolutionized the field of genetics, 18 years before the first bacterial genome was sequenced, and 26 years before completion of the human genome sequence. The intervening 30 years involved an explosion of knowledge of mammalian biology. Throughout this period, many members of the general public, especially those with incurable diseases or loved ones with incurable diseases, have been enthusiastic about the therapeutic application of these advances. Although curing disease is indeed one goal of genetic research, investigation of the overall safety of a treatment is also critical, as is consideration of the moral and ethical issues associated with a treatment. Although the first two tasks are reserved for scientists and clinical investigators, the latter is everyone's responsibility. This is particularly true when it comes to three recent "hot button" genetics-based therapies: gene therapy, stem cell therapy, and pharmacogenomics.
Gene Therapy
Human DNA is estimated to have approximately 12 million single nucleotide polymorphisms (SNPs) and thousands of copy number variants (CNVs), most of which are not harmful. However, genetic disorders do sometimes occur as a result of mutations that alter or inhibit protein function. Gene therapy focuses on correcting these mutated or defective genes by way of the following techniques:
- Random insertion of a normal gene into the genome (most common technique)
- Replacement of the abnormal gene with a normal one
- Repair of the abnormal gene
- Altering regulation of a particular gene
To date, the therapeutic results of gene therapy have been marginal. This approach holds much potential, however, especially for treating single-gene disorders, and it is therefore being actively investigated.
Ethics of Gene Therapy
The concept of changing a person's DNA, even to cure a fatal genetic disease, differs from more traditional remedies like surgery, pharmaceuticals, and physical therapy, and it is frightening to some people. Successful treatment approaches are available for a handful of single-gene disorders, most of which are enzyme deficiencies, including Gaucher's disease (a lysosomal storage disorder) and phenylketonuria (phenylalanine hydroxylase deficiency). Other disorders, including Duchenne muscular dystrophy (DMD), are the result of the complete loss of a functional protein. DMD is an X-linked recessive disorder that is caused by a mutation in the dystrophin gene. Mutation of this gene results in failure of muscle regeneration, leading to progressive muscle weakness and eventually causing fibrosis in essential organs (i.e., the heart and diaphragm). At this time, no medical, surgical, or other option exists to correct the underlying genetic cause of DMD and preserve muscle function in affected males. For individuals with DMD, the promise of gene therapy through one of the methods listed above means the possibility of leading a normal life.
Despite such potential benefits, many people oppose gene therapy on religious grounds, believing that altering genetic material is against God's will. This argument appears to hold the most sway because it raises the specter of "playing God". Table 1 lists some other frequently debated topics related to the inclusion of gene therapy in mainstream medical treatment. Many of these questions can be applied to any type of human research.
Table 1: Topics of Discussion in Gene Therapy Ethics
|
Adapted from Genetics Home Reference at http://ghr.nlm.nih.gov/handbook/therapy/ethics and from Kolehmainen, 2000
Scientific Obstacles
The scientific obstacles to gene therapy are related to the vehicles used to deliver normal genes. Most attempts at gene therapy involve inserting a normal or slightly modified version of a gene sequence into a viral vector. This virus then carries the gene into the patient's body and to the targeted tissue. Indeed, some success has been achieved in treating DMD using this method. However, this and other uses of viral vectors are often hindered by issues related to the patient's immune response, the specificity of delivery, and insertional mutagenesis.
As previously mentioned, one complicating factor in gene therapy is that many of the body's immunological defenses that are used to tackle infections are activated when a virus is present, including those viruses used as vectors to carry transgenes designed to cure genetic disease. Adenoviral vectors are the most immunogenic of all the viral vector groups, and they can have devastating effects. For example, an immune response to this vector was the cause of death in the case of Jesse Gelsinger, a young man who died during an experimental gene therapy trial in 1999.
Moreover, although natural infections with wild-type viruses are restricted to certain tissues based on the route of transmission, recombinant vectors are not subject to the same physical limitations. In other words, viral vectors are "promiscuous," and systemic delivery generally leads to unwanted vector uptake by many different cell types in multiple organs. Indeed, there are concerns that random insertion of these integrating viral vectors, many of which are derived from retroviruses, can inadvertently lead to mutagenesis and cancer (Hacein-Bey-Abina, 2003; Thomas, 2003).
Despite such problems, the realm of gene therapy has evolved since the 1999 study that took Gelsinger's life, and a number of successes have been documented. The first gene therapy success was in 2000, when three children were cured of a fatal immunodeficiency disorder. Later, in 2005, Qiao et al. reversed muscular dystrophy in mice using a single injection of the normal version of the dystrophin gene. More recently, two independent studies reported partial sight restoration in four young adults who were born with severe blindness, again with a single injection of a curative gene (Bainbridge et al., 2008). The years since the Gelsinger tragedy have thus been filled with a mixture of successes and failures on the gene therapy front. And, although advances continue to be made, much is left to be done before this type of therapy is ready for full clinical integration.
Stem Cell Therapy
Beyond gene therapy, another issue of much debate relates to the use of stem cells. These cells can be divided into two broad classes: embryonic and adult. Both classes are currently being explored for possible therapeutic applications.
Embryonic Stem Cells
Following IVF, chromatin from donor cells joins to form pronuclei in the oocyte cytoplasm. The membranes of these pronuclei eventually disintegrate, creating a single-celled zygote with a diploid number of chromosomes. Then, over the next few days in cell culture, a series of cleavage divisions take the zygote through the two-, four-, eight-, and sixteen-cell stages. Eventually, a hollow ball of cells (the blastocyst) is formed, and embryonic stem cells (ES cells) can be extracted from the inner cell mass within the blastocyst. After several more months, millions of stem cells exist, all derived from the dozens of cells that were part of the inner cell mass. If the resulting cells have not differentiated and are genetically normal, they constitute an embryonic stem cell line.
At this point, each ES cell is pluripotent, meaning that it retains the ability to differentiate into any of the three germ layers: endoderm (the layer that forms the organs and tissues associated with digestion and respiration), mesoderm (the layer that forms the muscles, bones, and reproductive structures), and ectoderm (the layer that forms the skin, brain and nervous system). Therein lies the value of ES cells.
ES cell lines can remain undifferentiated for years. However, because their fate is undetermined, they have the potential to become any type of cell in the human body under the right conditions. If these conditions are met, ES cells can be placed in any area of the body affected by disease to produce healthy cells and tissues (in a process known as cell-based therapy). To date, no human ES-cell-based therapies have made it to clinical trial.
Adult Stem Cells
All tissues and organs contain undifferentiated cells. Unlike ES cells, however, adult stem cells differentiate into the cell type from which they were derived. Thus far, the most successful adult stem cell therapies have been transplantations of bone marrow and peripheral blood stem cells to treat cancers and blood diseases such as Fanconi's anemia and severe combined immunodeficiency disease (SCID or "bubble boy disease").
Induced Pluripotent Stem Cells
In recent years, induced pluripotent stem (iPS) cells that rival ES cells in their ability to differentiate have been generated using adult human somatic cells (Takahashi et al., 2007; Yu et al., 2007). At this time, the process requires the use of retroviral vectors. Retroviruses insert randomly, which creates the risk of disrupting normal cell function and cell cycle regulation. Virus-free iPS cells will be required before they can be safely used in regenerative medicine.
Ethical Controversies Involving Stem Cells
Treatments using adult stem cells rarely generate controversy. As a matter of fact, bone marrow transplants have become so familiar as a cancer treatment that many people may not fully grasp the concept that stem cells are being used.
In contrast, ES cell technology has produced a firestorm of controversy, as outlined in Table 2. Arguments against ES-cell-based therapies arise from the belief that life/consciousness starts at the moment of conception or within the first six days after conception, as well as from concern regarding the source of the embryonic cells.
Table 2: Arguments For and Against Federal Funding of Embryonic Stem Cell Research
| For | Against |
| Adult stem cells lack the versatility of embryonic cells, making them less likely to lead to breakthrough medical discoveries than embryonic stem cells. The research is too important to be left to private researchers; researchers are required to share data when their work is federally funded. Using frozen human embryos that would otherwise be discarded is ethically acceptable given the potential that stem cells hold. | Performing research on embryonic stem cells is effectively destroying life, and it should therefore be avoided. Twenty years of research has not produced a single approved treatment or human trial using embryonic stem cells. The side effects of ES cell therapy are quite severe: It tends to produce tumors and malignant carcinomas, cause transplant rejection, and form the wrong kinds of cells. The necessity of harvesting a woman's eggs for further embryonic research increases the risks associated with superovulation or high-dose hormone therapies, such as cancer, infertility, memory loss, stroke, seizure, and death. Embryonic research brings about increased possibilities for future commercial exploitation of women (poor women, in particular) to collect their eggs. |
Adapted from http://www.sourcewatch.org/index.php?title=U.S._federal_stem_cell_legislation#_note-39
Some religious beliefs define life as beginning at the moment of conception. Therefore, individuals with these beliefs feel that all research using ES cells is immoral. This point of view is usually countered with the argument that the embryos that are used are completely outside a woman's body, which negates the usual view of conception. Some opponents also present the argument of consciousness in embryos, which has been countered with evidence that embryos lack a nervous system until after implantation.
Some scientists are trying to perfect techniques that use only one cell or just a few cells from an embryo, rather than all of them, similar to the techniques routinely performed in preimplantation genetic testing. Efforts are also underway to engineer ES cells that stop developing before they reach a certain point in development. The ability to induce pluripotency in adult somatic cells, however, might soon make the entire discussion of the uses of ES cells irrelevant.
The United States has attempted to legislate a middle ground in this debate by making a distinction between publicly and privately funded research entities. Publicly funded research is restricted to pre-existing cell lines, although ES cells can be generated anew if private funding is used. Although publicly funded research is generally made publicly available, private companies are under no obligation to provide open access to their data. As a result, two questions have arisen:
- What oversight is there for ES cell research that is privately funded?
- Does having two disparate policies that depend solely on the source of funding hinder the potential for research advances?
Pharmacogenomics
Today, the field of personalized medicine makes use of pharmacogenomics, or the science that predicts a person's response to a drug based upon that person's genetic makeup. Indeed, the U.S. Food and Drug Administration (FDA) signaled its commitment to personalized medicine with the decision to add a warning to the label of a widely used blood thinner stating that response to the drug might be influenced by a person's genetic makeup. Warfarin (Coumadin) is used as a blood thinner in the prevention of strokes, heart attacks, and blood clots. A suboptimal dose can lead to life-threatening blood clots, although too much of the drug can cause uncontrolled bleeding. Adverse reactions to warfarin are second only to insulin as a source of emergency room visits (Riley, 2007). Response to warfarin can be highly variable, with 30%-50% of negative reactions explained by mutations in the CYP2C9 (cytochrome 2C9) and VKORC1 (vitamin K epoxide reductase complex subunit 1) genes (Flockhart, 2008). There is much debate, however, about whether patients should be tested for these mutations, and whether such testing actually allows warfarin to be prescribed more effectively.
As pharmacogenomics increasingly moves into the marketplace, further research is needed to determine whether genomic study of many drugs, not just warfarin, is of benefit to patients. Several high-profile articles have pointed out that the time has come not just for more in-depth analysis of genomics and drug reaction, but for greater consideration of which drugs are worthy of study (Haga & Burke, 2004; Phillips & van Bebber, 2005; Ries Merikangas & Risch, 2004). The concern is that certain standards should be put in place to emphasize social and health benefits, rather than allowing the biotech and pharmaceutical industries to plot the course (Phillips & van Bebber, 2005; Webster et al., 2004).
The value of pharmacogenomics has recently taken on new importance for three reasons (Phillips & van Bebber, 2005):
- New tests are being made available for broad use, rather for only than narrowly targeted populations.
- Concerns about the safety of drugs such as Vioxx and Celebrex will likely lead the FDA to use pharmacogenomic testing to a greater extent to increase drug safety.
- So far, there has been little to support early assertions that pharmacogenomics will reduce health care costs and adverse drug reactions.
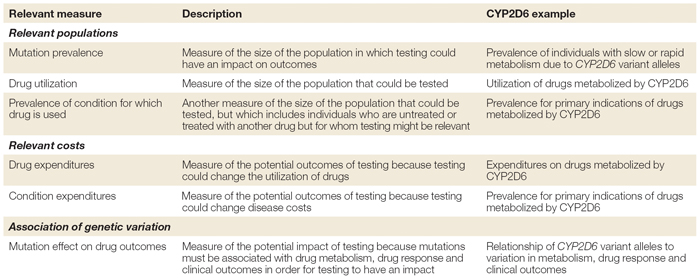
One solution to the dilemma of trying to judge the value of a particular drug is to formulate a resource allocation framework for each set of study results. Table 3 outlines the steps used to develop such a framework, using the drug metabolizing enzyme CYP2D6 as an example.
Following the steps outlined in Table 3, Phillips and van Bebber (2005) were able to detect glaring gaps in data about the association between genetic variation and drug metabolism, response, and clinical outcome. In addition, the attempts to inform citizens were inadequate and the estimated costs resulting from adverse reactions have been poorly addressed.
Flockhart et al. (2008) reached similar conclusions with regard to testing for CYP2C9 and VKORC1 in warfarin patients. In their words:
"There is insufficient evidence, at this time, to recommend for or against routine CYP2C9 and VKORC1 testing in warfarin-naive patients. Prospective clinical trials are needed that provide direct evidence of the benefits, disadvantages, and costs associated with this testing in the setting of initial warfarin dosing. Although the routine use of warfarin genotyping is not endorsed by this work group at this time, in certain situations, CYP2C9 and VKORC1 testing may be useful, and warranted, in determining the cause of unusual therapeutic responses to warfarin therapy."
Such issues of value and benefit must be addressed before the full potential of phamacogenomics research and personalized medicine can be realized. Indeed, the same could be said of many types of experimental therapy being investigated today.
References and Recommended Reading
Bainbridge, J. W. B., et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. New England Journal of Medicine 358, 2231–2239 (2008)
Flockhart, D. A., et al. Pharmacogenetic testing of CYP2C9 and VKORC1 alleles for warfarin. Genetics in Medicine 10, 139–150 (2008)
Genetics Home Reference. What are the ethical issues surrounding gene therapy? http://ghr.nlm.nih.gov/handbook/therapy/ethics (2008)
Hacein-Bey-Abina, S., et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. New England Journal of Medicine 348, 255–256 (2003)
Haga, S. B., & Burke, W. Using pharmacogenetics to improve drug safety and efficacy. Journal of the American Medical Association 291, 2869–2871 (2004)
Kolehmainen, S. M. The dangerous promise of gene therapy. http://www.actionbioscience.org/biotech/kolehmainen.html (2000)
Phillips, K. A., & van Bebber, S. L. Measuring the value of pharmacogenomics. Nature Reviews Drug Discovery 4, 500–509 (2005) (link to article)
Qiao, C., et al. Amelioration of laminin-ά2-deficient congenital muscular dystrophy by somatic gene transfer of miniagrin. Proceedings of the National Academy of Sciences 102, 11999–12004 (2005)
Ries Merikangas, K., & Risch, N. Genomic priorities and public health. Science 302, 599–601 (2004)
Riley, K. FDA approves updated warfarin (Coumadin) prescribing information. FDA News, August 17 (2007)
Takahashi, K., et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007)
Thomas, C. E., et al. Progress and problems with the use of viral vectors for gene therapy. Nature Reviews Genetics 4, 346–358 (2003) (link to article)
Webster, A., et al. Integrating pharmacogenetics into society: In search of a model. Nature Reviews Genetics 5, 663–669 (2004) (link to article)
Yu, J., et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007)



 Table 1: Summary of measures in a pharmacogenetics resource-allocation framework.
Table 1: Summary of measures in a pharmacogenetics resource-allocation framework.