« Prev Next »
Consider the humble origin from which we all started: the fertilized egg. How do all vertebrates develop from that single-cell state to a new embryo and then on to a mature adult? Cell proliferation and cell differentiation are two critical processes in metazoan development; from one cell we must have many cells, and from many cells we must have many types of cells to become the vital specialized tissues and organs that compose an organism. But perhaps the dominant element in this developmental process lies in cell migration and adhesion, which are the main forces morphing these cells into critical anatomical structures. The ability of a cell to migrate to its correct destination depends heavily on signaling at the cell membrane. At every level of detail — from the outgrowth of our limbs and the sculpting of our bodies, to the wiring of our nervous system and the development of our vasculature, down to the guidance and placement of stem cells to their proper niche — signaling at the cell membrane is the ultimate controller. What follows is a brief discussion of signaling during development and how the family of Eph receptors and their ligands, the ephrins, orchestrates the precise control necessary to guide a cell to its destination.
Cell-Surface Receptors Affect Cell Migration and Development
Cell migration is a considerable challenge to the developing organism. During migration, cells or extensions of cells must move over remarkable distances, in some cases covering many thousands of times the length of a single cell. Further, it is imperative that these migrating cells locate their target destination precisely; if cells do not do so properly during development, there can be serious repercussions and deformities, such as a cleft palate. How, then, are organisms able to orchestrate these remarkable migration events with such precision? Much the same way that you might rely on your sensory systems to guide you through your everyday travels, similar structures exist at the cellular level in the form of cell-surface receptors that guide a migrating cell to its destination. These receptors are located on the cell surface, and in response to an extracellular interaction with specific binding partners (ligands), they initiate intracellular signaling events that change the cell's activity. By interacting with cytoskeletal structures within the cell, these signaling events can instruct the migrating cell in a number of ways, ranging from "turn around" or "stay put" to "keep coming this way." By using a number of different cell-surface receptor types, all sensitive to different ligands and all with preferred signaling outcomes and instructions for the cell, it becomes clear that cells have their own navigational systems that permit them to migrate relatively great distances to very precise targets. The largest (by class) and most expansive (by role) navigational system used by cells during this complex and dynamic process is signaling through the Eph receptors and their cognate ligands, the ephrins.
The Eph Receptors and Their Ephrin Ligands
What about the Eph ligands? The ligands for the Eph receptors are the ephrins (also known as Eph-receptor-interacting proteins and spelled with a lowercase "e"). In a similar variety, ephrins comprise nine different molecules, also divided into A- and B-subclasses (Eph Nomenclature Committee 1997). Unlike the Eph receptors, which were fairly uniform across subclass, the structures of the six A-subclass ephrins (ephrin-A1 to ephrin-A6) and three B-subclass ephrins (ephrin-B1 to ephrin-B3) are radically different. Members of the ephrin A-subclass possess a globular extracellular domain that preferentially only binds EphA receptors and is tethered to the outer leaflet of the plasma membrane by a glycosylphosphatidylinositol linkage. In contrast, the three B-subclass ephrins have an extracellular structure that preferentially only binds to EphB receptors (except for EphA4, which can interact with both A- and B-subclass ephrins). Like the Eph receptors, these B-subclass ephrins possess a single-pass hydrophobic transmembrane domain. However, unlike the Eph receptors, these ligands (commonly referred to as ephrin Bs) do not have an intracellular catalytic domain. Instead, they have a short, highly conserved cytoplasmic tail.
Because the ephrin ligands are expressed on the cell surface, activation of Eph receptor signaling only occurs at sites of close cell-cell interaction. Close contact is required for signaling to occur, which in turn allows for highly specific spatial instruction, more so than is possible with a soluble ligand. An appropriate analogy for this precise spatial advantage would be how effectively a human navigates in the dark via touch compared with the more diffuse signals of sound or smell. Contact is everything.
How Does Signaling Between Ephs and Ephrins Work?
Signaling between Ephs and ephrins follows the RTK model. Following cell-cell contact, there is high-affinity binding between Eph receptors and ephrin ligands. Thereafter, the activation of the Eph receptor initiates signaling on the cytoplasmic side, in a similar fashion to other RTKs. In the absence of ligand interaction, the unphosphorylated tyrosines near the cell membrane of the Eph receptor interact tightly with the kinase domain, distorting it and preventing tyrosine kinase activity. But following ligand interaction, Eph receptors cluster together and become phosphorylated on these key "juxtamembrane" tyrosine residues, either by tyrosine phosphorylating one another in trans or through the activation of colocalized Src-family tyrosine kinases. This event is a critical step in up-regulating the catalytic activity of the kinase because the phosphorylation of these tyrosine residues disrupts their interaction with the kinase domain, thus relieving its distortion. This relief permits the Eph receptor to phosphorylate its downstream substrates and propagate its signal transduction cascades (Wybenga-Groot 2001). The simple binding of ephrin to Eph receptor radically transforms the intracellular region of the Eph receptor from a catalytically dormant and inaccessible molecule (in the "off" state) into a catalytically active and highly accessible center point of signal transduction into the cell (in the "on" state).
An Unexpected Discovery Reverses Our Understanding of Eph-ephrin Signaling
If ephrin binding to an Eph receptor initiates signaling events through the Eph receptor into the Eph-expressing cell, might the same also work in reverse? That is, could Eph binding to ephrin initiate signal transduction events into the ephrin-expressing cell as well? Amazingly, that turns out to be the case. The pioneering study demonstrating this phenomenon was carried out in 1996, in Tony Pawson's laboratory at Toronto's Mount Sinai Hospital. There, investigators were looking at the brains of mice with EphB2 completely knocked out (EphB2 null mice) to determine if EphB2 played any role in the development of neural structures. By cutting and staining thin sections of the brain, the investigators found that EphB2 null mice showed disrupted axon pathfinding among neurons in a major bundle of forebrain axons called the anterior commissure (AC). This bundle of axons connects the right and left hemispheres of the brain (Henkemeyer et al. 1996). Although the defects in the AC were likely due to the lack of EphB2, this finding was only the beginning of figuring out how and where Eph/ephrin binding had been disrupted. Where was the presence of EphB2 crucial for normal AC development? To determine where EphB2 was expressed in these neural structures, Mark Henkemeyer created mice with mutant EphB2, wherein the cytoplasmic domain of EphB2 (containing all the signaling structures) was deleted and replaced with conjugated b-galactosidase (EphB2lacZ mice). Placed in this conjugated position on the Eph receptor, the b-galactosidase served as a reporter gene to show anatomically where EphB2 is expressed, while at the same time disabling intracellular signaling through the EphB receptor. What did Henkemeyer find after creating this transgenic mouse? Amazingly, mice that were homozygous for the EphB2lacZ mutation did not present with any defects in the anterior commissure. Was the EphB2 receptor still somehow functional, even without an intracellular tyrosine kinase region? In fact, it was. Through anatomical analysis of the b-galactosidase reporter gene expression, the investigators found that EphB2 was not even expressed in the AC. Instead, they found EphB2 expression in a region of the brain just ventral to the migrating AC axons, the hypothalamus. Interestingly, further analysis of ligand expression showed that ephrin-B2 was strongly expressed in these migrating neural fibers that must pass by the EphB2-rich region to successfully form the AC. What did these data mean? This discovery was actually stunning to the investigators. Their results indicated that Eph receptors could somehow signal in a novel fashion through their extracellular domain interactions with the ephrins, because the extracellular domain appeared to be enough for normal development in this brain region. This novel form of signaling did not require the intracellular domain of the EphB2 receptors and therefore suggested an additional ligand-like role for the extracellular domain of Eph receptors and, by extension, a receptor-like role for the ephrins.
Confirmation of this new possibility soon followed, and we now know that ephrins have their own intracellular signaling capability when bound to Ephs with their extracellular domain. Specifically, binding of Eph by ephrin actually clusters the ephrin at the ephrin-expressing cell surface and thereby activates colocalized Src-family kinases (SFKs). In the case of the A-subclass ephrins, these SFKs activate and go on to tyrosine phosphorylate other molecules present at the membrane, initiating signaling events and subsequent physiological responses. Alternatively, with the B-subclass ephrins, investigators wondered if instead of tyrosine phosphorylating other membranous proteins, might the activated SFK actually phosphorylate conserved tyrosine residues on the cytoplasmic tail of the ephrin-B itself? Through a simple set of cell culture experiments, investigators from the Pawson laboratory found that to be true. They showed that B-subclass ephrins, following Eph receptor interaction, could be immunoprecipitated by antibodies recognizing only phospho-tyrosine residues, confirming that this phosphorylation activity was indeed there and was initiated by Eph receptor binding (Holland et al. 1996). More of this mechanism has since been worked out in detail. The phosphorylation of these tyrosine residues converts the ephrin-B cytoplasmic tail into a substrate for subsequent signal transduction by creating binding sites that can facilitate interactions between the ephrin-B and other intracellular proteins (Cowan & Henkemeyer 2001). Like the Eph receptor, Eph-ephrin interaction quickly converts the cytoplasmic presence of ephrin-B from a dormant state to a focal point of active signal transduction, even though the small cytoplasmic domain of ephrin-B lacks any catalytic activity. These unexpected results changed the way investigators asked questions about receptor function and the direction of signaling between cells.
The Phenomenon of Bidirectional Signaling
Not knowing the exact direction of signaling between molecules can be somewhat confusing. To properly keep track of signaling through ephrin activation of the Eph receptor and vice versa, biologists refer to signaling into the Eph receptor-expressing cell as the "forward signal," as distinguished from signaling into the ephrin-expressing cell, the "reverse signal." Thus, when an Eph-expressing cell encounters an ephrin-expressing cell, the potential exists for propagation of both forward signals into the Eph-bearing cell and reverse signals into the ephrin-bearing cell, a phenomenon biologists call bidirectional signaling (Figure 2). This potential for bidirectional tyrosine kinase signaling makes the Ephs and ephrins a very powerful guidance system (in much the way two people bouncing ideas off each other might achieve more than someone working alone). Bidirectional signaling also makes things a little more challenging for biologists working to better understand these molecules. Unlike with simpler unidirectional receptor-ligand systems, with Eph-ephrin systems scientists must determine whether any physiological response is the result of forward signaling, reverse signaling, or even some combination of both forward and reverse signaling.
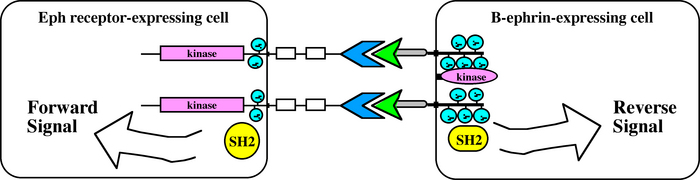
Cytoskeletal Activation via Tyrosine Kinase in the Eph-Ephrin System
An interesting thing about the Ephs and ephrins is that, unlike the majority of tyrosine kinase signaling in the cell, signal transduction through the Ephs and ephrins does not appear to principally target the nucleus to regulate transcription. When investigators first cloned cDNAs for the Eph receptors, they were surprised to find that the overexpression of Eph receptors in cultured cells did not transform the cells or make them more proliferative, as is the case with many other tyrosine kinases (Lhotak & Pawson 1993). Instead, when they activated Eph-ephrin signaling in tissue expressing Eph receptors, investigators found that they could dramatically alter the cytoskeletal structure of the cell (Drescher et al. 1995). A substantial body of work indicating that the principle function of Eph-ephrin signaling is to exert precise control over cytoskeletal dynamics has now emerged from those initial studies.
How might cell-surface receptors like the Ephs and ephrins work to restructure the cytoskeleton? To answer this question, we must review the molecules involved in cytoskeleton dynamics. The major class of intracellular molecules that regulate cytoskeletal behavior in the cell is the small GTPases of the Rho family, namely Rho, Rac, and Cdc42. These Rho family GTPases cycle between (1) an active conformation in which GTP is bound and they are capable of interacting with effector molecules and (2) an inactive conformation in which GTP has been hydrolyzed to GDP and they remain dormant. The activation state of these Rho family GTPases is controlled by the differential activity of guanine nucleotide exchange factors (GEFs), which catalyze the exchange of GDP for GTP to activate these molecules. The activation state is also controlled by GTPase-activating proteins (GAPs), which help stimulate the hydrolysis of GTP into GDP. When cell biologists have studied these molecules in cell culture systems, GTP-bound Rac and Cdc42 are often associated with the formation of long extensions from the cell, called lamellipodia and filopodia. Similarly, activated Rho appears to promote the formation of stress fibers by increasing contractility in the cell. However, ongoing research suggests that these understandings might be overly simplistic and that activation of Rho, Rac, and Cdc42 might promote different outcomes in cell shape and movement based on context (what else is happening in the cell) and the feedback these molecules have on one another. How these complex interactions work is still a mystery, and many open questions remain.
Therefore, to control cytoskeletal dynamics, Ephs and ephrins must have some way of regulating the activation levels of these Rho family proteins. Indeed, investigators have repeatedly shown through different biochemical and protein-protein interaction assays that both forward signaling and reverse signaling directly target GAPs and GEFs for these Rho family proteins and thereby control their activation states (GTP- versus GDP-bound), which in turn affects the cytoskeletal behavior of the cell (Shamah et al. 2001; Iwasato et al. 2007; Segura et al. 2007). Given this evidence about cytoskeletal movement regulation, it is possible to envision how simple ligand-receptor interactions at the cell surface could turn into instructions for a migrating cell, such as "Stop and go back" or "Keep coming this way."
What is a possible scenario for these molecular instructions? A cell expressing an Eph receptor might encounter an ephrin-expressing cell, turning on Eph receptor forward signals that then target GEFs for Rho and GAPs for Rac and Cdc42 to produce GTP-bound Rho and GDP-bound Rac and Cdc42, respectively. This process would stimulate cellular contractility while inhibiting the formation of cellular extensions and movement, causing the Eph-expressing cell to retract away from the ephrin-positive cell (i.e., "Stop and go back"). Similarly, a migrating cell expressing ephrin might come into contract with an Eph receptor, inducing reverse signals that activate GEFs for Rac or Cdc42 to produce GTP-bound Rac or Cdc42 and thus induce the cell to produce filopodia- or lamellipodia-like extensions that encourage further directional migration ("Come this way").
Eph-Ephrin Signaling Has Broad Relevance
Through these simple instructions, the Ephs and ephrins are able to control the development of many different tissue systems. Investigators have been able to identify these tissue systems primarily through the creation and analysis of Eph and ephrin knockout mouse models. Perhaps the system in which the Ephs and the ephrins are the most prevalent is the nervous system, particularly in the guidance of axons during their development to precise regions in the ultimate task of properly wiring neurons across long distances (e.g., visual, olfactory, corticalspinal tract, and motor neurons). Other notable areas of tissue development that are dependent on Eph-ephrin signaling include vascular morphogenesis, where the coordinated activity of ephrin-B2 (expressed principally on arterial vessels) and EphB4 (expressed on venous vessels) mold primitive vascular networks into mature, vital vasculature (Gerety et al. 1999; Cowan et al. 2004); cell sorting and boundary formation, notably in rhombomere segregation in the hindbrain and somite formation (Xu et al. 1995; Durbin et al. 1998); neural crest cell migration (Smith et al. 1997); and midline adhesion, where the closure of critical structures at the midline such as the neural tube, hindgut septum, ventral body wall, and palatal shelf all rely on Eph-ephrin signaling (Orioli et al. 1996; Holmberg, Clarke & Frisen 2000; Dravis et al. 2004). Furthermore, the Ephs and ephrins have a significant role in stem cell biology, where they appear responsible for guiding stem cells in the brain and intestine to their proper niches (Holmberg et al. 2006; Chumley et al. 2007). Amazingly, the instructions from Eph-ephrin signaling during these developmental events appear to cover a full range of responses, from cell/axon repulsion all the way to attraction and adhesion. (See movie of axon repulsion here).
The Future of Eph-Ephrin Research
As Eph-ephrin research continues, a major question facing the field is: How do the Ephs and ephrins achieve such a wide range of responses? Sometimes they are repulsive, sometimes they are attractive, and sometimes they can be both in the same population of cells. How these same molecules are used in different fashions at different checkpoints of development is a fascinating focus for developmental biologists. Sometimes it is just as much the question of when Eph-ephrins are expressed in development as it is where they are expressed. Investigators want to know how the signaling between migrating cell and the other cells it encounters occurs. How can Eph forward signaling (or ephrin reverse signaling or bidirectional signaling) first instruct a cell repulsion outcome and then later yield the polar opposite in a cell attraction response?
The answer is by no means trivial, because finding answers could help alleviate many different disease and injury states. As a regulator of cell migration, the Ephs and ephrins may be involved in different cancerous states (recall where the EPH name comes from, above), and ongoing research does seem to suggest that the misexpression of the Ephs and ephrins appears to figure prominently in cancer, particularly with aspects of metastasis (migration of cancer cells) and neovascularization (blood vessel growth in tumors). The most notable role for Eph-ephrin signaling among injury states is its potential role in the repair of spinal cord injuries. Many years of research in this area have shown that the Ephs and ephrins figure prominently in stunting the regeneration of damaged spinal cord tissue (and likely other nervous tissue elsewhere). Can this repulsion be switched to attraction and promote regeneration? How possible is it to toggle these repulsion versus attraction mechanisms between Eph and ephrin molecules? Finding the answer to these questions offers legitimate hopes that we can design therapeutics that control these signaling events in ways that repair injured or disease states.
For now, however, these questions remain largely unanswered. Defining how the context of a signaling event can promote or discourage repulsion or attraction is the largest challenge for biologists. Likely, this context is mostly controlled by the secondary molecules available to become tyrosine phosphorylated or recruited to the Eph or ephrin to propagate different signaling cascades. It may also have something to do with which other signaling pathways may become activated at this time, in addition to the Ephs and ephrins. Finally, it may also depend on the relative strength of these Eph-ephrin signaling events compared with other guidance cues or with other Ephs and ephrins. With hopes of filling in these details, significant research on these molecules continues. Using tools such as cell culture systems, small molecule inhibitors, and increasingly sophisticated mouse models to manipulate these signaling events in vivo, researchers are tackling multiple questions at once, hoping to better understand Eph-ephrin bidirectional signaling and to do so with an eye toward future therapeutic or bioengineering applications.
References and Recommended Reading
Chumley, M. J. et al. EphB receptors regulate stem/progenitor cell proliferation, migration, and polarity during hippocampal neurogenesis. Journal of Neuroscience 27, 13481–13490 (2007) doi:10.1523/JNEUROSCI.4158-07.2007.
Cowan, C. A. & Henkemeyer, M. The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals. Nature 413, 174–179 (2001) doi:10.1038/35093123.
Cowan, C. A. et al. Ephrin-B2 reverse signaling is required for axon pathfinding and cardiac valve formation but not early vascular development. Developmental Biology 271, 263–271 (2004) doi:10.1016/j.ydbio.2004.03.026.
Dravis, C. et al. Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Developmental Biology 271, 272–290 (2004) doi:10.1016/j.ydbio.2004.03.027.
Drescher, U. et al. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell 82, 359–370 (1995) doi:10.1016/0092-8674(95)90425-5.
Durbin, L. et al. Eph signaling is required for segmentation and differentiation of the somites. Genes and Development 12, 3096–3109 (1998).
Gerety, S. S. et al. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Molecular and Cellular Biology 4, 403–414 (1999) doi:10.1016/S1097-2765(00)80342-1.
Henkemeyer, M. et al. Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell 86, 35–46 (1996) doi:10.1016/S0092-8674(00)80075-6.
Hirai, H. et al. A novel putative tyrosine kinase receptor encoded by the eph gene. Science 238, 1717–1720 (1987) doi: 10.1126/science.2825356.
Holland, S. J. et al. Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature 383, 722–725 (1996) doi:10.1038/383722a0.
Holmberg, J., Clarke, D. L. & Frisen, J. Regulation of repulsion versus adhesion by different splice forms of an Eph receptor. Nature 408, 203–206 (2000) doi:10.1038/35041577.
Holmberg, J. et al. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell 125, 1151–1163 (2006) doi:10.1016/j.cell.2006.04.030.
Eph Nomenclature Committee (1997).
Iwasato, T. et al. Rac-GAP alpha-chimerin regulates motor-circuit formation as a key mediator of EphrinB3/EphA4 forward signaling. Cell 130, 742–753 (2007) doi:10.1016/j.cell.2007.07.022.
Lhotak, V. & Pawson, T. Biological and biochemical activities of a chimeric epidermal growth factor-Elk receptor tyrosine kinase. Molecular and Cellular Biology 13, 7071–7079 (1993).
Orioli, D. et al. Sek4 and Nuk receptors cooperate in guidance of commissural axons and in palate formation. Embo Journal 15, 6035–6049 (1996) doi:10.1523/JNEUROSCI.4158-07.
Segura, I. et al. Grb4 and GIT1 transduce ephrinB reverse signals modulating spine morphogenesis and synapse formation. Nature Neuroscience 10, 301–310 (2007) doi:10.1038/nn1858.
Shamah, S. M. et al. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell 105, 233–244 (2001).
Smith, A. et al. The EphA4 and EphB1 receptor tyrosine kinases and ephrin-B2 ligand regulate targeted migration of branchial neural crest cells. Current Biology 7, 561–570 (1997) doi:10.1016/S0960-9822(06)00255-7.
Wybenga-Groot, L. E. et al. Structural basis for autoinhibition of the Ephb2 receptor tyrosine kinase by the unphosphorylated juxtamembrane region. Cell 106, 745–757 (2001) doi:10.1016/S0092-8674(01)00496-2.
Xu, Q. et al. Expression of truncated Sek-1 receptor tyrosine kinase disrupts the segmental restriction of gene expression in the Xenopus and zebrafish hindbrain. Development 121, 4005–4016 (1995).



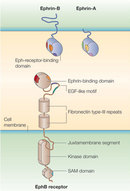
 Figure 1
Figure 1


























