Featured
-
-
Letter |
 Phosphodiesterase 9A controls nitric-oxide-independent cGMP and hypertrophic heart disease
Phosphodiesterase 9A controls nitric-oxide-independent cGMP and hypertrophic heart disease
The inhibition, in mice, of the phosphodiesterase PDE9A, which specifically regulates natriuretic-peptide-coupled cGMP signalling, is independent of nitric oxide and is upregulated in failing human hearts, and can reverse pre-established stress-induced heart disease.
- Dong I. Lee
- , Guangshuo Zhu
- & David A. Kass
-
Article |
 Mesenchymal–endothelial transition contributes to cardiac neovascularization
Mesenchymal–endothelial transition contributes to cardiac neovascularization
This study shows that cardiac injury induces cardiac fibroblasts to undergo mesenchymal–endothelial transition and acquire an endothelial-cell like fate, a process mediated, in part, by a p53-dependent mechanism — use of a small molecule activator of p53 increases mesenchymal–endothelial transition, leading to reduced scarring and better preservation of heart function.
- Eric Ubil
- , Jinzhu Duan
- & Arjun Deb
-
Letter |
 A long noncoding RNA protects the heart from pathological hypertrophy
A long noncoding RNA protects the heart from pathological hypertrophy
Here, a long noncoding RNA, termed Mhrt, is identified in the loci of myosin heavy chain (Myh) genes in mice and shown to be capable of suppressing cardiomyopathy in the animals, as well as being repressed in diseased human hearts.
- Pei Han
- , Wei Li
- & Ching-Pin Chang
-
Letter |
 Dichloroacetate prevents restenosis in preclinical animal models of vessel injury
Dichloroacetate prevents restenosis in preclinical animal models of vessel injury
During development of myointimal hyperplasia in human arteries, smooth muscle cells have hyperpolarized mitochondrial membrane potential (ΔΨm), high proliferation and apoptosis resistance; PDK2 is a key regulatory protein whose activation is necessary for myointima formation, and its blockade with dichloroacetate prevents Δψm hyperpolarization, facilitates apoptosis and reduces myointima formation in injured arteries, without preventing vessel re-endothelialization, possibly representing a novel strategy to prevent proliferative vascular diseases.
- Tobias Deuse
- , Xiaoqin Hua
- & Sonja Schrepfer
-
Letter |
 Inhibition of miR-25 improves cardiac contractility in the failing heart
Inhibition of miR-25 improves cardiac contractility in the failing heart
Reduced activity of the calcium-transporting ATPase SERCA2a is a hallmark of heart failure; here, microRNAs that downregulate SERCA2a function are identified, and antagonism of one, miR-25, is shown to halt heart failure in mice.
- Christine Wahlquist
- , Dongtak Jeong
- & Mark Mercola
-
Letter |
 The heterotaxy gene GALNT11 glycosylates Notch to orchestrate cilia type and laterality
The heterotaxy gene GALNT11 glycosylates Notch to orchestrate cilia type and laterality
The O-glycosylation enzyme Galnt11 has an important role in heterotaxy, a disorder of left–right body patterning or laterality: Galnt11 modulates Notch signalling which alters cilia types at the embryonic left–right organizer, therefore determining laterality.
- Marko T. Boskovski
- , Shiaulou Yuan
- & Mustafa K. Khokha
-
Letter |
 Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation
Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation
CaMKII is known to be pathologically activated in heart failure and arrhythmias; here it is shown that glucose-induced CaMKII activation via O-linked glycosylation might contribute to cardiac pathology in diabetes.
- Jeffrey R. Erickson
- , Laetitia Pereira
- & Donald M. Bers
-
Letter |
 MicroRNA-34a regulates cardiac ageing and function
MicroRNA-34a regulates cardiac ageing and function
A role is demonstrated for miR-34a, a microRNA that is upregulated in the ageing heart; miR-34a downregulates PNUTS, a protein that protects cardiomyocytes and telomeres, silencing of miR-34a is therefore a promising therapeutic target.
- Reinier A. Boon
- , Kazuma Iekushi
- & Stefanie Dimmeler
-
Outlook |
 Cardiovascular disease: Biochemistry to behaviour
Cardiovascular disease: Biochemistry to behaviour
Cardiovascular disease (CVD) remains the grim reaper's primary calling card, but people can take steps to keep the world's number one killer at bay.
- Bill Cannon
-
Outlook |
 Pathology: At the heart of the problem
Pathology: At the heart of the problem
Research is illuminating the molecular mechanisms that can cascade into debilitating heart disease.
- Cassandra Willyard
-
Outlook |
 Diagnostics: The new risk predictors
Diagnostics: The new risk predictors
New imaging methods and biomarkers may help identify people who are at risk for heart disease but are overlooked by standard risk assessments.
- Peter Gwynne
-
Outlook |
 Physiology: Beating stroke
Physiology: Beating stroke
New drugs and more focused therapy might cut down on atrial fibrillation and reduce the incidence of stroke.
- Neil Savage
-
Outlook |
 Drugs: Blood battles
Drugs: Blood battles
The standard medications for hypertension and cholesterol have lingering issues, but new drugs hold promise for high-risk patients.
- Katharine Gammon
-
Outlook |
 Psychology: Mind over myocardium
Psychology: Mind over myocardium
Mental factors beyond stress trigger physiological changes that can cause heart disease.
- Shailaja Neelakantan
-
Outlook |
 Public planning: Designs fit for purpose
Public planning: Designs fit for purpose
Better thought-out town planning and interior design can create healthier environments, but how to effectively implement the best designs remains uncertain.
- Duncan Graham-Rowe
-
Outlook |
 Perspective: The power of disease prevention
Perspective: The power of disease prevention
Improving lifestyles could halve the number of deaths caused by cardiovascular disease, says Joep Perk.
- Joep Perk
-
Outlook |
 Perspective: A tale of two receptors
Perspective: A tale of two receptors
A hormone system adapted for self-preservation can break and fix your heart, say Sébastien Foulquier, Ulrike Muscha Steckelings and Thomas Unger.
- Sébastien Foulquier
- , Ulrike Muscha Steckelings
- & Thomas Unger
-
Letter |
 Studying arrhythmogenic right ventricular dysplasia with patient-specific iPSCs
Studying arrhythmogenic right ventricular dysplasia with patient-specific iPSCs
This study demonstrates that an inheritable adult onset heart disease can be modelled in vitro within months with the help of metabolic maturation induction.
- Changsung Kim
- , Johnson Wong
- & Huei-Sheng Vincent Chen
-
Outlook |
 Perspective: Don't be superficial
Perspective: Don't be superficial
Severe psoriasis carries cardiovascular risks. Dermatologists should consider more than just patients' outer layers, argues Henning Boehncke.
- Wolf-Henning Boehncke
-
News |
 Heart cells coaxed to divide and conquer
Heart cells coaxed to divide and conquer
The heart does have a limited ability to heal itself — and a genetic 'trick' can harness this.
- Kerri Smith
-
Article |
 Functional screening identifies miRNAs inducing cardiac regeneration
Functional screening identifies miRNAs inducing cardiac regeneration
The human heart regenerates poorly, causing insufficient healing after injury; here, microRNAs screened for the ability to induce cardiomyocyte proliferation are shown to stimulate cardiac regeneration and almost complete recovery of the heart after infarction.
- Ana Eulalio
- , Miguel Mano
- & Mauro Giacca
-
Letter |
 Mammalian heart renewal by pre-existing cardiomyocytes
Mammalian heart renewal by pre-existing cardiomyocytes
During normal ageing a low rate of division of pre-existing cardiomyocytes, rather than progenitor cells, is responsible for cardiomyocyte genesis; this process is increased fourfold during myocardial infarction.
- Samuel E. Senyo
- , Matthew L. Steinhauser
- & Richard T. Lee
-
News |
 Chelation-therapy heart trial draws fire
Chelation-therapy heart trial draws fire
Critics not persuaded that metal-snaring treatment works.
- Ewen Callaway
-
News |
 Stem-cell transplant claims debunked
Stem-cell transplant claims debunked
Transplant of induced pluripotent stem cells to treat heart failure probably never happened.
- David Cyranoski
-
Letter |
 CaMKII determines mitochondrial stress responses in heart
CaMKII determines mitochondrial stress responses in heart
- Mei-ling A. Joiner
- , Olha M. Koval
- & Mark E. Anderson
-
Research Highlights |
 Fatty plaque link to inflammation
Fatty plaque link to inflammation
-
News |
 Animals engineered with pinpoint accuracy
Animals engineered with pinpoint accuracy
More accurate genetic modification has created allergen-free cow's milk and pigs that could serve as a model for atherosclerosis.
- Amy Maxmen
-
Research Highlights |
Nanofibres foster blood vessels
-
Q&A |
 Turning point: Nehal Mehta
Turning point: Nehal Mehta
Cardiologist is first winner of award supporting clinical research.
- Charlotte Schubert
-
Letter |
 Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts
Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts
A guinea-pig model of cardiac injury is used to show that human embryonic stem-cell-derived cardiomyocyte grafts can electrically integrate into the injured heart, improving mechanical function and reducing spontaneous and induced ventricular tachycardia; this is a major step towards clinical adoption of cell replacement therapies for cardiovascular diseases using human cardiomyocytes.
- Yuji Shiba
- , Sarah Fernandes
- & Michael A. Laflamme
-
News & Views |
 Bad matters made worse
Bad matters made worse
Heart attacks occur when lipoprotein-driven inflammation called atherosclerosis triggers blood clotting in the arteries. It seems that the attacks can, in turn, accelerate atherosclerosis by fanning the inflammation. See Letter p.325
- Ira Tabas
-
Letter |
 APJ acts as a dual receptor in cardiac hypertrophy
APJ acts as a dual receptor in cardiac hypertrophy
APJ is shown to be a bifunctional receptor for both mechanical stretch and the endogenous peptide apelin, a finding that is important for the development of APJ agonists to treat heart failure.
- Maria Cecilia Scimia
- , Cecilia Hurtado
- & Pilar Ruiz-Lozano
-
Research Highlights |
 Nanoparticles home in to clear clots
Nanoparticles home in to clear clots
-
Article |
 Myocardial infarction accelerates atherosclerosis
Myocardial infarction accelerates atherosclerosis
Myocardial infarction accelerates atherosclerosis through activation of the sympathetic nervous system, and the consequent release of haematopoietic stem and progenitor cells.
- Partha Dutta
- , Gabriel Courties
- & Matthias Nahrendorf
-
Research Highlights |
Stem cells from blood vessels
-
News & Views |
Reprogramming the injured heart
When the heart is injured, the muscle does not regenerate and scars are produced. This process can be attenuated in the hearts of live mice by forcing scar-forming cells to become muscle cells. See Articles p.593 & p.599
- Nathan J. Palpant
- & Charles E. Murry
-
Letter |
 Apolipoprotein E controls cerebrovascular integrity via cyclophilin A
Apolipoprotein E controls cerebrovascular integrity via cyclophilin A
The APOE4-mediated proinflammatory pathway is shown to initiate blood–brain barrier breakdown and resulting neurodegeneration in transgenic mice.
- Robert D. Bell
- , Ethan A. Winkler
- & Berislav V. Zlokovic
-
Article |
 Heart repair by reprogramming non-myocytes with cardiac transcription factors
Heart repair by reprogramming non-myocytes with cardiac transcription factors
A combination of four transcription factors, GATA4, HAND2, MEF2C and TBX5, can reprogram fibroblasts into cardiac-like myocytes in vitro and in vivo; expression of these factors ameliorated cardiac function in mice that had suffered myocardial infarction.
- Kunhua Song
- , Young-Jae Nam
- & Eric N. Olson
-
News & Views |
 Escaped DNA inflames the heart
Escaped DNA inflames the heart
High blood pressure can damage heart muscle cells and their mitochondrial organelles. DNA from degraded mitochondria has been shown to trigger inflammation leading to heart failure. See Letter p.251
- Klitos Konstantinidis
- & Richard N. Kitsis
-
Article |
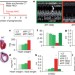 Cardiac angiogenic imbalance leads to peripartum cardiomyopathy
Cardiac angiogenic imbalance leads to peripartum cardiomyopathy
Evidence from mice and humans indicates that peripartum cardiomyopathy (PPCM) is a vascular disease caused by excessive anti-angiogenic signalling in the peripartum period of pregnancy and that pre-eclampsia and multiple gestation are important risk factors for the development of PPCM.
- Ian S. Patten
- , Sarosh Rana
- & Zoltan Arany
-
Research Highlights |
Watching risky blood clots form
-
Research Highlights |
 Patient-specific heart cells
Patient-specific heart cells
-
Letter |
 Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure
Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure
Mitochondrial DNA escaping from the autophagy pathway can trigger inflammation through Toll-like receptor (TLR) 9, leading to abnormalities in cardiac structure and function, and increased mortality.
- Takafumi Oka
- , Shungo Hikoso
- & Kinya Otsu
-
Research Highlights |
Cell clean-up for artery health
-
Letter |
 Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy
Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy
Pregnant corin- or ANP-deficient mice have impaired trophoblast invasion and uterine spiral artery remodelling, and patients with pre-eclampsia have lower uterine corin messenger RNA and protein levels than normal pregnancies, suggesting that defects in corin and ANP function may contribute to pre-eclampsia.
- Yujie Cui
- , Wei Wang
- & Qingyu Wu
-
News & Views |
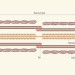 Broken giant linked to heart failure
Broken giant linked to heart failure
Genetic mutations can cause a type of heart disease called dilated cardiomyopathy, by predisposing the organ to enlarge and function poorly. It has now been found that 27% of cases are due to mutations that disrupt the muscle protein titin.
- Elizabeth M. McNally
-
Research Highlights |
Restore my beating heart
-
Letter |
 Circadian rhythms govern cardiac repolarization and arrhythmogenesis
Circadian rhythms govern cardiac repolarization and arrhythmogenesis
Circadian rhythmicity of cardiac ion-channel expression and of an index of myocardial repolarization is under the control of Klf15, a clock-dependent oscillator that is required for generating transient outward potassium current, and deficiencies or excesses of which cause loss of rhythmic variation in myocardial and abnormal repolarization, and an enhanced susceptibility to ventricular arrhythmias.
- Darwin Jeyaraj
- , Saptarsi M. Haldar
- & Mukesh K. Jain
-
Research Highlights |
Culprits in diabetic heart risk

 HIF-driven SF3B1 induces KHK-C to enforce fructolysis and heart disease
HIF-driven SF3B1 induces KHK-C to enforce fructolysis and heart disease