Abstract
Diet-induced weight loss is associated with improved beta-cell function in people with type 2 diabetes (T2D) with remaining secretory capacity. It is unknown if adding exercise to diet-induced weight loss improves beta-cell function and if exercise volume is important for improving beta-cell function in this context. Here, we carried out a four-armed randomized trial with a total of 82 persons (35% females, mean age (s.d.) of 58.2 years (9.8)) with newly diagnosed T2D (<7 years). Participants were randomly allocated to standard care (n = 20), calorie restriction (25% energy reduction; n = 21), calorie restriction and exercise three times per week (n = 20), or calorie restriction and exercise six times per week (n = 21) for 16 weeks. The primary outcome was beta-cell function as indicated by the late-phase disposition index (insulin secretion multiplied by insulin sensitivity) at steady-state hyperglycemia during a hyperglycemic clamp. Secondary outcomes included glucose-stimulated insulin secretion and sensitivity as well as the disposition, insulin sensitivity, and secretion indices derived from a liquid mixed meal tolerance test. We show that the late-phase disposition index during the clamp increases more in all three intervention groups than in standard care (diet control group, 58%; 95% confidence interval (CI), 16 to 116; moderate exercise dose group, 105%; 95% CI, 49 to 182; high exercise dose group, 137%; 95% CI, 73 to 225) and follows a linear dose–response relationship (P > 0.001 for trend). We report three serious adverse events (two in the control group and one in the diet control group), as well as adverse events in two participants in the diet control group, and five participants each in the moderate and high exercise dose groups. Overall, adding an exercise intervention to diet-induced weight loss improves glucose-stimulated beta-cell function in people with newly diagnosed T2D in an exercise dose-dependent manner (NCT03769883).
Similar content being viewed by others
Main
As the progressive deterioration of normal beta-cell function is regarded as a determining factor for the onset and subsequent progression of T2D, re-establishing beta-cell function is considered pivotal to improving the pathogenesis of T2D1.
Although a substantial diet-induced weight loss is consistent with improved beta-cell function2,3,4, the effects of exercise on beta-cell function in T2D are not well understood5,6,7,8,9. Inconsistent findings may relate to differences in concomitant pharmacological therapy, the participants’ pretrial insulin secretory capacity, or differences in exercise modality, intensity and/or volume10,11,12,13,14. The inconsistencies could also be related to a failure to correct for prevailing insulin sensitivity when assessing beta-cell function. As the normal physiological response to decreased insulin sensitivity is an increase in insulin secretion, the assessment of beta-cell function should incorporate both measures (that is, insulin sensitivity and secretion)15. A widely accepted measure of beta-cell function is the disposition index (DI), that is, the product of insulin sensitivity and insulin secretion15. Whereas there is evidence to suggest that exercise-induced improvements in DI are explained via improvements in insulin sensitivity and glucose disposal, the exercise-induced effects on insulin secretion in the context of prevailing insulin sensitivity remain to be clarified5,10,16.
Intensive structured weight management programs aiming for weight loss are recommended alongside pharmacological therapy to treat hyperglycemia17. Diet-induced weight loss is consistent with improvements in beta-cell function2,18, and glucose-lowering medications may increase insulin sensitivity, insulin secretion and incretin responses19,20,21. Therefore, potential interactions between these therapies and exercise should be considered when assessing the role of exercise on DI in people with T2D in a clinical setting. As such, there is a need to investigate the potential effects of exercise on DI in the context of standardized dietary weight loss and pharmacological therapy.
Accordingly, the primary objective of this study was to investigate the change in DI during the final 30 min of clamp-induced hyperglycemia (late-phase DI) after a 16-week intervention with different volumes of exercise in addition to diet-induced weight loss and algorithm-guided pharmacological management in people with newly diagnosed T2D. We hypothesized that late-phase DI would increase with increasing volumes of exercise in combination with diet-induced weight loss. Furthermore, we expected that both moderate and high volumes of exercise in combination with a diet-induced weight loss intervention would be superior to the control in improving late-phase DI22. The secondary objective was to assess the effects of the intervention on insulin sensitivity and secretion. Moreover, we aimed to explore the effects on cardiometabolic risk factors, postprandial glucose metabolism, glucose kinetics, glucagon-like peptide 1 (GLP-1) sensitivity and maximal insulin secretory capacity.
Results
Trial population and adherence to the intervention
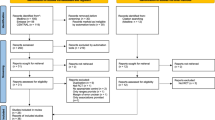
Eighty-two persons were included in the study (Fig. 1). Five participants were lost to follow-up: one was due to malignancy, one was dissatisfied with group allocation, one refrained from study testing due to COVID-19, and two were due to musculoskeletal injuries. The mean (s.d.) age was 58.2 years (9.8), body mass index (BMI) was 33 kg/m2 (3.7), and glycated hemoglobin (HbA1c) was 50.2 mmol/mol (6.6). Thirty-five percent (35%) of participants were females, and the median (interquartile range (IQR)) T2D duration was 4.0 years (1.9 to 5.5). Baseline characteristics are presented in Table 1. Mean (s.d.) adherence to the prescribed diet intervention (~25–30% energy deficit per day) was 92% (11) for the diet control group (DCON), 91% (18) for the moderate exercise dose group (MED), and 88% (13) for the high exercise dose group (HED) (Supplementary Table 1). Mean (s.d.) adherence to the prescribed exercise protocol was 86% (28) and 93% (18) for HED and MED, respectively (Supplementary Tables 2–7). No compensatory decrease in total free-living physical activity was seen in the intervention groups during the intervention period (Supplementary Table 8). In-study adherence to the predefined pharmacological treatment was similar among all groups (Supplementary Table 9).
Primary outcome
The late-phase DI increased in all intervention groups from baseline to 16-week follow-up with no change in the control group (CON) (Fig. 2a) in the intention-to-treat (ITT) analysis; as such, all intervention groups increased more than CON (P < 0.005 for all comparisons; Table 2). Compared with DCON, both MED and HED increased the late-phase DI (MED versus DCON, 29% (95% CI, −5 to 77), P = 0.11; HED versus DCON, 50% (95% CI, 10 to 104), P = 0.01) (Table 2). The magnitude of increases across groups was consistent with a linear dose–response relationship (P for trend <0.001). The per-protocol (PP) analysis set consisted of CON n = 18 (90%), DCON n = 21 (100%), MED n = 19 (95%) and HED n = 18 (86%), and followed the pattern observed in ITT (Table 2). The distribution of absolute values at baseline and follow-up are presented in Extended Data Fig. 1.
a–f, The bars represent estimated mean change from baseline for each intervention group from the n = 82 persons included in the study. Error bars represent 95% confidence intervals. Data were log(e)-transformed and back-transformed, and the results are presented as relative (percentage term) changes based on the ratio of geometric mean change from baseline to follow-up. Results were adjusted for sex. Data were analyzed using a constrained baseline longitudinal model. The dots represent the relative (percentage term) individual changes from baseline to follow-up. Left panel: Data are based on the final 30 min of the hyperglycemic clamp (stage 1). Right panel: Data are from 0 to 120 min of the MMTT. a, Change in late-phase DI by group. b, Change in late-phase ISI by group. c, Change in late-phase ISR by group. d, Change in oral DI of the MMTT by group. e, Change in oral ISI by group. f, Change in oral ISR by group. CON, n = 20; DCON, n = 21; MED, n = 20; HED, n = 21.
Secondary outcomes
The dose–response relationship observed for the late-phase DI was also reflected in the late-phase glucose-stimulated insulin sensitivity index (ISI) (P for trend <0.001), where both MED and HED increased more than CON, although the difference between DCON and CON was less pronounced (Fig. 2b). HED was associated with a greater increase in late-phase ISI compared with DCON (55% (95% CI, 15 to 109), P = 0.004). No differences were observed in late-phase ISI between DCON and CON. Late-phase glucose-stimulated insulin secretion rate (ISR) increased more in all intervention groups than in CON (Fig. 2c, Table 2 and Supplementary Tables 10 and 11), but no differences were observed among the remaining groups.
DI derived from the mixed meal tolerance test (MMTT) (oral DI) increased more in all intervention groups than in CON (Fig. 2d). The MED and HED groups increased more than DCON (MED versus DCON, 25% (95% CI, −5 to 65), P = 0.12; HED versus DCON, 29% (95% CI, −2 to 70), P = 0.065) with no signs of additional increases in HED versus MED (4% (95% CI, −22 to 37), P = 0.81).
All groups increased oral ISI compared with CON (P < 0.001) (Fig. 2e), with more pronounced increases in HED than DCON (29% (95% CI, 3 to 62), P = 0.025) (Table 2). No differences were observed in the oral ISR between the groups (Fig. 2f).
Safety outcomes
Three serious adverse events were observed: one case of transient ischemic attack and one case of malignant melanoma in the CON group, and one case of prolactinoma in the DCON group (Table 3). Two participants in the DCON group, and five participants each in the MED and HED groups reported adverse events. Beyond musculoskeletal complaints and overuse injuries in MED and HED, the nature and frequency were similar between groups.
Exploratory outcomes
Supporting clamp-derived indices of beta-cell function
The first-phase (0–10 min of clamp-induced hyperglycemia) DI increased in all intervention groups for all comparisons with CON (P ˂ 0.001; Supplementary Table 11). In addition, both HED and MED increased the first-phase DI more than DCON (HED versus DCON, 37% (95% CI, 6 to 77), P = 0.001; MED versus DCON, 58% (95% CI, 22 to 105), P = 0.017). No difference was observed between HED and MED (Supplementary Table 11). Peak and mean ISR in response to GLP-1 and GLP-1 + arginine infusion increased more from baseline to follow-up in all intervention groups compared with CON (Fig. 3 and Supplementary Tables 10 and 11). Whereas HED did not increase ISR in response to GLP-1 compared with DCON, MED was associated with increased ISR in response to GLP-1 compared with DCON (peak ISR, 0.2 (pmol/kg/min) × mM−1 (95% CI, 0.0 to 0.3), P = 0.019; mean ISR, 0.3 (pmol/kg/min) × mM−1 (95% CI, 0.05 to 0.6), P = 0.045). All intervention groups increased ISR in response to arginine, but no consistent differences were observed among the intervention groups (Supplementary Table 11).
Glucose kinetics
The change in basal rate of glucose appearance (Ra) and disappearance (Rd), and thus the basal endogenous glucose production (EGP), was increased only in HED compared with CON, but no additional differences between the groups were observed. Late-phase Rd and Ra increased more in all intervention groups than in CON (P < 0.001 for all comparisons); HED increased more than DCON (difference in Rd, 0.8 (95% CI, 0.2 to 1.4), P = 0.012; difference in Ra, 0.7 (95% CI, 0.1 to 1.3), P = 0.022). Complete data on glucose infusion rate (GIR), EGP, Rd and Ra are presented in Extended Data Fig. 2 and Supplementary Tables 10 and 11.
Postprandial glucose metabolism
Postprandial plasma glucose and insulin decreased more in all intervention groups than in CON (total area under the curve (tAUC), t = 0–120 min, P < 0.001). No differences were observed between intervention groups (Supplementary Tables 10 and 11; incremental AUC (iAUC) is shown in Supplementary Tables 14 and 15). The tAUC for GLP-1 and gastric inhibitory polypeptide (GIP) secretion increased more in CON than in the intervention groups from baseline to follow-up (Supplementary Tables 10 and 11). No differences were observed between the intervention groups (Supplementary Tables 10 and 11 and iAUC in Supplementary Tables 14 and 15). Postprandial responses are presented in Extended Data Figs. 3–8.
Body weight
Body weight decreased by 0%, 7%, 10% and 12% from baseline in CON, DCON, MED and HED, respectively, and decreased more in all intervention groups than in CON (Tables 4 and 5). Both MED and HED reduced the body weight by 3.2 kg (P = 0.043) and 4.5 kg (P = 0.004) more than DCON, respectively, with no difference in changes between the exercising groups. The same pattern was observed for BMI (Tables 4 and 5).
Other cardiometabolic markers
HbA1c decreased 0.6% (7 mmol/mol) more in all intervention groups than in CON (P < 0.001), but no differences were observed between intervention groups (Tables 4 and 5). The same pattern was observed for fasting glucose, fasting insulin, fasting C-peptide, fasting triglycerides and systolic blood pressure (Tables 4 and 5). All intervention groups had reduced diastolic blood pressure, and the reduction was greater in HED and MED than in DCON. No reductions in low-density lipoprotein cholesterol (LDL-C) were observed. Physical fitness defined as maximum oxygen consumption in ml O2/min (VO2max) increased in MED and HED compared with CON and DCON, and HED improved more than MED. VO2max relative to body weight defined as ml O2/min/kg (relative VO2max) changed by −3%, 8%, 23% and 39% in CON, DCON, MED and HED, respectively (Table 4). HED improved absolute and relative-to-body-weight 1 repetition maximum (RM) chest press compared with all of the other groups, whereas 1 RM leg extensions relative to body weight improved in HED only when compared with CON and DCON (Table 5).
Sensitivity analyses
The multiple imputation analyses on the primary and secondary outcomes agreed with the primary analyses (Supplementary Table 13).
Post hoc analyses
As a post hoc outcome, the need for medication, after completion of follow-up testing, was calculated based on the prespecified algorithm. Reductions of glucose-lowering medication were 11%, 92%, 81% and 89% in CON, DCON, MED and HED, respectively (Table 4). The corresponding numbers for discontinuations of glucose-lowering medication were 28%, 39%, 69% and 83%, respectively (Table 4). The odds of reductions and discontinuations were higher in all intervention groups than in CON (P < 0.05). No differences were observed in the odds of reductions of glucose-lowering medication between the intervention groups, but the odds of discontinuations were higher for HED than for CON (odds ratio (OR), 2.7 (95% CI, 1.1; 7.9); Table 5). Although the odds of discontinuation were higher in MED than in DCON (OR, 3.4 (95% CI, 0.6; 21.6)), it did not reach statistical significance (P = 0.2; Table 5). The odds of discontinuations were similar between HED and MED, and no differences were observed in any group comparison for other medications (Table 5).
The role of weight loss on the primary and secondary outcomes was explored in a post hoc statistical mediation analysis (Supplementary Table 16). It revealed that the treatment effect mediated by weight loss on late-phase DI was similar across the exercising groups and accounted for around 50–60% of the total effect, whereas 70% of the treatment effect was mediated by weight loss in DCON. Regarding late-phase ISI, the pattern was similar for the exercising groups, but for DCON, the weight loss was entirely responsible for the treatment effect. Weight loss did not explain the increase in late-phase ISR.
Discussion
One of our main findings is that all intervention groups improved beta-cell function, as expressed by late-phase DI, more than standard care. Furthermore, adding exercise to diet-induced weight loss improved beta-cell function more than diet-induced weight loss or standard care alone. This seemed to be achieved by additional increases in insulin sensitivity induced by exercise in a dose-dependent manner. However, the secondary and exploratory outcomes did not uniformly support the linear dose–response relationship observed for the primary outcome.
There is a paucity of studies investigating the role of exercise and exercise volume in conjunction with diet-induced weight loss, but our observations are in line with previous findings suggesting that high volumes of exercise without a concomitant dietary intervention improve first-phase and/or late-phase DI in people with prediabetes and T2D5,12,23. In line with other studies, our data support that the exercise component increases DI due to increases in insulin sensitivity rather than increased insulin secretion5,12,23,24. In contrast, other studies have shown that exercise may increase insulin secretion and not insulin sensitivity in people with dysglycemia6,8. One reason for the discrepancy may be the higher glucose clamp levels (25 mmol/l) used in ref. 6 compared with the ~13 mmol/l plasma glucose used in our study. At clamp levels closer to our target (~13 mmol/l plasma glucose), the researchers in ref. 6 also did not observe an increase in insulin secretion. The differences could also relate to the exercise intensities in the studies wherein, for a given increase in insulin sensitivity, it has been shown that high-intensity exercise results in a larger reduction in insulin secretion than low-intensity to moderate-intensity exercise12.
Although we did not observe a difference in late-phase ISR with increased exercise volume compared with diet-induced weight loss alone, all intervention groups exhibited similar increases in late-phase ISR. Therefore, it could be speculated that diet-induced weight loss alone might explain this observation and that a weight loss of ~7.5% body weight may be sufficient to re-establish late-phase ISR in this study population. Supporting this, a previous study from our group, using the same intervention protocol as for HED, also increased DI. Consistent with our current findings, only the improvement in insulin sensitivity index, and not the small increase in insulin secretion, was associated with exercise volume in that study10. Taken together, these findings suggest that changes in insulin sensitivity are more exercise driven, whereas changes in insulin secretion are primarily driven by weight loss. In further support of this, previous studies have shown an increased insulin secretory capacity after caloric restriction with no or marginal improvement in peripheral insulin sensitivity; thus, first-phase, late-phase and total-phase DI are explained mainly by increases in insulin secretion3,18,25. The increased first-phase ISR following diet-induced weight loss is consistent with findings of other studies3,26 and may relate to a decrease in fasting plasma glucose (which was comparable between the intervention groups in our study)27. However, it was surprising that exercise was associated with an attenuation of the increase in first-phase ISR induced by diet-induced weight loss. When first-phase DI was calculated (correcting the ISR for insulin sensitivity), the increase was larger in the exercising groups than with diet-induced weight loss alone. Although there seems to be a consensus that regaining first-phase insulin secretion is characteristic of T2D remission, we found a reduction when exercise was added to a diet-induced weight loss intervention. An explanation may relate to both improved insulin sensitivity and glucose effectiveness observed with exercise5,16, or could be ascribed to a blunted insulin secretion during hyperglycemia, GIP stimulation and arginine stimulation after high volumes of exercise28,29,30. The HED group may have experienced an exercise-induced blunting of insulin secretion whereas MED did not, which could also explain why there were no further increases in ISR, first-phase DI, GLP-1 stimulation and arginine stimulation despite the largest late-phase ISI being in HED. The metabolic consequences of a slightly blunted insulin secretion in people with T2D are unknown. Nevertheless, exercise-induced decreases in insulin secretion during concomitant increases in insulin sensitivity are consistently found in individuals with prediabetes and/or obesity, as well as in healthy people12,23,28. Given that insulin secretion physiologically counterbalances insulin sensitivity as a homeostatic response15,31, an exercise-induced increase in insulin sensitivity added to diet-induced weight loss may reduce the demand on beta cells, offering beta-cell rest and therefore preserving beta-cell health.
In this study, both late-phase DI and late-phase ISI increased linearly with increasing treatment intensity. However, a similar relationship was not observed during the MMTT nor in first-phase DI or the GLP-1 and arginine stimulations. Although speculative, this may relate to the route of glucose administration. Recently, it was described that the increased insulin secretion observed after oral administration of glucose compared with intravenous administration might be accompanied by a compensatory decrease in insulin sensitivity32. Furthermore, the incretin response during the MMTT might activate a GIP-induced vasoconstriction in the microvasculature of skeletal muscles33, which could dampen a difference in insulin action between the exercise groups achieved by exercise-induced skeletal muscle capillarization34.
These findings suggest that there is only a limited effect of increasing exercise volume from three to six sessions weekly in the context of diet-induced weight loss. Likewise, although weight loss was larger in both exercise groups compared with diet-induced weight loss alone, there was no apparent additional weight loss when doubling the exercise volume from three to six sessions per week. In contrast, the increase in both absolute and relative VO2max was positively associated with exercise dose, with only marginal differences in maximal strength.
Although a weight loss of ≥5% may increase beta-cell function slightly, a weight loss of ≥11% may be necessary to maximize an increase in peripheral insulin sensitivity in people with obesity2. However, diet-induced weight losses of ≥15 kg resulting in significant improvements in beta-cell function have been reported in people with T2D without concomitant increases in peripheral insulin sensitivity3.
Still, diet-induced weight loss may primarily improve hepatic (central) insulin sensitivity and beta-cell insulin secretory capacity through reductions in visceral and ectopic fat (that is, in liver and pancreas) that confer a dose-dependent increase in beta-cell function3,4. This might explain why the post hoc statistical mediation analysis suggested that the role of weight loss on the intervention effect mediated the entire effect of late-phase ISI in DCON. In contrast, exercise mainly improves peripheral insulin sensitivity35 and may explain why only 50–60% of the intervention effect was mediated by weight loss on late-phase ISI when exercise was added to the diet. As such, the small additional weight loss observed in the exercise groups compared with DCON most likely does not alone explain the add-on effect on late-phase ISI. These findings support that exercise may improve beta-cell function by increasing peripheral insulin sensitivity beyond the effects of weight reduction alone34,35,36.
Interestingly, weight loss completely mediated the intervention effects in the oral DI and oral ISI, suggesting that weight loss becomes the most important signal in the context of an oral mixed meal and normal homeostatic postprandial regulation. Although speculative, this may relate not only to the complex neuronal and endocrine organ crosstalk, but also to the meal composition wherein certain amino acids and fatty acids regulate insulin secretion, as well as skeletal muscle microvascular blood flow.
Limitations
Our findings must be interpreted in the context of the limitations of the study. First, the sample size was based on a previous study including up to five aerobic exercise sessions per week. Thus, the lack of differences between MED and HED or DCON and MED could be a type 2 error due to low statistical power. However, given the consistent signal across most beta-cell indices, the results can be interpreted with confidence. Second, we assessed beta-cell DI with a hyperglycemic clamp. Although the hyperglycemic clamp is the gold standard for beta-cell function15, it is an unphysiological assessment that limits physiological translation. However, we clamped the glucose level at only 5.4 mmol/l above the fasting glucose level, attempting to mimic postprandial glucose levels. Furthermore, we assessed beta-cell indices during an MMTT to compare the supraphysiological hyperglycemic clamp (glucose levels ~13 mmol/l at baseline and follow-up) to the physiological conditions during the MMTT (peak glucose levels ~16 mmol/l during MMTT at baseline). Because we observed a consistent pattern between the hyperglycemic clamp and MMTT, this allows for translating the findings from the hyperglycemic clamp to a physiological context. Third, we applied pharmacological constraints, and the participants were all relatively newly diagnosed and pharmacologically well regulated before randomization as well as throughout the study. Therefore, we cannot directly translate the results to people with longer T2D duration, treated with other pharmacological agents, or who have poor glycemic control. However, as our findings are consistent with pharmacological weight-loss trials37, they may still have clinical implications. Fourth, the intervention was only 16 weeks. Diminished benefits concerning beta-cell indices when going from three to six exercise sessions per week could be due to ceiling effects for the time course of the intervention. Hence, three exercise sessions per week combined with a 25% energy deficit may almost fully saturate the rate of adaptation for the mechanisms influencing beta-cell function. Furthermore, organ-specific changes in response to chronic exercise may occur on different time courses and will also reflect individual responses to exercise35,38. Thus, 16 weeks may have been too short to see significant deviations between MED and HED or even from DCON. Fifth, we did not use the gold standard hyperinsulinemic-euglycemic clamp to assess insulin sensitivity; however, the hyperglycemic clamp provides a reliable measure of glucose disposal15,39. Moreover, we observed that late-phase EGP did not change while Rd increased compared with CON, suggesting an increased peripheral insulin sensitivity and not hepatic insulin sensitivity. Sixth, we assessed dietary adherence using self-reporting, which may include information bias40. Seventh, although DI is considered the most accurate assessment of beta-cell function15, the relationship between insulin secretion and insulin sensitivity is not consistently hyperbolic across levels of glucose tolerance, BMI or measurements of DI31. Moreover, an increased beta-cell DI does not necessarily imply improved beta-cell health. This is because an increase in DI via an increased demand of insulin secretion to compensate for decreased insulin sensitivity (that is, higher allostatic load) has been associated with deterioration of beta-cell function compared with increasing DI via improved insulin sensitivity31,41,42. Therefore, we evaluated both insulin secretion and insulin sensitivity for calculating beta-cell DI, and our results are in line with preclinical and clinical studies suggesting that increasing beta-cell DI through insulin sensitivity is beneficial for beta-cell health.
Conclusion and perspectives
Among adults with T2D within 7 years of diagnosis, exercise in addition to diet-induced weight loss increases late-phase DI across a 16-week intervention. The most pronounced benefits were observed with exercise six times per week.
The direction of the exercise effects on the oral DI and oral ISI are consistent with an additional benefit when added to diet-induced weight loss. In contrast to the linear dose–response relationship observed with glucose stimulation only, the oral DI and oral ISI displayed a curve-linear relationship with diminished returns when comparing three and six exercise sessions per week. Hence, data from the meal stimulation suggest that increasing the exercise dose beyond three times per week may be redundant to gain additional benefits of exercise on beta-cell function when performed in conjunction with diet-induced weight loss. Further research is needed to confirm this.
Methods
Study design
The study was a 16-week, parallel-group, four-arm, assessor-blinded, randomized clinical trial conducted between February 2019 and October 2021 at the Centre for Physical Activity Research (CFAS), Rigshospitalet, Copenhagen, Denmark. The study was preregistered at ClinicalTrials.gov (NCT03769883) and was approved by the Scientific Ethical Committee of the Capital Region of Denmark (approval number H-18038298) before the commencement of any study procedures. Guidelines from the Helsinki Declaration were followed, and the data are reported following the CONSORT guideline for multi-arm trials43 and the REPORT standards43. The study protocol for this clinical trial is available in the Supplementary Information and has been published previously22. The prespecified full statistical analysis plan (SAP) was completed and uploaded to our website before commencing any statistical analyses (https://aktivsundhed.dk/images/docs/SAP_doseex_nov21.pdf).
Participants and eligibility criteria
Participants were recruited through the media, municipalities and the Danish Health Data Authorities. The potential participants contacted the study nurse and completed the screening process before the medical examination. The main inclusion criteria were (1) men and women aged 18–80 years, (2) diagnosed with T2D within <7 years, (3) no current treatment with insulin and (4) BMI > 27 kg/m2 and <40 kg/m2. All participants provided written and oral informed consent before any testing.
Interventions
CON received standard care and was encouraged to maintain habitual physical activity and dietary habits throughout the study. DCON received standard care and dietary intervention. MED received standard care, dietary intervention and an exercise intervention with two aerobic training sessions per week and one combined aerobic and resistance training session per week, totaling 150–165 min of exercise training per week. HED received the standard care and dietary interventions as described above but had twice as much exercise as MED, with a total of four aerobic training sessions per week and two combined aerobic and resistance training sessions per week, totaling 300–330 min of exercise training per week.
Standard care component
Standard care included pharmacological management of blood glucose, blood lipids and blood pressure according to a prespecified algorithm and was managed by an endocrinologist who was blinded for participant allocation22. To minimize an influence on the findings of poor glucose control upon study entry, medical standardization was introduced according to the prespecified treat-to-target algorithm for 6 weeks before the baseline measurements. Furthermore, the pharmacological treatment was evaluated according to the algorithm following baseline measurements and at week 12 of the intervention. The treatment targets were in line with current guidelines. In adjunct to the algorithm, pharmacological treatment was adapted to mitigate subjective signs of hypotension or hypoglycemia. Blood lipids, blood pressure and blood glucose were measured before the intervention and 4, 12 and 16 weeks into the intervention. In case of any adverse events, the participants were advised to contact the study nurse. At each visit, the study nurse interviewed all participants about potential adverse events. The adverse events definition followed ICH E2A guidelines44.
Dietary component
Daily energy requirements were estimated using the age-adjusted Oxford equation45. The dietary intervention aimed at ~25–30% energy deficit per day with a macronutrient distribution within the range of 45–60 energy percent (E%) carbohydrate, 15–20E% protein and 20–35E% fat (<7E% saturated fat). The intervention consisted of individualized recommendations and recipes. A clinical dietician implemented the plan at three sessions during the intervention, and adjustments were performed based on self-reported, 3-day food records.
Exercise component
The exercise intervention consisted of both aerobic and resistance training, and the first 2 weeks served as a familiarization period. The aerobic training sessions of 30-min duration had a target intensity of 60–100% of maximal heart rate (HRmax). Throughout the intervention, the relative time spent exercising in intensity zone 80–100% of HRmax was increased, and the relative time spent in the intensity zone 60–79% of HRmax was reduced accordingly. Resistance training was added in combined sessions with 30 min of aerobic training and 30–45 min of resistance training. The resistance training consisted of three sets in the main muscle groups, for example, chest press, leg press, back row, and leg extension. The 8–12 repetitions aimed at a resistance consistent with 0–3 repetitions in reserve46. All heart-rate profiles were recorded during the exercise interventions (Polar V800), and all training sessions were supervised by educated trainers.
Experimental days
Two experimental days were conducted at baseline and repeated at 16-week follow-up. Forty-eight hours before the experimental days, the participants were instructed to discontinue glucose-lowering medication use and refrain from any exercise. Moreover, no alcohol or caffeine was permitted 24 h before the visits, and the participants were instructed to maintain their habitual diet. The participants arrived at the testing facilities at 07:30 am after an overnight fast (≥10 h fasting). Experimental days 1 and 2 were planned to be separated by 1 week.
Experimental day 1
The participants completed a 3-h MMTT. The liquid meal was prepared using 400 ml of Nestlé Resource with an additional 36 g of dextrose (total energy content, 735 kcal; E%, 64/24/12 carbohydrate/fat/protein). Paracetamol (1.5 g) was added to assess gastric emptying. Body weight was measured with an electronic scale, and height was measured with a Holtain stadiometer according to standard procedures. VO2max was assessed using indirect calorimetry (Quark CPET, Cosmed) on a Monark LC4 bicycle (Monark Exercise). The test was performed with a 5-min warm-up followed by increases of 20 watts/min until exhaustion. Maximum muscle strength was assessed by two exercises performed in resistance training machines (chest press, leg extension) via estimating the maximum weight (kg) that could be lifted once with a full range of motion with proper form (that is, 1 RM).
Experimental day 2
A three-stage hyperglycemic clamp was performed. After baseline blood sampling, a priming bolus of [6,6-2H2]glucose was injected intravenously and a continuous tracer infusion was initiated. The bolus dose and infusion rate of the tracer depended on the participant’s fasting glucose level and body weight as described elsewhere5. After 2 h of tracer infusion, hyperglycemia was introduced by clamping glucose at 5.4 mM above fasting glucose (whereas the absolute postintervention clamp glucose level was equal to the preintervention clamp level). An initial increase in blood glucose was brought about by a square-wave glucose infusion lasting 15 min. After this, the glucose concentration was kept constant by adjusting GIRs based on blood glucose measurements (ABL 8 series, Radiometer) performed every 5 min according to an automated algorithm5. After 2 h of hyperglycemia, a continuous GLP-1 infusion was initiated at a rate of 0.5 pmol/kg/min, and after 1 h of hyperglycemia + GLP-1 infusion, an intravenous bolus of arginine hydrochloride (5 g given over 30 s) was administered to provide a maximal stimulus to the beta cells, leading to secretion of remaining intracellular vesicles of insulin. Before baseline sampling, the participant voided. Every time the participant voided during the clamp, the urine was accumulated, and urinary glucose concentration was measured at the end of the procedure.
Free-living measurements
Assessments of free-living physical activity and blood pressure were recorded by the participants between the 2 study days. Physical activity was also assessed with physical activity monitors (AX3, Axivity) for 7 consecutive days. Blood pressure was assessed with home-based resting measurements across 3 days, including three measurements morning and evening. Furthermore, a 3-day record of total dietary intake was completed at baseline, during the intervention period (at weeks 4 and 12), and during the 3 days leading up to follow-up testing.
Blood sample analyses
Blood samples (plasma insulin, C-peptide, glucose, HbA1c, LDL-C, triglycerides and paracetamol) were analyzed at the Department of Clinical Biochemistry, Rigshospitalet, using standard procedures. GLP-1 and GIP were analyzed using in-house carboxy-terminal radioimmunoassays. The total GLP-1 assay (codename 89390) is based on the amidated COOH terminus and therefore measures GLP-1(7–36)NH2 and GLP-1(9–36)NH2. The assay results, therefore, reflect the secretion rate of GLP-1 (refs. 47,48). The total GIP assay (codename 80867) reacts fully with intact GIP and amino-terminally truncated forms49. The glucose tracer [6,6-2H2]glucose was used for whole-body measurements of Ra and Rd of glucose during steady-state hyperglycemia and was calculated using non-steady-state equations50 adapted for stable isotopes51,52.
Participant compensation
All participants received up to DKK 6,000 (€800) in total to cover lost earnings, transport and discomfort. The transaction was completed upon completion of the study (all four full laboratory days (V1, V2, V6 and V7) or upon withdrawal). For every completed day of laboratory testing, participants received DKK 1,000. Moreover, DKK 500 in compensation was added per biopsy (up to four in total). To prevent loss to follow-up in the CON group, we offered three supervised training sessions and a free 16-week membership in a fitness center following final testing.
Outcomes
Primary outcome
The primary outcome was the change in late-phase DI from baseline to the 16-week follow-up, reflecting the beta-cell response during the last 30 min of the hyperglycemic stage15. DI was calculated as the product of late-phase ISR and late-phase ISI (designated secondary outcomes, see below).
Secondary outcomes
Secondary outcomes were prespecified in the SAP (designated ‘Major secondary outcomes’ in the SAP) and included the late-phase ISR, late-phase ISI derived during the last 30 min of the hyperglycemic stage, and the oral DI, oral ISI and oral ISR derived from the MMTT53. Late-phase ISR was calculated from the deconvoluted C-peptide measurements54 and subsequently normalized to ambient blood glucose concentrations. Late-phase ISI was calculated as the GIR divided by the product of insulin and glucose39. Oral DI was calculated as the product of oral ISI and oral ISR. Oral ISI (the Matsuda index) was calculated as 10,000/√(fasting glucose × fasting insulin) × (mean glucose0–120min × mean insulin0–120min), and oral ISR was calculated as the tAUC for glucose divided by the tAUC for insulin from time 0 to 120 min during the MMTT53.
Exploratory outcomes
The exploratory outcomes (designated ‘Other secondary outcomes’ in the SAP) included the change (baseline to 16-week follow-up) in first-phase ISR, EGP, first-phase DI, ISI and ISR, as well as HbA1c, LDL-C, fasting glucose, fasting insulin, fasting C-peptide, fasting triglycerides, systolic blood pressure, diastolic blood pressure, body weight, absolute VO2max, relative VO2max, 1 RM for chest press and leg extension (both absolute and relative to body weight), and tAUC and iAUC in glucose, insulin, C-peptide, GLP-1, GIP and paracetamol from the MMTT. AUCs for the different time periods were calculated using the trapezoidal rule. Ra and Rd were calculated from glucose tracers during clamp-induced steady-state hyperglycemia. Adverse events were self-reported.
Post hoc outcomes
Post hoc outcomes included intensification (yes or no), reduction (yes or no) and discontinuation (yes or no) for glucose-lowering and blood pressure-lowering medications. Due to restrictions in our pharmacological treatment algorithm regarding lipid-lowering medications, only intensifications were assessed for this outcome.
Randomization and blinding
The participants were randomly allocated to the four intervention arms upon successful completion of the baseline measurements. An independent statistician (author R.C.) prepared a computer-generated randomization schedule in a ratio of 1:1:1:1, stratified by sex. To ensure concealment, the (permuted) block sizes were not disclosed. The schedule was forwarded to a secretary who was not involved in any study procedures and stored on a password-protected computer. Sequentially numbered, opaque, sealed envelopes were prepared and stored in a locked cabinet before commencing the recruitment. The envelopes were lined with aluminum foil to render the envelope impermeable to intense light. Following the conclusion of the hyperglycemic clamp, the appropriate envelope was opened by a study nurse, and the participant was informed about the allocation stated on the card inside the envelope. The participant received the allocation in a closed room. As such, the participants were blinded for treatment allocation until after the completion of the hyperglycemic clamp. Following the baseline assessment, blinding of the participants was no longer possible. Both study personnel involved with the data collection and the study endocrinologist managing pharmacological treatment and safety were blinded to allocation. The clinical results used for pharmacological management and safety assessment were presented to the endocrinologist by the study nurse without disclosing participant allocation.
Sample size and power considerations
We expected that an exercise intervention would increase the late-phase DI by 1.5 arbitrary units (a.u.) more than the control group, with a standard deviation of 1.5 a.u. of the change in the exercise and 1.0 a.u. in the control group5. For a contrast in a one-way analysis of variance (ANOVA) with four means (1.5, 1.0, 0.5, 0.0) and contrast coefficients (1, 0, 0, −1) using a two-sided significance level of 0.05, assuming an error standard deviation of 1.5 and a balanced design, a total sample size of 80 participants in the PP population (approximately 20 participants in each group) would yield statistical power of 87.7%.
Statistical analysis
According to the protocol and the SAP, the analysis of the primary outcome was based on the as-observed population (missing data were not imputed in the primary analysis)55,56, as well as the PP population. The ‘Full Analysis Set’ for the ITT population included all randomized participants irrespective of their compliance with the interventions. The PP population criteria included (1) completion of the primary outcome assessment (all groups), (2) compliance with the diet protocol defined as being within ±30% of the prescribed energy intake (DCON, MED and HED), and (3) compliance with the exercise training protocol defined as completing ≥70% of the prescribed exercise volume across the intervention period (from weeks 2 to 16) (MED and HED). Missing data were assumed to be missing at random. Continuous data, including the primary, secondary and exploratory outcomes, were analyzed using constrained baseline longitudinal analysis via a linear mixed model57. As the baseline value is a part of the outcome vector, all participants with at least one measurement (baseline or follow-up) were included in the analyses57. The model included fixed effects for time (two levels), treatment (coded 0 for all groups at baseline and coded 0, 1, 2 or 3 at follow-up for CON, DCON, MED and HED, respectively) and sex (two levels), as well as the unique patient identifier as a random effect. The potentially biased PP population analysis was further adjusted for putative confounders: diabetes duration and baseline maximal oxygen consumption (ml O2/kg/min). Data are presented as the difference in the mean changes with 95% confidence intervals unless stated otherwise. The adequacy of the models was investigated via the predicted values and residuals. If the model assumptions were violated, the analyses were conducted using the log-transformed data and subsequently exponentiated for interpretation. Back-transformed data were expressed as the ratio of the geometric mean and interpreted as either percent change from baseline (within group) or difference in change between groups. A linear trend (interpreted as a linear dose–response relationship) was examined by treating each treatment category as a continuous variable in the main model and tested using a Wald test (P value reported). Linearity was inspected visually, and the P for trend was calculated only for the primary and secondary outcomes to the extent that the relationship was linear (that is, for late-phase DI and late-phase ISI). Sensitivity analyses were performed using multiple linear imputation procedures with the change in outcomes (post-pre values)55. The model included all covariates included in the main model, and beta coefficient and standard errors were based on 30 imputed data sets and adjusted for between-imputation variability58. Dichotomous outcomes were analyzed using logistic regression analyses. As sparsity of dichotomous outcomes (as expected for medications) invalidates the confidence intervals, exact logistic regression (exlogistic in Stata) was used when cases were <559,60. A post hoc statistical mediation analysis was performed to examine the extent to which the observed treatment effect (in the intervention groups) on the primary and secondary outcomes was mediated by the change in body weight. An exploratory statistical mediation analysis was performed in R61 to examine the extent to which the observed treatment effect (in the intervention groups) on the primary and key secondary outcomes was mediated by the change in body weight. The lme4 package was used to construct the linear mixed models for the analysis62. This simple mediation analysis partitions the total causal effect into average direct effects (ADE) and average causal mediation effects (ACME; otherwise known as indirect effects). Bias-corrected and accelerated 95% confidence intervals were generated via nonparametric bootstrap analysis (2,000 resamples with replacement).
All non-hypothesis-based comparisons (that is, on the secondary and exploratory outcomes) are per definition considered exploratory and supportive of the interpretation of the primary outcome. If the global test of significance indicated between-group differences (P < 0.1)63, all outcomes (primary, secondary, exploratory and post hoc) on pairwise comparisons were explored. Although no corrections for multiplicity were performed, family-wise type 1 error rate on the primary outcome was retained by using a hierarchical analytic approach63. In accordance with our prespecified SAP, the six prespecified hierarchical hypotheses (based on a superiority assumption) were tested using the prespecified sequence: (1) CON versus HED, (2) CON versus MED, (3) CON versus DCON, (4) DCON versus HED, (5) DCON versus MED, (6) MED versus HED. If we failed to progress from any of the prior between-group comparisons (P > 0.05), the subsequent P values and confidence intervals were regarded as indicators of associations rather than causality. The statistical significance level (for superiority) was set at α < 0.05 (two-sided). The statistical analyses were performed using Stata/SE (StataCorp), version 17.1.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data are not available for download owing to privacy and ethical restrictions under the European Union’s General Data Protection Regulation (EU GDPR). Specific requests for access to the trial and individual-level and unique biological data included in this article may be sent to mathias.ried-larsen@regionh.dk. Based on the request, access may be provided to a named individual in agreement with the rules and regulations of the Danish Data Protection Agency and the National Committee on Health Research Ethics. Requests will be considered from the date of publication of this article.
Code availability
The syntax files (Stata) are available upon request to mathias.ried-larsen@regionh.dk.
References
Schwartz, S. S. et al. A unified pathophysiological construct of diabetes and its complications. Trends Endocrinol. Metab. 28, 645–655 (2017).
Magkos, F. et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 23, 591–601 (2016).
Lim, E. L. et al. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 54, 2506–2514 (2011).
Taylor, R. et al. Remission of human type 2 diabetes requires decrease in liver and pancreas fat content but is dependent upon capacity for β cell recovery. Cell Metab. 28, 547–556.e3 (2018).
Karstoft, K. et al. Mechanisms behind the superior effects of interval vs continuous training on glycaemic control in individuals with type 2 diabetes: a randomised controlled trial. Diabetologia 57, 2081–2093 (2014).
Dela, F., von Linstow, M. E., Mikines, K. J. & Galbo, H. Physical training may enhance beta-cell function in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 287, E1024–E1031 (2004).
Rogers, M. A. et al. Improvement in glucose tolerance after 1 wk of exercise in patients with mild NIDDM. Diabetes Care 11, 613–618 (1988).
Krotkiewski, M. et al. The effects of physical training on insulin secretion and effectiveness and on glucose metabolism in obesity and type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 28, 881–890 (1985).
Eriksen, L., Dahl-Petersen, I., Haugaard, S. B. & Dela, F. Comparison of the effect of multiple short-duration with single long-duration exercise sessions on glucose homeostasis in type 2 diabetes mellitus. Diabetologia 50, 2245–2253 (2007).
Johansen, M. Y. et al. Effects of an intensive lifestyle intervention on the underlying mechanisms of improved glycaemic control in individuals with type 2 diabetes: a secondary analysis of a randomised clinical trial. Diabetologia 63, 2410–2422 (2020).
Curran, M. et al. The benefits of physical exercise for the health of the pancreatic β-cell: a review of the evidence. Exp. Physiol. 105, 579–589 (2020).
Slentz, C. A. et al. Effects of exercise training intensity on pancreatic beta-cell function. Diabetes Care 32, 1807–1811 (2009).
Zhang, S., Wei, Y. & Wang, C. Impacts of an exercise intervention on the health of pancreatic beta-cells: a review. Int. J. Environ. Res. Public Health 19, 7229 (2022).
AbouAssi, H. et al. The effects of aerobic, resistance, and combination training on insulin sensitivity and secretion in overweight adults from STRRIDE AT/RT: a randomized trial. J. Appl. Physiol. (1985) 118, 1474–1482 (2015).
Hannon, T. S. et al. Review of methods for measuring β-cell function: design considerations from the Restoring Insulin Secretion (RISE) Consortium. Diabetes Obes. Metab. 20, 14–24 (2018).
Karstoft, K. et al. Glucose effectiveness, but not insulin sensitivity, is improved after short-term interval training in individuals with type 2 diabetes mellitus: a controlled, randomised, crossover trial. Diabetologia 60, 2432–2442 (2017).
Davies, M. J. et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 45, 2753–2786 (2022).
Sathananthan, M. et al. Six and 12 weeks of caloric restriction increases β cell function and lowers fasting and postprandial glucose concentrations in people with type 2 diabetes. J. Nutr. 145, 2046–2051 (2015).
Goto, Y. et al. Improvement of skeletal muscle insulin sensitivity by 1 week of SGLT2 inhibitor use. Endocr. Connect. 9, 599–606 (2020).
Omar, B. & Ahrén, B. Pleiotropic mechanisms for the glucose-lowering action of DPP-4 inhibitors. Diabetes 63, 2196–2202 (2014).
Bahne, E. et al. Metformin-induced glucagon-like peptide-1 secretion contributes to the actions of metformin in type 2 diabetes. JCI Insight 3, e93936 (2018).
Lyngbaek, M. P. P. et al. The effects of different doses of exercise on pancreatic β-cell function in patients with newly diagnosed type 2 diabetes: study protocol for and rationale behind the “DOSE-EX” multi-arm parallel-group randomised clinical trial. Trials 22, 244 (2021).
Malin, S. K. et al. Pancreatic β-cell function increases in a linear dose-response manner following exercise training in adults with prediabetes. Am. J. Physiol. Endocrinol. Metab. 305, E1248–E1254 (2013).
Madsen, S. M., Thorup, A. C., Overgaard, K. & Jeppesen, P. B. High intensity interval training improves glycaemic control and pancreatic β cell function of type 2 diabetes patients. PLoS ONE 10, e0133286 (2015).
Malandrucco, I. et al. Very-low-calorie diet: a quick therapeutic tool to improve β cell function in morbidly obese patients with type 2 diabetes. Am. J. Clin. Nutr. 95, 609–613 (2012).
Lean, M. E. J. et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet 391, 541–551 (2018).
Kanat, M. et al. Impaired early- but not late-phase insulin secretion in subjects with impaired fasting glucose. Acta Diabetol. 48, 209–217 (2011).
King, D. S. et al. Insulin secretory capacity in endurance-trained and untrained young men. Am. J. Physiol. Endocrinol. Metab. 259, E155–E161 (1990).
Dela, F. Functional adaptation of the human β-cells after frequent exposure to noradrenaline. J. Physiol. 593, 3199–3206 (2015).
Shima, K., Hirota, M., Sato, M., Iwami, T. & Oshima, I. Effect of exercise training on insulin and glucagon release from perfused rat pancreas. Horm. Metab. Res 19, 395–399 (1987).
Vazquez Arreola, E., Hanson, R. L., Bogardus, C. & Knowler, W. C. Relationship between insulin secretion and insulin sensitivity and its role in development of type 2 diabetes: beyond the disposition index. Diabetes 71, 128–141 (2022).
Mingrone, G. et al. Insulin sensitivity depends on the route of glucose administration. Diabetologia 63, 1382–1395 (2020).
Roberts-Thomson, K. M. et al. Oral and intravenous glucose administration elicit opposing microvascular blood flow responses in skeletal muscle of healthy people: role of incretins. J. Physiol. 600, 1667–1681 (2022).
Akerstrom, T. et al. Increased skeletal muscle capillarization enhances insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 307, E1105–E1116 (2014).
Sylow, L. & Richter, E. A. Current advances in our understanding of exercise as medicine in metabolic disease. Curr. Opin. Physiol. 12, 12–19 (2019).
Dubé, J. J. et al. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia 54, 1147–1156 (2011).
Frías, J. P. et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N. Engl. J. Med. 385, 503–515 (2021).
Lundby, C., Montero, D. & Joyner, M. Biology of VO2 max: looking under the physiology lamp. Acta Physiol. (Oxf.) 220, 218–228 (2017).
Meneilly, G. S. & Elliott, T. Assessment of insulin sensitivity in older adults using the hyperglycemic clamp technique. J. Am. Geriatr. Soc. 46, 88–91 (1998).
Trabulsi, J. & Schoeller, D. A. Evaluation of dietary assessment instruments against doubly labeled water, a biomarker of habitual energy intake. Am. J. Physiol. Endocrinol. Metab. 281, E891–E899 (2001).
Kahn, S. E. et al. Effects of rosiglitazone, glyburide, and metformin on β-cell function and insulin sensitivity in ADOPT. Diabetes 60, 1552–1560 (2011).
Boland, B. B. et al. Pancreatic β-cell rest replenishes insulin secretory capacity and attenuates diabetes in an extreme model of obese type 2 diabetes. Diabetes 68, 131–140 (2019).
Atlas Collaboration. Reconstruction of hadronic decay products of tau leptons with the ATLAS experiment. Eur. Phys. J. C 76, 295 (2016).
ICH E2A Clinical safety data management: definitions and standards for expedited reporting (European Medicines Agency, 1995).
Henry, C. J. K. Basal metabolic rate studies in humans: measurement and development of new equations. Public Health Nutr. 8, 1133–1152 (2005).
Helms, E. R., Cronin, J., Storey, A. & Zourdos, M. C. Application of the repetitions in reserve-based rating of perceived exertion scale for resistance training. Strength Cond. J. 38, 42–49 (2016).
Ørskov, C., Rabenhøj, L., Wettergren, A., Kofod, H. & Holst, J. J. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes 43, 535–539 (1994).
Wewer Albrechtsen, N. J. et al. Stability of glucagon-like peptide 1 and glucagon in human plasma. Endocr. Connect. 4, 50–57 (2015).
Lindgren, O. et al. Incretin hormone and insulin responses to oral versus intravenous lipid administration in humans. J. Clin. Endocrinol. Metab. 96, 2519–2524 (2011).
Steele, R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann. N. Y. Acad. Sci. 82, 420–430 (1959).
Plomgaard, P. et al. Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes 54, 2939–2945 (2005).
Matthews, D. E. Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis: by Robert R Wolfe, 1992, 471 pages, hardcover, $89.95. John Wiley & Sons, Inc, Somerset, NJ. Am. J. Clin. Nutr. 58, 452 (1993).
Maki, K. C., McKenney, J. M., Farmer, M. V., Reeves, M. S. & Dicklin, M. R. Indices of insulin sensitivity and secretion from a standard liquid meal test in subjects with type 2 diabetes, impaired or normal fasting glucose. Nutr. J. 8, 22 (2009).
Van Cauter, E., Mestrez, F., Sturis, J. & Polonsky, K. S. Estimation of insulin secretion rates from C-peptide levels: comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 41, 368–377 (1992).
White, I. R., Horton, N. J., Carpenter, J. & Pocock, S. J. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 342, d40 (2011).
Detry, M. A. & Lewis, R. J. The intention-to-treat principle: how to assess the true effect of choosing a medical treatment. JAMA 312, 85–86 (2014).
Coffman, C. J., Edelman, D. & Woolson, R. F. To condition or not condition? Analysing ‘change’ in longitudinal randomised controlled trials. BMJ Open 6, e013096 (2016).
Rubin, D. B. Multiple imputation after 18+ years. J. Am. Stat. Assoc. 91, 473–489 (1996).
Greenland, S., Mansournia, M. A. & Altman, D. G. Sparse data bias: a problem hiding in plain sight. BMJ 352, i1981 (2016).
Kirk, S., Scott, B. J. & Daniels, S. R. Pediatric obesity epidemic: treatment options. J. Am. Diet. Assoc. 105, S44–S51 (2005).
R: a language and environment for statistical computing. (R Foundation for Statistical Computing, 2018).
Bates, D., Machler, M., Bolker, B. & Walker, S. lme4: linear mixed-effects models using Eigen and S4; https://github.com/lme4/lme4 (2022).
Dmitrienko, A. & D’Agostino, R. B.Sr. Multiplicity considerations in clinical trials. N. Engl. J. Med. 378, 2115–2122 (2018).
Acknowledgements
We thank the study participants for their time and engagement in this study, as well as current and former staff at CFAS, Rigshospitalet. We also thank the current and former staff at the Centre for Diabetes Research at the Municipality of Copenhagen for their support in recruitment and intervention delivery. The project was supported by a grant from TrygFonden and Svend Andersen Fonden. CFAS is supported by TrygFonden (grants ID 101390, ID 20045 and ID 125132). R.C. is from the Section for Biostatistics and Evidence-Based Research, the Parker Institute, Bispebjerg and Frederiksberg Hospital, which is supported by a core grant from the Oak Foundation (OCAY-18-774-OFIL). M.P.P.L. was supported by a research grant from the Danish Diabetes Academy (grant no. NNF17SA0031406), which is funded by the Novo Nordisk Foundation.
Author information
Authors and Affiliations
Contributions
B.K.P., K.K., T.P.A. and M.R.-L. conceived the study. M.P.P.L., G.E.L. and M.R.-L. wrote the first draft. M.R.-L. is the principal investigator. M.R.-L., M.P.P.L. and G.E.L. had full access to the data in the study, verified the data, and had full responsibility for the decision to submit and publish. M.P.P.L., G.E.L., M.R.-L., T.P.A., K.K., B.K.P., T.P.J.S., R.C., G.V.H. and J.J.H. contributed to protocol development and study design. G.E.L., M.P.P.L., S.L.B., C.S.F., N.S.N., B.L., U.N., M.Ø., K.T. and B.T. performed the experiments and collected the data. T.P.A. and K.T. performed pharmacological management. B.H., J.J.H. and G.V.H. performed biochemical analyses of incretins or stable isotopes. J.C.B. performed the accelerometer analyses. M.R.-L., K.T., N.S.N., G.E.L. and M.P.P.L. integrated and quality-checked the data. M.R.-L. and C.G.D. performed the statistical analyses. G.E.L., M.P.P.L., R.C. and M.R.-L. wrote the SAP. All authors read the SAP, critically revised it for important intellectual content and approved the final version. All authors read the manuscript, critically revised it for important intellectual content and approved the final version.
Corresponding author
Ethics declarations
Competing interests
J.J.H. is a member of advisory boards for Novo Nordisk. All other authors declare that they have no competing interests.
Peer review
Peer review information
Nature Metabolism thanks the anonymous reviewers for their contribution to the peer review of this work. Primary handling editor: Isabella Samuelson, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Box-plots for baseline values and by group at follow-up.
As the primary analyses are performed using a constrained baseline model, where all groups are assumed to be similar a baseline, the baseline values are not depicted by group. Circles denotes the individual participant values. Center line is the median values, light grey area is the lower inter quartile range, dark grey area is the upper interquartile range, the whiskers show + /-1.5 x the interquartile range, CON: control group (N = 20 independent samples), DCON: Dietary control group (N = 21 independent samples), MED: Moderate volume exercise (N = 20 independent samples), HED: High volume exercise (N = 21 independent samples), DI: Disposition index, ISI: Insulin sensitivity index, ISR: Insulin secretion rate, pmol: pico mol, mmol: milli mol, a.u.: arbitrary units, kg: kilograms.
Extended Data Fig. 2 The black circles represent baseline values, and the red circles represents the 16-week follow-up values.
Data are represented as the estimated means. Error bars are 95% confidence intervals. Results are adjusted for sex. Time 0-120 minutes is the hyperglycemic phase. Time 120-180 minutes is the hyperglycemic - and GLP-1 stimulation phase. Time 180-190 minutes is the hyperglycemic, GLP-1, and Arginine HCl stimulation phase. CON: control group (N = 20 independent samples), DCON: Dietary control group (N = 21 independent samples), MED: Moderate volume exercise (N = 20 independent samples), HED: High volume exercise (N = 21 independent samples).
Extended Data Fig. 3 The black circles represent baseline values, and the red circles represents the 16-week follow-up values.
Data are represented as the estimated means. Error bars are 95% confidence intervals. Results are adjusted for sex. CON: control group (N = 20 independent samples), DCON: Dietary control group (N = 21 independent samples), MED: Moderate volume exercise (N = 20 independent samples), HED: High volume exercise (N = 21 independent samples).
Extended Data Fig. 4 The black circles represent baseline values, and the red circles represents the 16-week follow-up values.
Data are represented as the estimated means. Error bars are 95% confidence intervals. Results are adjusted for sex. CON: control group (N = 20 independent samples), DCON: Dietary control group (N = 21 independent samples), MED: Moderate volume exercise (N = 20 independent samples), HED: High volume exercise (N = 21 independent samples).
Extended Data Fig. 5 The black circles represent baseline values, and the red circles represents the 16-week follow-up values.
Data are represented as the estimated means. Error bars are 95% confidence intervals. Results are adjusted for sex. CON: control group (N = 20 independent samples), DCON: Dietary control group (N = 21 independent samples), MED: Moderate volume exercise (N = 20 independent samples), HED: High volume exercise (N = 21 independent samples).
Extended Data Fig. 6 Represented as the estimated means.
Error bars are 95% confidence intervals. Results are adjusted for sex. CON: control group (N = 20 independent samples), DCON: Dietary control group (N = 21 independent samples), MED: Moderate volume exercise (N = 20 independent samples), HED: High volume exercise (N = 21 independent samples).
Extended Data Fig. 7 The black circles represent baseline values, and the red circles represents the 16-week follow-up values.
Data are represented as the estimated means. Error bars are 95% confidence intervals. Results are adjusted for sex. CON: control group (N = 20 independent samples), DCON: Dietary control group (N = 21 independent samples), MED: Moderate volume exercise (N = 20 independent samples), HED: High volume exercise (N = 21 independent samples).
Extended Data Fig. 8 The black circles represent baseline values, and the red circles represents the 16-week follow-up values.
Data are represented as the estimated means. Error bars are 95% confidence intervals. Results are adjusted for sex. CON: control group (N = 20 independent samples), DCON: Dietary control group (N = 21 independent samples), MED: Moderate volume exercise (N = 20 independent samples), HED: High volume exercise (N = 21 independent samples).
Supplementary information
Supplementary Information
CONSORT checklist, published protocol, overview of preplanned statistical analysis and approved study protocol.
Supplementary Tables
Supplementary table overview and Supplementary Tables 1–16.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Legaard, G.E., Lyngbæk, M.P.P., Almdal, T.P. et al. Effects of different doses of exercise and diet-induced weight loss on beta-cell function in type 2 diabetes (DOSE-EX): a randomized clinical trial. Nat Metab 5, 880–895 (2023). https://doi.org/10.1038/s42255-023-00799-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00799-7