Abstract
The published results of the post-marketing ORAL Surveillance study, which compared the Janus kinase (JAK) inhibitor tofacitinib with anti-TNF therapy in older patients with rheumatoid arthritis who have cardiovascular risk factors, have led to changes in the recommendations for the use of JAK inhibitors. Although new safety signals have emerged for tofacitinib, namely malignancy and cardiovascular disease, it should be noted that these signals are relative to those seen with TNF blockers. The new data further raise our intrigue that venous thromboembolism might be a true risk related to JAK inhibition. Reassuringly, the totality of the findings from this newly published study and the other data collected to date suggest that JAK inhibitors can be used safely at approved doses by many patients with rheumatoid arthritis.
Similar content being viewed by others
Introduction
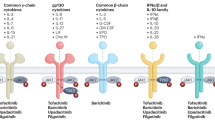
Tofacitinib was the first Janus kinase (JAK) inhibitor to be approved for the treatment of rheumatoid arthritis (RA) nearly 10 years ago. This oral medication, which diminishes the activity of JAK1, JAK2 and JAK3, was proven in phase III trials to offer an alternative of similar efficacy to injectable biologic DMARDs (bDMARDs). Since that time, other, more selective JAK inhibitor compounds have been approved, each with the idea that greater selectivity for one or more kinases might offer improvements in efficacy or safety (Fig. 1). So, where are we today in understanding the relative safety of these compounds? In the ‘old days’ we compared the safety of TNF inhibitors with non-biologic DMARDs. Over time, as more bDMARDs were approved, we began to shift our frame of reference and compare the safety of TNF inhibitors with other bDMARDs, and now compare JAK inhibitors and their relative safety with the bDMARDs. With so many choices for patients starting therapy, these relative comparisons are important, and very recently, the large post-marketing ORAL Surveillance (ORALSURV) trial has reported its outcomes in the peer-reviewed literature1. Although ORALSURV studied only patients with RA aged >50 years old with cardiovascular risk factors who started treatment with either tofacitinib or anti-TNF therapy (etanercept or adalimumab, depending on the region), the FDA extrapolated the study’s findings beyond tofacitinib to all JAK inhibitors currently in use for immune-mediated inflammatory diseases, and restricted use of this class of drugs to patients with RA only after TNF inhibitor failure2. While the practicing rheumatologist attempts to digest this information, which will no doubt change clinical practice recommendations, it is useful to put the ORALSURV findings in the context of the JAK inhibitor studies to date.
In 2012 tofacitinib became the first Janus kinase (JAK) inhibitor to be indicated for a rheumatic disease, when the FDA approved its use in the treatment of rheumatoid arthritis (RA); EMA approval came in 2017. In addition to tofacitinib, other JAK inhibitors (baricitinib, upadacitinib and filgotinib) have also been approved for use in the treatment of RA, and the indications for JAK inhibitors have expanded to include psoriatic arthritis (PsA), ankylosing spondylitis (AS) and ulcerative colitis (UC). MTX, methotrexate.
The ORALSURV study
In 2012, when tofacitinib received FDA approval for use in RA, the agency mandated the drug’s manufacturer, Pfizer, to conduct an additional post-marketing clinical trial owing to concerns regarding a potential increased risk of cancer, cardiovascular events and serious infections (SIEs) observed in the developmental programme in patients who received the higher, unapproved dose of 10 mg twice daily. Dose-dependent safety signals were noted in relation to a number of adverse events of special interest, leading to the conclusion that the benefit:risk ratio of tofacitinib was optimal with 5 mg twice daily, leading to the approval of only this dosage for use in RA3. ORALSURV was this FDA-mandated post-marketing phase IIIb–IV study, which enrolled 4,362 patients with RA aged >50 years who had at least one cardiovascular risk factor. Patients on background methotrexate therapy were randomly allocated to receive treatment either with tofacitinib at a dose of 5 mg or 10 mg twice daily or with a TNF inhibitor (etanercept or adalimumab, depending on the region). The trial’s primary end points were major adverse cardiovascular events (MACEs) and malignancy, and the trial was designed as an event-driven, non-inferiority study with regard to these two outcomes. The trial could only be concluded when at least 1,500 patients had been followed for 3 years, and 103 MACEs (including cardiovascular death, non-fatal myocardial infarction and non-fatal stroke) and 138 malignancies (excluding non-melanoma skin cancers) had occurred. Non-inferiority of the tofacitinib regimens to the TNF inhibitor control regimen was to be concluded if the upper confidence limits for the hazard ratios for malignancy (total time analysis) or MACEs (on-treatment time analysis) were less than 1.8 (ref.4).
Interestingly, in 2019, during the ORALSURV trial, a statistically significant elevation in the risk for pulmonary embolism was noted with the 10-mg dose of tofacitinib relative to TNF inhibitor treatment, and all patients on this dose were moved to the 5-mg dosage, although their data continued to be analysed as part of the 10-mg cohort1. This switch perhaps complicates interpretation of dose effect, and potentially brings the risk estimates for the two doses closer together, given the blending of these groups. Although this study was conceived primarily with cardiovascular and malignancy outcomes in mind, the study focused on the usual adverse events of interest for JAK inhibitor therapies: SIEs, herpes zoster infection, venous thromboembolism (VTE), MACEs, malignancy and mortality. For each of these adverse events, we lend context below, and in the end, conclude that the data are not dissimilar to those from the original developmental programme, which suggested additional safety concerns at the 10-mg dosage and that resulted in the 5-mg twice daily dosage as the approved dose for RA.
Infection
ORALSURV’s findings are consistent with clinical-trial and real-world studies of JAK inhibitors to date, and highlight that JAK inhibitors confer similar risk of infection to TNF inhibitors, with the exception of their propensity to reactivate latent viruses (such as varicella zoster virus, herpes simplex virus and cytomegalovirus). Unsurprisingly, ORALSURV reported rates of varicella zoster virus reactivation (that is, herpes zoster) several fold higher than for tofacitinib. To date, all JAK inhibitors approved in the USA seem to confer similar herpes zoster risk based on experience from phase II–III trials5, although direct comparator data between the different JAK inhibitor compounds are lacking. Filgotinib, a compound with selectivity for JAK1 and approved for use in Europe, had a lower herpes zoster incidence in more recently conducted phase II–III trials, although a dose-dependent elevation was observed6. With regard to SIEs, importantly, ORALSURV reported a similar risk for the tofacitinib 5-mg dose and TNF inhibitors, even in patients >65 years old. Although incidence rates were slightly higher in those >65 years old than in those 50–65 years old, there was no effect modification due to age, and the hazard ratios comparing tofacitinib with TNF inhibitors were similar in older and younger individuals. The ORALSURV SIE data were reassuring, and consistent with data from the RA development programmes of currently approved JAK inhibitors and bDMARDs, in which SIE rates are similar7 and generally in the range of 3–4 events per 100 patient-years, with elevated rates as expected in more elderly individuals.
Venous thromboembolism
ORALSURV’s finding of an increased risk of VTE with the tofacitinib 10-mg dose relative to TNF inhibition supports the idea, first raised in RA clinical trials of baricitinib’s 4-mg dose5, that VTE might be a true JAK inhibitor-related adverse event. Although a biological explanation is currently lacking, it is tempting to speculate that greater modulation of JAK2, which would be observed with higher doses of tofacitinib and baricitinib, could offer an eventual explanation8. Reassuringly, however, JAK inhibitors used in RA at their currently approved doses do not yet seem to carry excess risk. ORALSURV reported the tofacitinib 5-mg dose and TNF inhibitors to be associated with a similar risk of VTE, and this is consistent with real-world data (from the CORRONA registry) indicating that patients starting treatment with tofacitinib and those starting treatment with TNF inhibitors had a similar risk9. Furthermore, the incidence rates of VTE observed in pivotal trials of tofacitinib and upadacitinib were similar (and were even lower in filgotinib trials), and the rates within the active comparator groups of those programmes (that is, methotrexate and adalimumab) were similar or in some cases even higher6,10,11. Even for baricitinib, for which the initial imbalance in VTE risk between the 2-mg and 4-mg doses in the first 12 weeks of phase III trials raised eyebrows, similar long-term incidence rates were reported for both doses, of 0.5 events per 100 patient-years, a rate in line with RA population-based studies5,12. Lastly, baricitinib 4 mg given for 2 weeks did not increase the risk of VTE in the treatment of COVID-19, a condition with heightened VTE risk at baseline13. As the findings of ORALSURV suggest that there is a dose-dependent risk with tofacitinib relative to TNF inhibitors, until more basic and population-based research has been conducted with each of these compounds it seems at least prudent to steer JAK inhibitors away from those with a strong risk of VTE, particularly those with a history of VTE who are not presently anti-coagulated.
Major adverse cardiovascular events
Within ORALSURV, tofacitinib at both the 5-mg and the 10-mg twice daily doses failed to demonstrate non-inferiority for MACEs in comparison with TNF inhibitors, as the 95% CI exceeded the pre-specified upper boundary of 1.8. The incidence rate for the tofacitinib 5-mg dose was 0.91 per 100 patient-years and for the TNF inhibitors it was 0.73 per 100 patient-years (HR 1.24; 95% CI 0.81–1.91). Interestingly, the MACE incidence rate for TNF inhibitors in ORALSURV was markedly lower than that seen for etanercept (1.70 per 100 patient-years) in a similar trial evaluating patients with RA and cardiovascular risk factors14. Real-world data have established that TNF inhibitors are protective with regard to MACEs compared with non-biologic DMARDs, and some studies suggest that tocilizumab (an IL-6 receptor inhibitor) or even abatacept (a selective co-stimulation modulator) might be more protective than TNF inhibitors15. The 2022 STAR-RA population-based study, evaluating commercial and Medicare data on patients with RA initiating treatment with tofacitinib or TNF inhibitors, found no difference in the incidence rates of myocardial infarction and stroke between these treatment groups (HR 1.01; 95% CI 0.83–1.23)16. Of interest, however, when the analysis was restricted to patients with similar cardiovascular risk factors to those of patients enrolled in the ORALSURV study, again, no statistical difference in cardiovascular outcomes was found, but the HR of 1.24 (95% CI 0.90–1.69) for tofacitinib compared with TNF inhibitors was the same as that reported in the ORALSURV for tofacitinib 5-mg twice daily, noted above. As for JAK inhibitor data from studies prior to ORALSURV, data from the RA developmental programme for all approved JAK inhibitors suggest incidence rates similar to those observed for the bDMARD comparators in those phase III trials10,13,17.
Malignancy
The overall rate of malignancy for JAK inhibitors in RA randomized clinical trials and long-term extension studies has been reported to be similar to that seen with bDMARDs, and lower than that observed for tofacitinib in this study. In the ORALSURV study, the incidence rate for malignancy was 1.13 (95% CI 0.87–1.14) for patients treated with tofacitinib 5 mg twice daily and 1.13 (95% CI 0.86–1.14) for those treated with tofacitinib 10 mg twice daily, compared with 0.77 (95% CI 0.55–1.04) for the TNF inhibitor-treated patients (HR 1.48; 95% CI 1.04–2.09). This signal was driven by differential rates of several cancers (particularly lung cancer and lymphoma) primarily seen in the North American strata of the study (compared with the rest of the world), and among older individuals and in those with a history of tobacco smoking. An increased risk of non-melanoma skin cancer was also noted, which has been noted previously with use of the tofacitinib 10-mg dose in ulcerative colitis18. Conversely, numerically higher rates of melanoma (per 100 patient-years) were observed for patients using TNF inhibitors (0.09 (95% CI 0.03–0.21) versus 0.02 (95% CI 0.0–0.10) for either tofacitinib dosage. The mechanism by which JAK inhibitors could be associated with some types of cancer is unknown, but we would speculate that some JAK inhibitors, depending on their selectivity and effect on natural killer cells, could potentially diminish the host’s immunosurveillance for cancer, making an existing or de novo cancer more likely to progress19. In general, long-term data from large numbers of individuals is required to evaluate these long-latency events, and the real world RA data evaluated to date suggest no difference in cancer risk between patients treated with tofacitinib 5 mg or bDMARDs9. The extent to which tofacitinib or other JAK inhibitors might increase the risk of malignancy within specific high-risk groups (for example, elderly smokers) deserves further study.
Mortality
For all the approved JAK inhibitors, mortality rates have generally been reported to be similar to those associated with bDMARDs including TNF inhibitors, with standard incidence ratios in the Surveillance, Epidemiology, and End Results (SEER) database of around 1 with no statistical difference5,6,10,17. In the ORALSURV study, there was a statistically significant increase in overall mortality for the 10-mg dose (HR 2.37; 95% CI 1.34–4.18) and non-statistically significant trend for the 5-mg dose (HR 1.49; 95% CI 0.81–2.74) compared with TNF inhibitor-treated patients. These data were reflective of the differential rates of MACEs and malignancy observed in the trial.
How should we use JAK inhibitors?
In our minds, ORALSURV raises more questions than it answers, but it does help to inform treatment decision-making for physicians and patients, particularly if patients are at a high risk of certain outcomes. The issues raised by this study are reminiscent of the concerns raised about the risk of tuberculosis and other opportunistic infections with the use of TNF inhibitors, which was recognized in post-marketing surveillance, or of the association between cyclooxygenase 2 (COX2) inhibitors and increased cardiovascular risk, which resulted in rofecoxib and valdecoxib being withdrawn from the market20,21. As with ORALSURV, when signals of concern arose with TNF inhibitors and COX2 inhibitors there was substantial controversy and conflicting opinions. Over time, research confirmed these signals, and we look forward to additional mechanistic and clinical research to confirm or refute the observations from ORALSURV. What we do have to acknowledge is that we clearly have a signal of concern in a high-risk population and need to grapple with how this signal should influence our treatment decision-making. We must also acknowledge that ORALSURV reflects only a comparison of TNF inhibitors relative to JAK inhibitors in a specific population of patients with RA, and that both treatments might be protective against many of the outcomes under study if compared with no therapy, non-biologic DMARDs, or even other bDMARDs. It all boils down to the population under study and the referent group, and the fact that RA disease control is protective against all of the outcomes under study.
What does the clinician do now? Regulatory authorities such as the FDA and EMA have arrived at different conclusions with different modifications of JAK inhibitor utilization (Box 1). The EMA recommended that for patients ≥65 years old with a history of smoking or risk factors for cardiovascular disease or malignancy, tofacitinib should be used only if no suitable alternatives exist22; the FDA extrapolated the ORALSURV data beyond tofacitinib to include baricitinib and upadacitinib, with use of these agents recommended in such patients only in the case of prior TNF inhibition failure2. We believe that the ORALSURV data highlight the already described narrow safety window of JAK inhibitors, which was noted in the clinical trials, where the higher doses of the JAK inhibitors were not approved owing to increased toxicity. In general, these medicines, like all medicines, should be steered towards patients for whom the benefit:risk ratio is maximal, and it underscores the importance of screening patients for various risk factors prior to therapy selection. Fortunately, a number of highly effective alternatives are available for the treatment of rheumatic disease that we can utilize for patients at an increased risk of certain outcomes. For now, however, it seems that JAK inhibitors can be used at approved doses with safety similar to that of TNF blockade in many patients with RA, particularly in younger individuals and in older individuals who lack certain risk factors (for example, smoking).
Conclusions
Although the FDA’s ‘better to be safe than sorry’ approach might ultimately prove correct, there is certainly a need for studies comparing JAK inhibitor compounds, and comparing JAK inhibitors with other DMARDs, as well as further mechanistic studies to explain the safety signals observed in comparison with TNF inhibitors to date. How JAK inhibitor compounds compare with one another, and how they compare with other bDMARDs beyond TNF inhibitors, in regard to these outcomes is unknown. Although we clinicians navigate now perhaps murkier waters in the wake of this one study, we remind ourselves that we must always remember our frame of reference.
References
Ytterberg, S. R. et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N. Engl. J. Med. 386, 316–326 (2022).
U.S. Food and Drug Administration. FDA approves Boxed Warning about increased risk of blood clots and death with higher dose of arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR). https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-boxed-warning-about-increased-risk-blood-clots-and-death-higher-dose-arthritis-and (2021).
U.S. Food and Drug Administration. Center for Drug Evaluation and Research: NDA 203,214 Tofacitinib for Rheumatoid Arthritis, Addendum to Primary Clinical Review. Edited by Department of Health and Human Services, 26 September 2012 edn. Silver Spring, MD. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203214Orig1s000Approv.pdf (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/results/NCT02092467 (2021).
Genovese, M. C. et al. Safety profile of baricitinib for the treatment of rheumatoid arthritis over a median of 3 years of treatment: an updated integrated safety analysis. Lancet Rheumatol. 2, e347–e357 (2020).
Winthrop, K. L. et al. Integrated safety analysis update for filgotinib in patients with moderately to severely active rheumatoid arthritis receiving treatment over a median of 2.2 years [abstract]. Arthritis Rheumatol. 73, 1698 (2021).
Strand, V. et al. Systematic review and meta-analysis of serious infections with tofacitinib and biologic disease-modifying antirheumatic drug treatment in rheumatoid arthritis clinical trials. Arthritis Res. Ther. 17, 362 (2015).
Parra-Izquierdo, I. et al. Janus kinase inhibitors ruxolitinib and baricitinib impair glycoprotein-VI mediated platelet function. Platelets https://doi.org/10.1080/09537104.2021.1934665 (2021).
Kremer, J. M. et al. Postapproval comparative safety study of tofacitinib and biological disease-modifying antirheumatic drugs: 5-year results from a United States-based rheumatoid arthritis registry. ACR Open Rheumatol. 3, 173–184 (2021).
Cohen, S. B. et al. Long-term safety of tofacitinib up to 9.5 years: a comprehensive integrated analysis of the rheumatoid arthritis clinical development programme. RMD Open 6, e001395 (2020).
Winthrop, K. L. et al. Integrated safety analysis of filgotinib in patients with moderately to severely active rheumatoid arthritis receiving treatment over a median of 1.6 years. Ann. Rheum. Dis. 81, 184–192 (2022).
Desai, R. J., Pawar, A., Khosrow-Khavar, F., Weinblatt, M. E. & Kim, S. C. Risk of venous thromboembolism associated with tofacitinib in patients with rheumatoid arthritis: a population-based cohort study. Rheumatology 61, 121–130 (2021).
Marconi, V. C. et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir. Med. 9, 1407–1418 (2021).
Giles, J. T. et al. Cardiovascular safety of tocilizumab versus etanercept in rheumatoid arthritis: a randomized controlled trial. Arthritis Rheumatol. 72, 31–40 (2020).
Singh, S. et al. Comparative risk of cardiovascular events with biologic and synthetic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res. 72, 561–576 (2020).
Khosrow-Khavar, F., Kim, S. C., Lee, H., Lee, S. B. & Desai, R. J. Tofacitinib and risk of cardiovascular outcomes: results from the Safety of TofAcitinib in Routine care patients with Rheumatoid Arthritis (STAR-RA) study. Ann. Rheum. Dis. https://doi.org/10.1136/annrheumdis-2021-221915 (2022).
Cohen, S. B. et al. Safety profile of upadacitinib in rheumatoid arthritis: integrated analysis from the SELECT phase III clinical programme. Ann. Rheum. Dis. 80, 304–311 (2020).
Sands, B. E. et al. Tofacitinib for the treatment of ulcerative colitis: analysis of nonmelanoma skin cancer rates from the Ulcerative Colitis Clinical Program. Inflamm. Bowel Dis. 2, 234–245 (2022).
Nocturne, G., Pascaud, J., Ly, B., Tahmasebi, F. & Mariette, X. JAK inhibitors alter NK cell functions and may impair immunosurveillance against lymphomagenesis. Cell. Mol. Immunol. 17, 552–553 (2020).
Bhala, N. et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 382, 769–779 (2013).
Wallis, R. S., Broder, M. S., Wong, J. Y., Hanson, M. E. & Beenhouwer, D. O. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin. Infect. Dis. 38, 1261–1265 (2004).
European Medicines Agency. Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 7–10 June 2021 https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-7-10-june-2021 (2021).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
K.L.W. declares that he has acted as a consultant for AbbVie, Union AstraZeneca, Bristol Myers Squibb (BMS), Chimique Belge (UCB), Eli Lilly & Company, Galapagos, Gilead, GlaxoSmithKline, Novartis, Pfizer, Regeneron, Roche and Sanofi, and has received research funding from Bristol Myers Squibb and Pfizer. S.B.C. declares that he has acted as a consultant for and received research funding from AbbVie, Amgen, Gilead, Lilly and Pfizer.
Peer review
Peer review information
Nature Reviews Rheumatology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Winthrop, K.L., Cohen, S.B. Oral surveillance and JAK inhibitor safety: the theory of relativity. Nat Rev Rheumatol 18, 301–304 (2022). https://doi.org/10.1038/s41584-022-00767-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-022-00767-7
This article is cited by
-
Vascular damage in systemic lupus erythematosus
Nature Reviews Nephrology (2024)
-
Filgotinib Demonstrates Efficacy in Rheumatoid Arthritis Independent of Smoking Status: Post Hoc Analysis of Phase 3 Trials and Claims-Based Analysis
Rheumatology and Therapy (2024)
-
Comparative risk of infections between JAK inhibitors versus TNF inhibitors among patients with rheumatoid arthritis: a cohort study
Arthritis Research & Therapy (2023)
-
Treatment escalation patterns to start biologics in refractory moderate juvenile dermatomyositis among members of the Childhood Arthritis and Rheumatology Research Alliance
Pediatric Rheumatology (2023)
-
Janus kinase inhibitors are potential therapeutics for amyotrophic lateral sclerosis
Translational Neurodegeneration (2023)