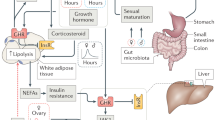
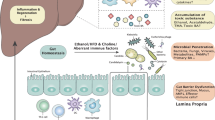
Abstract
Chronic liver disease is a major cause of morbidity and mortality worldwide. Epidemiology, clinical phenotype and response to therapies for gastrointestinal and liver diseases are commonly different between women and men due to sex-specific hormonal, genetic and immune-related factors. The hepatic immune system has unique regulatory functions that promote the induction of intrahepatic tolerance, which is key for maintaining liver health and homeostasis. In liver diseases, hepatic immune alterations are increasingly recognized as a main cofactor responsible for the development and progression of chronic liver injury and fibrosis. In this Review, we discuss the basic mechanisms of sex disparity in hepatic immune regulation and how these mechanisms influence and modify the development of autoimmune liver diseases, genetic liver diseases, portal hypertension and inflammation in chronic liver disease. Alterations in gut microbiota and their crosstalk with the hepatic immune system might affect the progression of liver disease in a sex-specific manner, creating potential opportunities for novel diagnostic and therapeutic approaches to be evaluated in clinical trials. Finally, we identify and propose areas for future basic, translational and clinical research that will advance our understanding of sex disparities in hepatic immunity and liver disease.
Key points
-
Increasing evidence indicates that biological sex modifies hepatic immune regulation, thereby contributing to hepatic immune outcomes in health and disease.
-
Changes in gut microbiota might substantially affect liver diseases in a sex-specific manner through the regulation of sex hormones, bile acid, lipid and hepatic xenobiotic metabolism.
-
Genetics have revealed few clues to sex disparities in liver disease, but dedicated studies are scarce; only for primary biliary cholangitis has a dedicated chromosome X-wide association study been performed.
-
Incidence, phenotype and disease progression in autoimmune liver diseases vary according to sex: men have an increased risk of disease progression, suboptimal treatment response and hepatobiliary malignancies.
-
Sex influences haemodynamics and portal hypertension in animal models; however, whether this translates into sex-specific responses to treatments lowering portal pressure in individuals with chronic liver disease is unclear.
-
Marked differences in body mass composition exist between sexes, both in health and chronic liver disease, potentially affecting the risk of hepatic decompensation and survival.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The publicly available GWAS catalogue referred to in Fig. 2 can be accessed, and the summary statistics contained therein can be downloaded from https://www.ebi.ac.uk/gwas/. For generation of the panels contained in Fig. 2, two publicly available tools were used: PhenoGram (https://ritchielab.org/software/phenogram-downloads) and the resource at https://bioinformatics.psb.ugent.be/webtools/Venn/.
References
Burra, P., Zanetto, A. & Germani, G. Sex bias in clinical trials in gastroenterology and hepatology. Nat. Rev. Gastroenterol. Hepatol. 19, 413–414 (2022).
Mauvais-Jarvis, F. et al. Sex and gender: modifiers of health, disease, and medicine. Lancet 396, 565–582 (2020).
Bizzaro, D. et al. Influence of sex in alcohol-related liver disease: pre-clinical and clinical settings. United Eur. Gastroenterol. J. 11, 218–227 (2023).
Burra, P. et al. Clinical impact of sexual dimorphism in non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). Liver Int. 41, 1713–1733 (2021).
Goodman, W. A., Erkkila, I. P. & Pizarro, T. T. Sex matters: impact on pathogenesis, presentation and treatment of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 17, 740–754 (2020).
Guarino, M. et al. Sarcopenia in chronic advanced liver diseases: a sex-oriented analysis of the literature. Dig. Liver Dis. 54, 997–1006 (2022).
Toniutto, P. et al. Role of sex in liver tumor occurrence and clinical outcomes: a comprehensive review. Hepatology 79, 1141–1157 (2023).
Zanetto, A. et al. Vascular liver diseases: a sex-oriented analysis of the literature. Dig. Liver Dis. 55, 178–186 (2023).
Lefebvre, P. & Staels, B. Hepatic sexual dimorphism – implications for non-alcoholic fatty liver disease. Nat. Rev. Endocrinol. 17, 662–670 (2021).
Knolle, P. A. & Thimme, R. Hepatic immune regulation and its involvement in viral hepatitis infection. Gastroenterology 146, 1193–1207 (2014).
Jenne, C. N. & Kubes, P. Immune surveillance by the liver. Nat. Immunol. 14, 996–1006 (2013).
Doherty, D. G. Immunity, tolerance and autoimmunity in the liver: a comprehensive review. J. Autoimmun. 66, 60–75 (2016).
Crispe, I. N. The liver as a lymphoid organ. Annu. Rev. Immunol. 27, 147–163 (2009).
Gao, B., Jeong, W. I. & Tian, Z. Liver: an organ with predominant innate immunity. Hepatology 47, 729–736 (2008).
Racanelli, V. & Rehermann, B. The liver as an immunological organ. Hepatology 43, S54–S62 (2006).
Tiegs, G. & Lohse, A. W. Immune tolerance: what is unique about the liver. J. Autoimmun. 34, 1–6 (2010).
Costa, D. et al. Systemic inflammation increases across distinct stages of advanced chronic liver disease and correlates with decompensation and mortality. J. Hepatol. 74, 819–828 (2021).
Liberal, R., Grant, C. R., Mieli-Vergani, G. & Vergani, D. Autoimmune hepatitis: a comprehensive review. J. Autoimmun. 41, 126–139 (2013).
Shin, E. C., Sung, P. S. & Park, S. H. Immune responses and immunopathology in acute and chronic viral hepatitis. Nat. Rev. Immunol. 16, 509–523 (2016).
Zanetto, A. et al. Severity of systemic inflammation is the main predictor of ACLF and bleeding in individuals with acutely decompensated cirrhosis. J. Hepatol. 78, 301–311 (2023).
Zanetto, A. et al. Toward a more precise prognostic stratification in acute decompensation of cirrhosis: the Padua model 2.0. United Eur. Gastroenterol. J. 11, 815–824 (2023).
Zhang, S., Lu, S. & Li, Z. Extrahepatic factors in hepatic immune regulation. Front. Immunol. 13, 941721 (2022).
Meijnikman, A. S. et al. Microbiome-derived ethanol in nonalcoholic fatty liver disease. Nat. Med. 28, 2100–2106 (2022).
Schwabe, R. F. & Greten, T. F. Gut microbiome in HCC – mechanisms, diagnosis and therapy. J. Hepatol. 72, 230–238 (2020).
Yin, Y. et al. Gut microbiota promote liver regeneration through hepatic membrane phospholipid biosynthesis. J. Hepatol. 78, 820–835 (2023).
Karlsen, T. H. et al. The EASL-Lancet Commission on liver health in Europe: prevention, case-finding, and early diagnosis to reduce liver-related mortality. Lancet 403, 1522–1524 (2024).
Karlsen, T. H. et al. The EASL-Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet 399, 61–116 (2022).
Heidari, S. et al. WHO’s adoption of SAGER guidelines and GATHER: setting standards for better science with sex and gender in mind. Lancet 403, 226–228 (2024).
Heymann, F. & Tacke, F. Immunology in the liver – from homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 13, 88–110 (2016).
Kasarinaite, A., Sinton, M., Saunders, P. T. K. & Hay, D. C. The influence of sex hormones in liver function and disease. Cells 12, 1604 (2023).
Della Torre, S. & Maggi, A. Sex differences: a resultant of an evolutionary pressure. Cell Metab. 25, 499–505 (2017).
Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 79, 1542–1556 (2023).
Catala-Senent, J. F. et al. Hepatic steatosis and steatohepatitis: a functional meta-analysis of sex-based differences in transcriptomic studies. Biol. Sex. Differ. 12, 29 (2021).
Giles, D. A. et al. Thermoneutral housing exacerbates nonalcoholic fatty liver disease in mice and allows for sex-independent disease modeling. Nat. Med. 23, 829–838 (2017).
Sommars, M. A. et al. Dynamic repression by BCL6 controls the genome-wide liver response to fasting and steatosis. Elife 8, e43922 (2019).
Nikkanen, J. et al. An evolutionary trade-off between host immunity and metabolism drives fatty liver in male mice. Science 378, 290–295 (2022).
Waxman, D. J. & Kineman, R. D. Sex matters in liver fat regulation. Science 378, 252–253 (2022).
Roelfsema, F. & Veldhuis, J. D. Growth hormone dynamics in healthy adults are related to age and sex and strongly dependent on body mass index. Neuroendocrinology 103, 335–344 (2016).
Guillaume, M. et al. Selective liver estrogen receptor α modulation prevents steatosis, diabetes, and obesity through the anorectic growth differentiation factor 15 hepatokine in mice. Hepatol. Commun. 3, 908–924 (2019).
Oliva, M. et al. The impact of sex on gene expression across human tissues. Science 369, eaba3066 (2020).
Lin, H. Y. et al. Increased hepatic steatosis and insulin resistance in mice lacking hepatic androgen receptor. Hepatology 47, 1924–1935 (2008).
Lee, H. S. et al. The effect of testosterone replacement therapy on nonalcoholic fatty liver disease in older hypogonadal men. J. Clin. Endocrinol. Metab. 109, e757–e764 (2024).
Sarkar, M. et al. Low testosterone is associated with nonalcoholic steatohepatitis and fibrosis severity in men. Clin. Gastroenterol. Hepatol. 19, 400–402.e2 (2021).
Chen, K. W., Chen, Y. S., Chen, P. J. & Yeh, S. H. Androgen receptor functions in pericentral hepatocytes to decrease gluconeogenesis and avoid hyperglycemia and obesity in male mice. Metabolism 135, 155269 (2022).
Wu, X. N. et al. Sex-determining region Y gene promotes liver fibrosis and accounts for sexual dimorphism in its pathophysiology. J. Hepatol. 80, 928–940 (2024).
Dong, J. et al. SRY is a key mediator of sexual dimorphism in hepatic ischemia/reperfusion injury. Ann. Surg. 276, 345–356 (2022).
Liu, C. et al. Activation of SRY accounts for male-specific hepatocarcinogenesis: implication in gender disparity of hepatocellular carcinoma. Cancer Lett. 410, 20–31 (2017).
Jaillon, S., Berthenet, K. & Garlanda, C. Sexual dimorphism in innate immunity. Clin. Rev. Allergy Immunol. 56, 308–321 (2019).
Shepherd, R., Cheung, A. S., Pang, K., Saffery, R. & Novakovic, B. Sexual dimorphism in innate immunity: the role of sex hormones and epigenetics. Front. Immunol. 11, 604000 (2020).
Gal-Oz, S. T. et al. ImmGen report: sexual dimorphism in the immune system transcriptome. Nat. Commun. 10, 4295 (2019).
Hammerich, L. & Tacke, F. Hepatic inflammatory responses in liver fibrosis. Nat. Rev. Gastroenterol. Hepatol. 20, 633–646 (2023).
Peiseler, M. et al. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease – novel insights into cellular communication circuits. J. Hepatol. 77, 1136–1160 (2022).
Saviano, A., Henderson, N. C. & Baumert, T. F. Single-cell genomics and spatial transcriptomics: discovery of novel cell states and cellular interactions in liver physiology and disease biology. J. Hepatol. 73, 1219–1230 (2020).
Ministrini, S., Montecucco, F., Sahebkar, A. & Carbone, F. Macrophages in the pathophysiology of NAFLD: the role of sex differences. Eur. J. Clin. Invest. 50, e13236 (2020).
Han, Y. H., Choi, H., Kim, H. J. & Lee, M. O. Chemotactic cytokines secreted from Kupffer cells contribute to the sex-dependent susceptibility to non-alcoholic fatty liver diseases in mice. Life Sci. 306, 120846 (2022).
Naugler, W. E. et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 317, 121–124 (2007).
Becerra-Diaz, M., Strickland, A. B., Keselman, A. & Heller, N. M. Androgen and androgen receptor as enhancers of M2 macrophage polarization in allergic lung inflammation. J. Immunol. 201, 2923–2933 (2018).
Bizzaro, D. et al. Sex-dependent differences in inflammatory responses during liver regeneration in a murine model of acute liver injury. Clin. Sci. 132, 255–272 (2018).
Chen, K. E., Lainez, N. M. & Coss, D. Sex differences in macrophage responses to obesity-mediated changes determine migratory and inflammatory traits. J. Immunol. 206, 141–153 (2021).
Petrescu, A. D. et al. Glucocorticoids cause gender-dependent reversal of hepatic fibrosis in the MDR2-knockout mouse model. Int. J. Mol. Sci. 18, 2389 (2017).
Krishnan, A. et al. tumor necrosis factor-related apoptosis-inducing ligand receptor deficiency promotes the ductular reaction, macrophage accumulation, and hepatic fibrosis in the Abcb4−/− mouse. Am. J. Pathol. 190, 1284–1297 (2020).
Lotter, H., Jacobs, T., Gaworski, I. & Tannich, E. Sexual dimorphism in the control of amebic liver abscess in a mouse model of disease. Infect. Immun. 74, 118–124 (2006).
Lotter, H. et al. Testosterone increases susceptibility to amebic liver abscess in mice and mediates inhibition of IFNγ secretion in natural killer T cells. PLoS ONE 8, e55694 (2013).
Sellau, J. et al. Androgens predispose males to monocyte-mediated immunopathology by inducing the expression of leukocyte recruitment factor CXCL1. Nat. Commun. 11, 3459 (2020).
Helk, E. et al. TNFα-mediated liver destruction by Kupffer cells and Ly6Chi monocytes during Entamoeba histolytica infection. PLoS Pathog. 9, e1003096 (2013).
Er-Lukowiak, M. et al. Testosterone affects type I/type II interferon response of neutrophils during hepatic amebiasis. Front. Immunol. 14, 1279245 (2023).
Groneberg, M. et al. HIF-1α modulates sex-specific Th17/Treg responses during hepatic amoebiasis. J. Hepatol. 76, 160–173 (2022).
Liedtke, C. et al. Experimental liver fibrosis research: update on animal models, legal issues and translational aspects. Fibrogenes. Tissue Repair. 6, 19 (2013).
Gallage, S. et al. A researcher’s guide to preclinical mouse NASH models. Nat. Metab. 4, 1632–1649 (2022).
Cuno-Gomiz, C. et al. Sex-based differences in natural killer T cell-mediated protection against diet-induced steatohepatitis in Balb/c mice. Biol. Sex. Differ. 14, 85 (2023).
Osonoi, S. & Takebe, T. Organoid-guided precision hepatology for metabolic liver disease. J. Hepatol. 80, 805–821 (2024).
Rezvani, M., Vallier, L. & Guillot, A. Modeling nonalcoholic fatty liver disease in the dish using human-specific platforms: strategies and limitations. Cell Mol. Gastroenterol. Hepatol. 15, 1135–1145 (2023).
Trivedi, P. J., Hirschfield, G. M., Adams, D. H. & Vierling, J. M. Immunopathogenesis of primary biliary cholangitis, primary sclerosing cholangitis and autoimmune hepatitis: themes and concepts. Gastroenterology 166, 995–1019 (2024).
Papenfuss, T. L. et al. Estriol generates tolerogenic dendritic cells in vivo that protect against autoimmunity. J. Immunol. 186, 3346–3355 (2011).
Seillet, C. et al. Estradiol promotes functional responses in inflammatory and steady-state dendritic cells through differential requirement for activation function-1 of estrogen receptor α. J. Immunol. 190, 5459–5470 (2013).
Henze, L., Schwinge, D. & Schramm, C. The effects of androgens on T cells: clues to female predominance in autoimmune liver diseases? Front. Immunol. 11, 1567 (2020).
Meester, I. et al. SeXY chromosomes and the immune system: reflections after a comparative study. Biol. Sex. Differ. 11, 3 (2020).
Zhang, X., Chen, B. D., Zhao, L. D. & Li, H. The gut microbiota: emerging evidence in autoimmune diseases. Trends Mol. Med. 26, 862–873 (2020).
Werner, M. et al. Epidemiology and the initial presentation of autoimmune hepatitis in Sweden: a nationwide study. Scand. J. Gastroenterol. 43, 1232–1240 (2008).
Gronbaek, L. et al. Incidence, prevalence and mortality of autoimmune hepatitis in England 1997-2015. A population-based cohort study. Liver Int. 40, 1634–1644 (2020).
Webb, G. J., Ryan, R. P., Marshall, T. P. & Hirschfield, G. M. The epidemiology of UK autoimmune liver disease varies with geographic latitude. Clin. Gastroenterol. Hepatol. 19, 2587–2596 (2021).
Hahn, J. W. et al. Global incidence and prevalence of autoimmune hepatitis, 1970-2022: a systematic review and meta-analysis. EClinicalMedicine 65, 102280 (2023).
Groribæk, L., Vilstrup, H. & Jepsen, P. Autoimmune hepatitis in Denmark: incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J. Hepatol. 60, 612–617 (2014).
Al-Chalabi, T., Underhill, J. A., Portmann, B. C., McFarlane, I. G. & Heneghan, M. A. Impact of gender on the long-term outcome and survival of patients with autoimmune hepatitis. J. Hepatol. 48, 140–147 (2008).
Donaldson, P. T. et al. Susceptibility to autoimmune chronic active hepatitis: human leukocyte antigens DR4 and A1-B8-DR3 are independent risk factors. Hepatology 13, 701–706 (1991).
Plagiannakos, C. P. et al. Treatment response and clinical event-free survival in autoimmune hepatitis: a Canadian multicentre cohort study. J. Hepatol. 81, 227–237 (2024).
Slooter, C. D. et al. Lack of complete biochemical response in autoimmune hepatitis leads to adverse outcome: first report of the IAIHG retrospective registry. Hepatology 79, 538–550 (2024).
Montano-Loza, A. J., Carpenter, H. A. & Czaja, A. J. Predictive factors for hepatocellular carcinoma in type 1 autoimmune hepatitis. Am. J. Gastroenterol. 103, 1944–1951 (2008).
Yan, L. J. et al. Sex and regional disparities in incidence of hepatocellular carcinoma in autoimmune hepatitis: a systematic review and meta-analysis. Hepatol. Int. 15, 1413–1420 (2021).
Colapietro, F. et al. Incidence and predictors of hepatocellular carcinoma in patients with autoimmune hepatitis. J. Hepatol. 80, 53–61 (2024).
Terrabuio, D. R. B., Abrantes-Lemos, C. P., Carrilho, F. J. & Cançado, E. L. R. Follow-up of pregnant women with autoimmune hepatitis. J. Clin. Gastroenterol. 43, 350–356 (2009).
Trivedi, P. J. & Hirschfield, G. M. Recent advances in clinical practice: epidemiology of autoimmune liver diseases. Gut 70, 1989–2003 (2021).
McNally, R. J., James, P. W., Ducker, S., Norman, P. D. & James, O. F. No rise in incidence but geographical heterogeneity in the occurrence of primary biliary cirrhosis in North East England. Am. J. Epidemiol. 179, 492–498 (2014).
Lleo, A. et al. Evolving trends in female to male incidence and male mortality of primary biliary cholangitis. Sci. Rep. 6, 25936 (2016).
Lv, T. et al. Regional variation and temporal trend of primary biliary cholangitis epidemiology: a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 36, 1423–1434 (2021).
Hirschfield, G. M. et al. EASL Clinical Practice Guidelines: the diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 67, 145–172 (2017).
Smyk, D. S. et al. Sex differences associated with primary biliary cirrhosis. Clin. Dev. Immunol. 2012, 610504 (2012).
Carbone, M. et al. Sex and age are determinants of the clinical phenotype of primary biliary cirrhosis and response to ursodeoxycholic acid. Gastroenterology 144, 560–569.e7 (2013).
Chen, S. et al. Prognosis of 732 ursodeoxycholic acid-treated patients with primary biliary cholangitis: a single center follow-up study from China. J. Gastroenterol. Hepatol. 34, 1236–1241 (2019).
Cheung, A. C. et al. Effects of age and sex of response to ursodeoxycholic acid and transplant-free survival in patients with primary biliary cholangitis. Clin. Gastroenterol. Hepatol. 17, 2076–2084.e2 (2019).
Trivedi, P. J. et al. Stratification of hepatocellular carcinoma risk in primary biliary cirrhosis: a multicentre international study. Gut 65, 321–329 (2016).
Rong, G. H. et al. Incidence and risk factors for hepatocellular carcinoma in primary biliary cirrhosis. Clin. Rev. Allerg. Immunol. 48, 132–141 (2015).
Roberts, S. B. et al. Ethnicity, disease severity, and survival in Canadian patients with primary biliary cholangitis. Hepatology 76, 303–316 (2022).
Boonstra, K. et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology 58, 2045–2055 (2013).
Trivedi, P. J. et al. Effects of primary sclerosing cholangitis on risks of cancer and death in people with inflammatory bowel disease, based on sex, race, and age. Gastroenterology 159, 915–928 (2020).
European Association for the Study of the Liver EASL Clinical Practice Guidelines on sclerosing cholangitis. J. Hepatol. 77, 761–806 (2022).
Karlsen, T. H., Folseraas, T., Thorburn, D. & Vesterhus, M. Primary sclerosing cholangitis – a comprehensive review. J. Hepatol. 67, 1298–1323 (2017).
Lunder, A. K. et al. Prevalence of sclerosing cholangitis detected by magnetic resonance cholangiography in patients with long-term inflammatory bowel disease. Gastroenterology 151, 660–669.e4 (2016).
Weismuller, T. J. et al. Patient age, sex, and inflammatory bowel disease phenotype associate with course of primary sclerosing cholangitis. Gastroenterology 152, 1975–1984.e8 (2017).
Toy, E., Balasubramanian, S., Selmi, C., Li, C. S. & Bowlus, C. L. The prevalence, incidence and natural history of primary sclerosing cholangitis in an ethnically diverse population. BMC Gastroenterol. 11, 83 (2011).
Barberio, B. et al. Prevalence of primary sclerosing cholangitis in patients with inflammatory bowel disease: a systematic review and meta-analysis. Gastroenterology 161, 1865–1877 (2021).
Rupp, C. et al. Impact of age at diagnosis on disease progression in patients with primary sclerosing cholangitis. United Eur. Gastroenterol. J. 6, 255–262 (2018).
Sinha, T. et al. Analysis of 1135 gut metagenomes identifies sex-specific resistome profiles. Gut Microbes 10, 358–366 (2019).
Hsu, C. L. & Schnabl, B. The gut-liver axis and gut microbiota in health and liver disease. Nat. Rev. Microbiol. 21, 719–733 (2023).
Saboo, K. et al. Sex is associated with differences in gut microbial composition and function in hepatic encephalopathy. J. Hepatol. 74, 80–88 (2021).
Markle, J. G. et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339, 1084–1088 (2013).
Collden, H. et al. The gut microbiota is a major regulator of androgen metabolism in intestinal contents. Am. J. Physiol. Endocrinol. Metab. 317, E1182–E1192 (2019).
Cross, T. L., Kasahara, K. & Rey, F. E. Sexual dimorphism of cardiometabolic dysfunction: gut microbiome in the play? Mol. Metab. 15, 70–81 (2018).
Collins, S. L., Stine, J. G., Bisanz, J. E., Okafor, C. D. & Patterson, A. D. Bile acids and the gut microbiota: metabolic interactions and impacts on disease. Nat. Rev. Microbiol. 21, 236–247 (2023).
Bennion, L. J. et al. Sex differences in the size of bile acid pools. Metabolism 27, 961–969 (1978).
Xie, G. et al. Profiling of serum bile acids in a healthy Chinese population using UPLC-MS/MS. J. Proteome Res. 14, 850–859 (2015).
Baars, A. et al. Sex differences in lipid metabolism are affected by presence of the gut microbiota. Sci. Rep. 8, 13426 (2018).
Xie, G. et al. Sex-dependent effects on gut microbiota regulate hepatic carcinogenic outcomes. Sci. Rep. 7, 45232 (2017).
Wankhade, U. D. et al. Maternal high-fat diet programs offspring liver steatosis in a sexually dimorphic manner in association with changes in gut microbial ecology in mice. Sci. Rep. 8, 16502 (2018).
Paik, J. M. et al. The burden of nonalcoholic fatty liver disease (NAFLD) is rapidly growing in every region of the world from 1990 to 2019. Hepatol. Commun. 7, e0251 (2023).
Zanetto, A. et al. New indications for liver transplantation. J. Clin. Med. 10, 3867 (2021).
Bjorkholm, B. et al. Intestinal microbiota regulate xenobiotic metabolism in the liver. PLoS ONE 4, e6958 (2009).
Chen, P. et al. Microbiota protects mice against acute alcohol-induced liver injury. Alcohol. Clin. Exp. Res. 39, 2313–2323 (2015).
Mazagova, M. et al. Commensal microbiota is hepatoprotective and prevents liver fibrosis in mice. FASEB J. 29, 1043–1055 (2015).
Weger, B. D. et al. The mouse microbiome is required for sex-specific diurnal rhythms of gene expression and metabolism. Cell Metab. 29, 362–382.e8 (2019).
Jourova, L. et al. Gut microbiome alters the activity of liver cytochromes p450 in mice with sex-dependent differences. Front. Pharmacol. 11, 01303 (2020).
Fu, C. et al. Sex different effect of antibiotic and probiotic treatment on intestinal microbiota composition in chemically induced liver injury rats. Genomics 115, 110647 (2023).
Cirulli, E. T. et al. A missense variant in PTPN22 is a risk factor for drug-induced liver injury. Gastroenterology 156, 1707–1716.e2 (2019).
Daly, A. K. Genetics of drug-induced liver injury: current knowledge and future prospects. Clin. Transl. Sci. 16, 37–42 (2023).
Ellinghaus, D. How genetic risk contributes to autoimmune liver disease. Semin. Immunopathol. 44, 397–410 (2022).
Eslam, M., Valenti, L. & Romeo, S. Genetics and epigenetics of NAFLD and NASH: clinical impact. J. Hepatol. 68, 268–279 (2018).
Karlsen, T. H., Lammert, F. & Thompson, R. J. Genetics of liver disease: from pathophysiology to clinical practice. J. Hepatol. 62, S6–S14 (2015).
Trepo, E. & Valenti, L. Update on NAFLD genetics: from new variants to the clinic. J. Hepatol. 72, 1196–1209 (2020).
Gerussi, A., Cristoferi, L., Carbone, M., Asselta, R. & Invernizzi, P. The immunobiology of female predominance in primary biliary cholangitis. J. Autoimmun. 95, 124–132 (2018).
Hirschfield, G. M., Karlsen, T. H., Lindor, K. D. & Adams, D. H. Primary sclerosing cholangitis. Lancet 382, 1587–1599 (2013).
Cooper, K. M., Delk, M., Devuni, D. & Sarkar, M. Sex differences in chronic liver disease and benign liver lesions. JHEP Rep. 5, 100870 (2023).
Dixon, P. H. et al. GWAS meta-analysis of intrahepatic cholestasis of pregnancy implicates multiple hepatic genes and regulatory elements. Nat. Commun. 13, 4840 (2022).
Dixon, P. H. et al. A comprehensive analysis of common genetic variation around six candidate loci for intrahepatic cholestasis of pregnancy. Am. J. Gastroenterol. 109, 76–84 (2014).
Adams, P. C., Jeffrey, G. & Ryan, J. Haemochromatosis. Lancet 401, 1811–1821 (2023).
Feder, J. N. et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat. Genet. 13, 399–408 (1996).
Girelli, D. et al. Clinical and pathologic findings in hemochromatosis type 3 due to a novel mutation in transferrin receptor 2 gene. Gastroenterology 122, 1295–1302 (2002).
Kato, J. et al. A mutation, in the iron-responsive element of H ferritin mRNA, causing autosomal dominant iron overload. Am. J. Hum. Genet. 69, 191–197 (2001).
Montosi, G. et al. Autosomal-dominant hemochromatosis is associated with a mutation in the ferroportin (SLC11A3) gene. J. Clin. Invest. 108, 619–623 (2001).
Njajou, O. T. et al. A mutation in SLC11A3 is associated with autosomal dominant hemochromatosis. Nat. Genet. 28, 213–214 (2001).
Papanikolaou, G. et al. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat. Genet. 36, 77–82 (2004).
Roetto, A. et al. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat. Genet. 33, 21–22 (2003).
Risch, N. Haemochromatosis, HFE and genetic complexity. Nat. Genet. 17, 375–376 (1997).
Anderson, G. J. & Bardou-Jacquet, E. Revisiting hemochromatosis: genetic vs. phenotypic manifestations. Ann. Transl. Med. 9, 731 (2021).
Bell, S. et al. A genome-wide meta-analysis yields 46 new loci associating with biomarkers of iron homeostasis. Commun. Biol. 4, 156 (2021).
Abdellaoui, A., Yengo, L., Verweij, K. J. H. & Visscher, P. M. 15 years of GWAS discovery: realizing the promise. Am. J. Hum. Genet. 110, 179–194 (2023).
Crouch, D. J. M. & Bodmer, W. F. Polygenic inheritance, GWAS, polygenic risk scores, and the search for functional variants. Proc. Natl Acad. Sci. USA 117, 18924–18933 (2020).
Mathieson, I. The omnigenic model and polygenic prediction of complex traits. Am. J. Hum. Genet. 108, 1558–1563 (2021).
Wainschtein, P. et al. Assessing the contribution of rare variants to complex trait heritability from whole-genome sequence data. Nat. Genet. 54, 263–273 (2022).
Daly, A. K. et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat. Genet. 41, 816–819 (2009).
Lucena, M. I. et al. Susceptibility to amoxicillin-clavulanate-induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology 141, 338–347 (2011).
Nicoletti, P. et al. Association of liver injury from specific drugs, or groups of drugs, with polymorphisms in HLA and other genes in a genome-wide association study. Gastroenterology 152, 1078–1089 (2017).
Singer, J. B. et al. A genome-wide study identifies HLA alleles associated with lumiracoxib-related liver injury. Nat. Genet. 42, 711–714 (2010).
Chen, Y. et al. Genome-wide association meta-analysis identifies 17 loci associated with nonalcoholic fatty liver disease. Nat. Genet. 55, 1640–1650 (2023).
Romeo, S. et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 40, 1461–1465 (2008).
Anstee, Q. M. et al. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically characterised cohort. J. Hepatol. 73, 505–515 (2020).
Valenti, L., Dongiovanni, P., Ginanni Corradini, S., Burza, M. A. & Romeo, S. PNPLA3 I148M variant and hepatocellular carcinoma: a common genetic variant for a rare disease. Dig. Liver Dis. 45, 619–624 (2013).
Chung, B. K. & Karlsen, T. H. Genetic discoveries highlight environmental factors as key drivers of liver disease. Dig. Dis. 35, 323–333 (2017).
Choi, S. W., Mak, T. S. & O’Reilly, P. F. Tutorial: a guide to performing polygenic risk score analyses. Nat. Protoc. 15, 2759–2772 (2020).
Ji, S. G. et al. Genome-wide association study of primary sclerosing cholangitis identifies new risk loci and quantifies the genetic relationship with inflammatory bowel disease. Nat. Genet. 49, 269–273 (2017).
Gerussi, A. et al. Improving predictive accuracy in primary biliary cholangitis: a new genetic risk score. Liver Int. 44, 1952–1960 (2024).
Khera, A. V. et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet. 50, 1219–1224 (2018).
Invernizzi, P. et al. Frequency of monosomy X in women with primary biliary cirrhosis. Lancet 363, 533–535 (2004).
Lleo, A. et al. Y chromosome loss in male patients with primary biliary cirrhosis. J. Autoimmun. 41, 87–91 (2013).
Keur, N., Ricano-Ponce, I., Kumar, V. & Matzaraki, V. A systematic review of analytical methods used in genetic association analysis of the X-chromosome. Brief. Bioinform 23, bbac287 (2022).
Asselta, R. et al. X chromosome contribution to the genetic architecture of primary biliary cholangitis. Gastroenterology 160, 2483–2495.e26 (2021).
Sumida, T. S., Cheru, N. T. & Hafler, D. A. The regulation and differentiation of regulatory T cells and their dysfunction in autoimmune diseases. Nat. Rev. Immunol. 24, 503–517 (2024).
Iwakiri, Y. & Trebicka, J. Portal hypertension in cirrhosis: pathophysiological mechanisms and therapy. JHEP Rep. 3, 100316 (2021).
Sandahl, T. D. et al. The macrophage activation marker sCD163 combined with markers of the Enhanced Liver Fibrosis (ELF) score predicts clinically significant portal hypertension in patients with cirrhosis. Aliment. Pharmacol. Ther. 43, 1222–1231 (2016).
Zhang, B., Ji, L. H., Zhang, C. G., Zhao, G. & Wu, Z. Y. Gender differences in vascular reactivity of mesenteric arterioles in portal hypertensive and non-portal hypertensive rats. World J. Gastroenterol. 25, 5953–5960 (2019).
Robert, R., Chagneau-Derrode, C., Carretier, M., Mauco, G. & Silvain, C. Gender differences in vascular reactivity of aortas from rats with and without portal hypertension. J. Gastroenterol. Hepatol. 20, 890–894 (2005).
Wiest, R. & Groszmann, R. J. The paradox of nitric oxide in cirrhosis and portal hypertension: too much, not enough. Hepatology 35, 478–491 (2002).
Kajita, M. et al. iNOS expression in vascular resident macrophages contributes to circulatory dysfunction of splanchnic vascular smooth muscle contractions in portal hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 300, H1021–H1031 (2011).
Park, E. M. et al. Inducible nitric oxide synthase contributes to gender differences in ischemic brain injury. J. Cereb. Blood Flow. Metab. 26, 392–401 (2006).
Sacerdoti, D. et al. Arachidonic acid metabolites and endothelial dysfunction of portal hypertension. Prostaglandins Other Lipid Mediat. 120, 80–90 (2015).
Graupera, M. et al. Cyclooxygenase-derived products modulate the increased intrahepatic resistance of cirrhotic rat livers. Hepatology 37, 172–181 (2003).
Hou, M. C. et al. Enhanced cyclooxygenase-1 expression within the superior mesenteric artery of portal hypertensive rats: role in the hyperdynamic circulation. Hepatology 27, 20–27 (1998).
Wu, Y., Burns, R. C. & Sitzmann, J. V. Effects of nitric oxide and cyclooxygenase inhibition on splanchnic hemodynamics in portal hypertension. Hepatology 18, 1416–1421 (1993).
Reiberger, T. et al. Carvedilol for primary prophylaxis of variceal bleeding in cirrhotic patients with haemodynamic non-response to propranolol. Gut 62, 1634–1641 (2013).
Jachs, M. et al. Carvedilol achieves higher hemodynamic response and lower rebleeding rates than propranolol in secondary prophylaxis. Clin. Gastroenterol. Hepatol. 21, 2318–2326.e7 (2023).
Villanueva, C. et al. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 393, 1597–1608 (2019).
Villanueva, C. et al. Carvedilol reduces the risk of decompensation and mortality in patients with compensated cirrhosis in a competing-risk meta-analysis. J. Hepatol. 77, 1014–1025 (2022).
Thalheimer, U., Bosch, J. & Burroughs, A. K. How to prevent varices from bleeding: shades of grey – the case for nonselective β blockers. Gastroenterology 133, 2029–2036 (2007).
Reiberger, T. et al. Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. J. Hepatol. 58, 911–921 (2013).
Jachs, M. et al. Amelioration of systemic inflammation in advanced chronic liver disease upon beta-blocker therapy translates into improved clinical outcomes. Gut 70, 1758–1767 (2021).
Walle, T., Walle, U. K., Cowart, T. D. & Conradi, E. C. Pathway-selective sex differences in the metabolic clearance of propranolol in human subjects. Clin. Pharmacol. Ther. 46, 257–263 (1989).
Burza, M. A., Marschall, H. U., Napoleone, L. & Molinaro, A. The 35-year odyssey of beta blockers in cirrhosis: any gender difference in sight? Pharmacol. Res. 119, 20–26 (2017).
Mortensen, C., Andersen, O., Krag, A., Bendtsen, F. & Moller, S. High-sensitivity C-reactive protein levels predict survival and are related to haemodynamics in alcoholic cirrhosis. Eur. J. Gastroenterol. Hepatol. 24, 619–626 (2012).
Bernardi, M., Moreau, R., Angeli, P., Schnabl, B. & Arroyo, V. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J. Hepatol. 63, 1272–1284 (2015).
Zanetto, A. et al. Increased platelet aggregation in patients with decompensated cirrhosis indicates higher risk of further decompensation and death. J. Hepatol. 77, 660–669 (2022).
D’Amico, G. et al. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment. Pharmacol. Ther. 39, 1180–1193 (2014).
Deltenre, P., Zanetto, A., Saltini, D., Moreno, C. & Schepis, F. The role of transjugular intrahepatic portosystemic shunt in patients with cirrhosis and ascites: recent evolution and open questions. Hepatology 77, 640–658 (2023).
Ripoll, C. et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 133, 481–488 (2007).
Simbrunner, B. et al. Bacterial translocation occurs early in cirrhosis and triggers a selective inflammatory response. Hepatol. Int. 17, 1045–1056 (2023).
Austin, A. S., Mahida, Y. R., Clarke, D., Ryder, S. D. & Freeman, J. G. A pilot study to investigate the use of oxpentifylline (pentoxifylline) and thalidomide in portal hypertension secondary to alcoholic cirrhosis. Aliment. Pharmacol. Ther. 19, 79–88 (2004).
Caraceni, P., Abraldes, J. G., Gines, P., Newsome, P. N. & Sarin, S. K. The search for disease-modifying agents in decompensated cirrhosis: from drug repurposing to drug discovery. J. Hepatol. 75, S118–S134 (2021).
Jepsen, P. & Younossi, Z. M. The global burden of cirrhosis: a review of disability-adjusted life-years lost and unmet needs. J. Hepatol. 75, S3–S13 (2021).
Cherubini, A., Della Torre, S., Pelusi, S. & Valenti, L. Sexual dimorphism of metabolic dysfunction-associated steatotic liver disease. Trends Mol. Med., https://doi.org/10.1016/j.molmed.2024.05.013 (2024).
Hofer, B. S. et al. Acute hemodynamic response to propranolol predicts bleeding and nonbleeding decompensation in patients with cirrhosis. Hepatol. Commun. 6, 2569–2580 (2022).
Sanyal, A. J. et al. The natural history of advanced fibrosis due to nonalcoholic steatohepatitis: data from the simtuzumab trials. Hepatology 70, 1913–1927 (2019).
Garcia-Tsao, G. et al. Randomized placebo-controlled trial of emricasan for non-alcoholic steatohepatitis-related cirrhosis with severe portal hypertension. J. Hepatol. 72, 885–895 (2020).
Reiberger, T. et al. The rationale and study design of two phase II trials examining the effects of BI 685,509, a soluble guanylyl cyclase activator, on clinically significant portal hypertension in patients with compensated cirrhosis. Trials 24, 293 (2023).
Harrison, S. A. et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N. Engl. J. Med. 390, 497–509 (2024).
Burghart, L. et al. Distinct prognostic value of different portal hypertension-associated features in patients with primary biliary cholangitis. J. Gastroenterol. 57, 99–110 (2022).
Geer, E. B. & Shen, W. Gender differences in insulin resistance, body composition, and energy balance. Gend. Med. 6, 60–75 (2009).
Bredella, M. A. Sex differences in body composition. Adv. Exp. Med. Biol. 1043, 9–27 (2017).
Fain, J. N., Madan, A. K., Hiler, M. L., Cheema, P. & Bahouth, S. W. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 145, 2273–2282 (2004).
Heymsfield, S. B. & Wadden, T. A. Mechanisms, pathophysiology, and management of obesity. N. Engl. J. Med. 376, 254–266 (2017).
Ebadi, M. et al. Review article: prognostic significance of body composition abnormalities in patients with cirrhosis. Aliment. Pharmacol. Ther. 52, 600–618 (2020).
Mitsiopoulos, N. et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J. Appl. Physiol. 85, 115–122 (1998).
Kim, G., Kang, S. H., Kim, M. Y. & Baik, S. K. Prognostic value of sarcopenia in patients with liver cirrhosis: a systematic review and meta-analysis. PLoS ONE 12, e0186990 (2017).
van Vugt, J. L. et al. Systematic review and meta-analysis of the impact of computed tomography-assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am. J. Transpl. 16, 2277–2292 (2016).
Carey, E. J. et al. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl. 23, 625–633 (2017).
Carey, E. J. et al. A North American expert opinion statement on sarcopenia in liver transplantation. Hepatology 70, 1816–1829 (2019).
Nishikawa, H. et al. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 46, 951–963 (2016).
van Vugt, J. L. A. et al. Low skeletal muscle mass is associated with increased hospital costs in patients with cirrhosis listed for liver transplantation – a retrospective study. Transpl. Int. 31, 165–174 (2018).
Montano-Loza, A. J. et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl. 20, 640–648 (2014).
Ando, Y. et al. Sarcopenia impairs health-related quality of life in cirrhotic patients. Eur. J. Gastroenterol. Hepatol. 31, 1550–1556 (2019).
Kaido, T. et al. Impact of sarcopenia on survival in patients undergoing living donor liver transplantation. Am. J. Transpl. 13, 1549–1556 (2013).
Montano-Loza, A. J. et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 10, 166–173.e1 (2012).
Montano-Loza, A. J. et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J. Cachexia, Sarcopenia Muscle 7, 126–135 (2016).
Baracos, V. E. & Arribas, L. Sarcopenic obesity: hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann. Oncol. 29, ii1–ii9 (2018).
Eslamparast, T., Montano-Loza, A. J., Raman, M. & Tandon, P. Sarcopenic obesity in cirrhosis – the confluence of 2 prognostic titans. Liver Int. 38, 1706–1717 (2018).
Pou, K. M. et al. Patterns of abdominal fat distribution: the Framingham Heart Study. Diabetes Care 32, 481–485 (2009).
Kim, D. et al. Body fat distribution and risk of incident and regressed nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 14, 132–138.e134 (2016).
Koo, B. K. et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J. Hepatol. 66, 123–131 (2017).
Pan, X. Y. et al. Low skeletal muscle mass is associated with more severe histological features of non-alcoholic fatty liver disease in male. Hepatol. Int. 16, 1085–1093 (2022).
Cao, Y. T. et al. Sex- and reproductive status-specific relationships between body composition and non-alcoholic fatty liver disease. BMC Gastroenterol. 23, 364 (2023).
Montano-Loza, A. J. et al. Visceral adiposity increases risk for hepatocellular carcinoma in male patients with cirrhosis and recurrence after liver transplant. Hepatology 67, 914–923 (2018).
Terjimanian, M. N. et al. Abdominal adiposity, body composition and survival after liver transplantation. Clin. Transplant. 30, 289–294 (2016).
Ha, N. B. et al. Sarcopenic visceral obesity is associated with increased post-liver transplant mortality in acutely ill patients with cirrhosis. Am. J. Transpl. 22, 2195–2202 (2022).
Saponaro, C., Gaggini, M., Carli, F. & Gastaldelli, A. The subtle balance between lipolysis and lipogenesis: a critical point in metabolic homeostasis. Nutrients 7, 9453–9474 (2015).
Johannsen, D. L. et al. Effect of 8 weeks of overfeeding on ectopic fat deposition and insulin sensitivity: testing the “adipose tissue expandability” hypothesis. Diabetes Care 37, 2789–2797 (2014).
Ebadi, M. et al. Low subcutaneous adiposity associates with higher mortality in female patients with cirrhosis. J. Hepatol. 69, 608–616 (2018).
Rodrigues, S. G., Brabandt, B., Stirnimann, G., Maurer, M. H. & Berzigotti, A. Adipopenia correlates with higher portal pressure in patients with cirrhosis. Liver Int. 39, 1672–1681 (2019).
Labeur, T. A. et al. Body composition is an independent predictor of outcome in patients with hepatocellular carcinoma treated with sorafenib. Liver Cancer 8, 255–270 (2018).
Alberino, F. et al. Nutrition and survival in patients with liver cirrhosis. Nutrition 17, 445–450 (2001).
Quiroz-Aldave, J. E. et al. From liver to hormones: the endocrine consequences of cirrhosis. World J. Gastroenterol. 30, 1073–1095 (2024).
Foresta, C., Schipilliti, M., Ciarleglio, F. A., Lenzi, A. & D’Amico, D. Male hypogonadism in cirrhosis and after liver transplantation. J. Endocrinol. Invest. 31, 470–478 (2008).
Lundsgaard, A. M. & Kiens, B. Gender differences in skeletal muscle substrate metabolism – molecular mechanisms and insulin sensitivity. Front. Endocrinol. 5, 195 (2014).
Harimoto, N. et al. Sarcopenia is a poor prognostic factor following hepatic resection in patients aged 70 years and older with hepatocellular carcinoma. Hepatol. Res. 46, 1247–1255 (2016).
Acknowledgements
The authors thank NIH center P30 DK120515 for support to B.S. and the German Research Foundation (DFG Ta434/8-1, SFB/TRR 296 and CRC1382, project ID 403224013) for support to F.T.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
P.B. has received lecture and consulting fees from Biotest, Chiesi Farmaceutici and Sandoz. B.S. has been consulting for Ambys Medicines, Ferring Research Institute, Gelesis, HOST Therabiomics, Intercept Pharmaceuticals, Mabwell Therapeutics, Patara Pharmaceuticals, Surrozen and Takeda. B.S. is founder of Nterica Bio. UC San Diego has filed several patents with B.S. as inventor related to this work. B.S.’s institution, UC San Diego, has received research support from Axial Biotherapeutics, BiomX, ChromoLogic, CymaBay Therapeutics, Intercept Pharmaceuticals, NGM Biopharmaceuticals, Prodigy Biotech and Synlogic Operating Company. T.R. received grant support from Abbvie, Boehringer Ingelheim, Gilead, Intercept/Advanz Pharma, MSD, Myr Pharmaceuticals, Philips Healthcare, Pliant, Siemens and W. L. Gore & Associates; speaking honoraria from Abbvie, Gilead, Intercept/Advanz Pharma, Roche, MSD, W. L. Gore & Associates; consulting/advisory board fee from Abbvie, Astra Zeneca, Bayer, Boehringer Ingelheim, Gilead, Intercept/Advanz Pharma, MSD, Resolution Therapeutics, Siemens; and travel support from Abbvie, Boehringer Ingelheim, Dr. Falk Pharma, Gilead and Roche. T.H.K. received speaker fees from Gilead and did consulting for Falk Pharma, Albireo, MSD and Boeringer Ingelheim – consulting. F.T.’s laboratory has received research funding from Gilead, AstraZeneca, and MSD (funding to the institution). F.T. has received honoraria for consulting or lectures from AstraZeneca, Gilead, AbbVie, BMS, Boehringer, Madrigal, Intercept, Falk, Inventiva, MSD, GSK, Orphalan, Merz, Pfizer, Alnylam, CSL Behring, Novo Nordisk, Sanofi, and Novartis. A.Z., A.J.M.-L. and R.A. have nothing to disclose.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Joost Drenth and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
GnomAD: https://gnomad.broadinstitute.org/
NHGRI-EBI GWAS: https://www.ebi.ac.uk/gwas/
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Burra, P., Zanetto, A., Schnabl, B. et al. Hepatic immune regulation and sex disparities. Nat Rev Gastroenterol Hepatol (2024). https://doi.org/10.1038/s41575-024-00974-5
Accepted:
Published:
DOI: https://doi.org/10.1038/s41575-024-00974-5