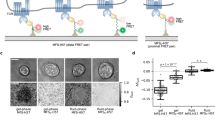
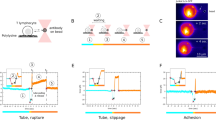
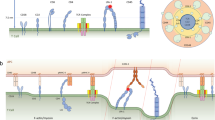
Abstract
The T cell receptor (TCR) is thought to be a mechanosensor, meaning that it transmits mechanical force to its antigen and leverages the force to amplify the specificity and magnitude of TCR signalling. Although a variety of molecular probes have been proposed to quantify TCR mechanics, these probes are immobilized on hard substrates, and thus fail to reveal fluid TCR–antigen interactions in the physiological context of cell membranes. Here we developed DNA origami tension sensors (DOTS) which bear force sensors on a DNA origami breadboard and allow mapping of TCR mechanotransduction at dynamic intermembrane junctions. We quantified the mechanical forces at fluid TCR–antigen bonds and observed their dependence on cell state, antigen mobility, antigen potency, antigen height and F-actin activity. The programmability of DOTS allows us to tether these to microparticles to mechanically screen antigens in high throughput using flow cytometry. Additionally, DOTS were anchored onto live B cells, allowing quantification of TCR mechanics at immune cell–cell junctions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the Article and its Supplementary Information. Raw imaging data have been deposited into the Dataverse repository and can be accessed via https://doi.org/10.15139/S3/BTO70R. Any other data are available upon request from the corresponding author. Source data are provided with this paper.
Code availability
The code used for oxDNA modelling and subsequent analysis is available online via https://github.com/SalaitaLab/DNA_Origami_Tension_Sensors.git
References
Huang, J. et al. A single peptide-major histocompatibility complex ligand triggers digital cytokine secretion in CD4(+) T cells. Immunity 39, 846–857 (2013).
Sasmal, D. K. et al. TCR–pMHC bond conformation controls TCR ligand discrimination. Cell Mol. Immunol. 17, 203–217 (2020).
Kim, S. T. et al. The αβ T cell receptor is an anisotropic mechanosensor. J. Biol. Chem. 284, 31028–31037 (2009).
Lee, M. S. et al. A mechanical switch couples T cell receptor triggering to the cytoplasmic juxtamembrane regions of CD3ζζ. Immunity 43, 227–239 (2015).
Liu, B. et al. Accumulation of dynamic catch bonds between TCR and agonist peptide-MHC triggers T cell signaling. Cell 157, 357–368 (2014).
Feng, Y. et al. Mechanosensing drives acuity of αβ T cell recognition. Proc. Natl Acad. Sci. USA 114, E8204–E8213 (2017).
Zhang, Y. et al. DNA-based digital tension probes reveal integrin forces during early cell adhesion. Nat. Commun. 5, 5167 (2014).
Stabley, D. R. et al. Visualizing mechanical tension across membrane receptors with a fluorescent sensor. Nat. Methods 9, 64–67 (2011).
Chang, A. C. et al. Single molecule force measurements in living cells reveal a minimally tensioned integrin state. ACS Nano 10, 10745–10752 (2016).
Hong, J. et al. A TCR mechanotransduction signaling loop induces negative selection in the thymus. Nat. Immunol. 19, 1379–1390 (2018).
Liu, Y. et al. DNA-based nanoparticle tension sensors reveal that T cell receptors transmit defined pN forces to their antigens for enhanced fidelity. Proc. Natl Acad. Sci. USA 113, 5610–5615 (2016).
Wang, M. S. et al. Mechanically active integrins target lytic secretion at the immune synapse to facilitate cellular cytotoxicity. Nat. Commun. 13, 3222 (2022).
Ma, V. P. et al. Ratiometric tension probes for mapping receptor forces and clustering at intermembrane junctions. Nano Lett. 16, 4552–4559 (2016).
Gohring, J. et al. Temporal analysis of T cell receptor-imposed forces via quantitative single molecule FRET measurements. Nat. Commun. 12, 2502 (2021).
Nowosad, C. R. et al. Germinal center B cells recognize antigen through a specialized immune synapse architecture. Nat. Immunol. 17, 870–877 (2016).
Sage, P. T. et al. Antigen recognition is facilitated by invadosome-like protrusions formed by memory/effector T cells. J. Immunol. 188, 3686–3699 (2012).
Aramesh, M. et al. Functionalized bead assay to measure three-dimensional traction forces during T cell activation. Nano Lett. 21, 507–514 (2021).
Wahl, A. et al. Biphasic mechanosensitivity of T cell receptor-mediated spreading of lymphocytes. Proc. Natl Acad. Sci. USA 116, 5908–5913 (2019).
Saitakis, M. et al. Different TCR-induced T lymphocyte responses are potentiated by stiffness with variable sensitivity. eLife 6, e23190 (2017).
Hellmeier, J. et al. DNA origami demonstrate the unique stimulatory power of single pMHCs as T cell antigens. Proc. Natl Acad. Sci. USA 118, e2016857118 (2021).
Dong, R. et al. DNA origami patterning of synthetic T cell receptors reveals spatial control of the sensitivity and kinetics of signal activation. Proc. Natl Acad. Sci. USA 118, e2109057118 (2021).
Fang, T. et al. Spatial regulation of T cell signaling by programmed death-ligand 1 on wireframe DNA origami flat sheets. ACS Nano 15, 3441–3452 (2021).
Damjanovich, S. et al. Distribution and mobility of murine histocompatibility H-2Kk antigen in the cytoplasmic membrane. Proc. Natl Acad. Sci. USA 80, 5985–5989 (1983).
Glazier, R. et al. DNA mechanotechnology reveals that integrin receptors apply pN forces in podosomes on fluid substrates. Nat. Commun. 10, 4507 (2019).
Monks, C. R. F. et al. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395, 82–86 (1998).
James, J. R. et al. Biophysical mechanism of T cell receptor triggering in a reconstituted system. Nature 487, 64–69 (2012).
Jenkins, E. et al. Antigen discrimination by T cells relies on size-constrained microvillar contact. Nat. Commun. 14, 1611 (2023).
Cai, E. et al. Visualizing dynamic microvillar search and stabilization during ligand detection by T cells. Science 356, eaal3118 (2017).
Hatch, K. et al. Demonstration that the shear force required to separate short double-stranded DNA does not increase significantly with sequence length for sequences longer than 25 base pairs. Phys. Rev. E 78, 011920 (2008).
Woodside, M. T. et al. Nanomechanical measurements of the sequence-dependent folding landscapes of single nucleic acid hairpins. Proc. Natl Acad. Sci. USA 103, 6190–6195 (2006).
Ma, R. et al. DNA probes that store mechanical information reveal transient piconewton forces applied by T cells. Proc. Natl Acad. Sci. USA 116, 16949–16954 (2019).
Glazier, R. et al. Spectroscopic analysis of a library of DNA tension probes for mapping cellular forces at fluid interfaces. ACS Appl. Mater. Interfaces 13, 2145–2164 (2021).
Wang, X. et al. Defining single molecular forces required to activate integrin and notch signaling. Science 340, 991–994 (2013).
Whitton, J. L. et al. Functional avidity maturation of CD8+ T cells without selection of higher affinity TCR. Nat. Immunol. 2, 711–717 (2001).
Thauland, T. J. et al. Cytoskeletal adaptivity regulates T cell receptor signaling. Sci. Signal 10, eaah3737 (2017).
Fletcher, D. A. et al. Cell mechanics and the cytoskeleton. Nature 463, 485–492 (2010).
Borroto, A. et al. First-in-class inhibitor of the T cell receptor for the treatment of autoimmune diseases. Sci. Transl. Med. 8, 370ra184 (2016).
Barda-Saad, M. et al. Dynamic molecular interactions linking the T cell antigen receptor to the actin cytoskeleton. Nat. Immunol. 6, 80–89 (2005).
Su, X. et al. Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 352, 595–599 (2016).
Al-Aghbar, M. A. et al. The interplay between membrane topology and mechanical forces in regulating T cell receptor activity. Commun. Biol. 5, 40 (2022).
Allard, J. F. et al. Mechanical modulation of receptor–ligand interactions at cell–cell interfaces. Biophys. J. 102, 1265–1273 (2012).
Cespedes, P. F. et al. T cell trans-synaptic vesicles are distinct and carry greater effector content than constitutive extracellular vesicles. Nat. Commun. 13, 3460 (2022).
Olden, B. R. et al. Cell-templated silica microparticles with supported lipid bilayers as artificial antigen-presenting cells for T cell activation. Adv. Health. Mater. 8, e1801188 (2019).
Zhao, X. et al. Tuning T cell receptor sensitivity through catch bond engineering. Science 376, eabl5282 (2022).
Feng, Y. et al. A bead-based method for high-throughput mapping of the sequence- and force-dependence of T cell activation. Nat. Methods 19, 1295–1305 (2022).
Grakoui, A. et al. The immunological synapse—a molecular machine controlling T cell activation. Science 285, 221–227 (1999).
Kumari, S. et al. T cell antigen receptor activation and actin cytoskeleton remodeling. Biochim. Biophys. Acta 1838, 546–556 (2014).
Ge, Z. et al. Programming cell–cell communications with engineered cell origami clusters. J. Am. Chem. Soc. 142, 8800–8808 (2020).
Zhao, W. et al. Cell-surface sensors for real-time probing of cellular environments. Nat. Nanotechnol. 6, 524–531 (2011).
Liu, Y. et al. The effects of overhang placement and multivalency on cell labeling by DNA origami. Nanoscale 13, 6819–6828 (2021).
Akbari, E. et al. Engineering cell surface function with DNA origami. Adv. Mater. 29, 1703632 (2017).
Lei, K. et al. Cancer-cell stiffening via cholesterol depletion enhances adoptive T cell immunotherapy. Nat. Biomed. Eng. 5, 1411–1425 (2021).
Bashour, K. T. et al. CD28 and CD3 have complementary roles in T cell traction forces. Proc. Natl Acad. Sci. USA 111, 2241–2246 (2014).
Cai, H. et al. Full control of ligand positioning reveals spatial thresholds for T cell receptor triggering. Nat. Nanotechnol. 13, 610–617 (2018).
Deeg, J. et al. T cell activation is determined by the number of presented antigens. Nano Lett. 13, 5619–5626 (2013).
Amiri, S. et al. Intracellular tension sensor reveals mechanical anisotropy of the actin cytoskeleton. Nat. Commun. 14, 8011 (2023).
Galush, W. J. et al. Quantitative fluorescence microscopy using supported lipid bilayer standards. Biophys. J. 95, 2512–2519 (2008).
Acknowledgements
K.S. acknowledges financial support from NIH R01 AI172452 and R01 GM131099. Y.H. is a recipient of the National Cancer Institute Predoctoral to Postdoctoral Fellow Transition Award (F99CA274690). Y.D. is a recipient of an American Heart Association Postdoctoral Fellowship (23POST1028975). We thank the National Institutes of Health (NIH) Tetramer Facility at Emory University for providing the biotinylated pMHC monomers. We thank L. Finzi and D. Dunlap for granting us access to their AFM and providing valuable instructions. We thank J. Mancuso and H. Ogasawara for helping with the electrospray ionization mass spectrometry characterizations of DNA oligos. We thank A. Kellner for suggestions on the drug AX-024. This research project was supported in part by the Emory University Integrated Cellular Imaging Core. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the NIH.
Author information
Authors and Affiliations
Contributions
Y.H. and K.S. designed the research. Y.H. performed the experiments and analysed the data. J.R. helped purify T cells and conduct the intermolecular FRET experiments. Y.D. designed the DNA origami structures and helped make DNA origami. A.V. conducted computational modelling experiments to analyse the height and mechanical properties of DOTS. S.N. helped conduct the FLIM imaging and analysed the FLIM data. S.A.A. helped make DNA origami. Y.H. and K.S. wrote the manuscript, with all the authors providing inputs.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Li Tang, Byoung Choul Kim and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–24, Notes 1–5 and Tables 1–3.
Supplementary Video 1
Time lapse video showing origami exclusion from the cell spreading area. A raw 6-min time lapse showing DOTS fluorescence signal changes under T cells. In the video, three T cells landed on the DOTS SLB surface and excluded DOTS from the spreading area resulting in a dark signal. The remaining DOTS clustered and centralized to form cSMAC. Scale bar, 5 µm.
Supplementary Video 2
Single molecule experiments showing the spatiotemporal dynamics of DOTS in the immune synapse. A raw 5-min time lapse of naive T cells seeded on low-density DOTS SLB surface. Right shows the single molecule DOTS signal and left is the RICM channel showing the T cell spreading. Scale bar, 5 µm.
Supplementary Video 3
The dynamics of DNA hairpin on DOTS in the presence of force. The terminus of the DNA hairpin on DOTS was attached to a trap (stiffness - 0.2) which was effectively spring in oxDNA. Each of the eight anchor strands on the structure were also attached to a trap (stiffness - 0.1). A repulsion plane (stiffness - 2) was constructed just below the anchor strand traps to mimic a hard surface. The hairpin trap was moved at a loading rate of a 1.41 × 104 nm s−1 to simulate the stretching forces while the other traps were rigidly fixed in position. A snapshot of the structure’s configuration was captured every 2×106 steps (~30.3 ns) and a video was generated.
Supplementary Video 4
The distribution of F-actin and DOTS at the effector T cell immune synapse. Cell spreading (RICM channel), DOTS (red channel), LifeAct-GFP (green channel) were imaged, after 2 min of spreading, for a duration of 20 min. Scale bar, 5 µm.
Supplementary Video 5
3D view of DOTS and tension patterns at the SSLB–T cell interface. The video represents a 360-degree rotation of the SSLB engaging an OT-1 naive T cell. Tension signal (grey channel) and DOTS Cy3B signal (green channel) of the SSLB were imaged after 30 min incubation. Scale bar, 1 µm.
Supplementary Data
Source data for supplementary figures.
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 1
Unprocessed gel
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 4
Statistical Source Data.
Source Data Fig. 5
Statistical Source Data.
Source Data Fig. 6
Statistical Source Data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, Y., Rogers, J., Duan, Y. et al. Quantifying T cell receptor mechanics at membrane junctions using DNA origami tension sensors. Nat. Nanotechnol. (2024). https://doi.org/10.1038/s41565-024-01723-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41565-024-01723-0
This article is cited by
-
DNA origami force probes illuminate T cell receptor forces at the immune synapse
Nature Nanotechnology (2024)