Abstract
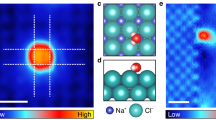
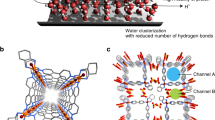
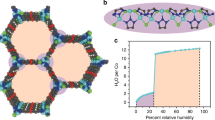
The permeability and selectivity of biological and artificial ion channels correlate with the specific hydration structure of single ions. However, fundamental understanding of the effect of ion–ion interaction remains elusive. Here, via non-contact atomic force microscopy measurements, we demonstrate that hydrated alkali metal cations (Na+ and K+) at charged surfaces could come into close contact with each other through partial dehydration and water rearrangement processes, forming one-dimensional chain structures. We prove that the interplay at the nanoscale between the water–ion and water–water interaction can lead to an effective ion–ion attraction overcoming the ionic Coulomb repulsion. The tendency for different ions to become closely packed follows the sequence K+ > Na+ > Li+, which is attributed to their different dehydration energies and charge densities. This work highlights the key role of water molecules in prompting close packing and concerted movement of ions at charged surfaces, which may provide new insights into the mechanism of ion transport under atomic confinement.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Source data are provided with this paper. All other data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Hummer, G., Rasaiah, J. C. & Noworyta, J. P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature 414, 188–190 (2001).
Tunuguntla, R. H. et al. Enhanced water permeability and tunable ion selectivity in subnanometer carbon nanotube porins. Science 357, 792–796 (2017).
Garaj, S. et al. Graphene as a subnanometre trans-electrode membrane. Nature 467, 190–193 (2010).
Joshi, R. K. et al. Precise and ultrafast molecular sieving through graphene oxide membranes. Science 343, 752–754 (2014).
Shen, J., Liu, G. P., Han, Y. & Jin, W. Q. Artificial channels for confined mass transport at the sub-nanometre scale. Nat. Rev. Mater. 6, 294–312 (2021).
Radha, B. et al. Molecular transport through capillaries made with atomic-scale precision. Nature 538, 222–225 (2016).
Suo, L. M. et al. ‘Water-in-salt’ electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 350, 938–943 (2015).
Chao, D. L. et al. Roadmap for advanced aqueous batteries: from design of materials to applications. Sci. Adv. 6, eaba4098 (2020).
Liu, T. C. et al. In situ quantification of interphasial chemistry in Li-ion battery. Nat. Nanotechnol. 14, 50–56 (2019).
Cheng, X. B., Zhang, R., Zhao, C. Z. & Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: a review. Chem. Rev. 117, 10403–10473 (2017).
Doyle, D. A. et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280, 69–77 (1998).
Payandeh, J., Scheuer, T., Zheng, N. & Catterall, W. A. The crystal structure of a voltage-gated sodium channel. Nature 475, 353–358 (2011).
Zhou, Y. F., Morais-Cabral, J. H., Kaufman, A. & MacKinnon, R. Chemistry of ion coordination and hydration revealed by a K+ channel–Fab complex at 2.0 Å resolution. Nature 414, 43–48 (2001).
Chen, L. et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 550, 415–418 (2017).
Schoch, R. B., Han, J. Y. & Renaud, P. Transport phenomena in nanofluidics. Rev. Mod. Phys. 80, 839–883 (2008).
Zhu, Z. P., Wang, D. Y., Tian, Y. & Jiang, L. Ion/molecule transportation in nanopores and nanochannels: from critical principles to diverse functions. J. Am. Chem. Soc. 141, 8658–8669 (2019).
Xue, Y. et al. Atomic-scale ion transistor with ultrahigh diffusivity. Science 372, 501–503 (2021).
Kopfer, D. A. et al. Ion permeation in K+ channels occurs by direct Coulomb knock-on. Science 346, 352–355 (2014).
Robin, P., Kavokine, N. & Bocquet, L. Modeling of emergent memory and voltage spiking in ionic transport through angstrom-scale slits. Science 373, 687–691 (2021).
Kopec, W. et al. Direct knock-on of desolvated ions governs strict ion selectivity in K+ channels. Nat. Chem. 10, 813–820 (2018).
Zheng, J. X. et al. Understanding thermodynamic and kinetic contributions in expanding the stability window of aqueous electrolytes. Chem 4, 2872–2882 (2018).
Giessibl, F. J. The qPlus sensor, a powerful core for the atomic force microscope. Rev. Sci. Instrum. 90, 011101 (2019).
Carrasco, J., Hodgson, A. & Michaelides, A. A molecular perspective of water at metal interfaces. Nat. Mater. 11, 667–674 (2012).
Gross, L., Mohn, F., Moll, N., Liljeroth, P. & Meyer, G. The chemical structure of a molecule resolved by atomic force microscopy. Science 325, 1110–1114 (2009).
Peng, J. B. et al. The effect of hydration number on the interfacial transport of sodium ions. Nature 557, 701–705 (2018).
Tian, Y. et al. Visualizing Eigen/Zundel cations and their interconversion in monolayer water on metal surfaces. Science 377, 315–319 (2022).
Peng, J. B. et al. Weakly perturbative imaging of interfacial water with submolecular resolution by atomic force microscopy. Nat. Commun. 9, 122 (2018).
Ma, R. Z. et al. Atomic imaging of the edge structure and growth of a two-dimensional hexagonal ice. Nature 577, 60–63 (2020).
Shiotari, A. & Sugimoto, Y. Ultrahigh-resolution imaging of water networks by atomic force microscopy. Nat. Commun. 8, 14313 (2017).
Mita, K. et al. Conductance selectivity of Na+ across the K+ channel via Na+ trapped in a tortuous trajectory. Proc. Natl Acad. Sci. USA 118, e2017168118 (2021).
Hribar, B., Southall, N. T., Vlachy, V. & Dill, K. A. How ions affect the structure of water. J. Am. Chem. Soc. 124, 12302–12311 (2002).
Chandler, D. Interfaces and the driving force of hydrophobic assembly. Nature 437, 640–647 (2005).
Meyer, E. E., Rosenberg, K. J. & Israelachvili, J. Recent progress in understanding hydrophobic interactions. Proc. Natl Acad. Sci. USA 103, 15739–15746 (2006).
Xu, X. Z. et al. Ultrafast epitaxial growth of metre-sized single-crystal graphene on industrial Cu foil. Sci. Bull. 62, 1074–1080 (2017).
Ohta, T., Bostwick, A., Seyller, T., Horn, K. & Rotenberg, E. Controlling the electronic structure of bilayer graphene. Science 313, 951–954 (2006).
Kresse, G. Ab-initio molecular-dynamics for liquid-metals. J. Non-Cryst. Solids 193, 222–229 (1995).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Klimes, J., Bowler, D. R. & Michaelides, A. Chemical accuracy for the van der Waals density functional. J. Phys. Condens. Matter 22, 022201 (2010).
Klimes, J., Bowler, D. R. & Michaelides, A. Van der Waals density functionals applied to solids. Phys. Rev. B 83, 195131 (2011).
Neugebauer, J. & Scheffler, M. Adsorbate–substrate and adsorbate–adsorbate interactions of Na and K adlayers on Al(111). Phys. Rev. B 46, 16067–16080 (1992).
Makov, G. & Payne, M. C. Periodic boundary-conditions in ab-initio calculations. Phys. Rev. B 51, 4014–4022 (1995).
Henkelman, G., Arnaldsson, A. & Jonsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 36, 354–360 (2006).
Hapala, P. et al. Mechanism of high-resolution STM/AFM imaging with functionalized tips. Phys. Rev. B 90, 085421 (2014).
Berendsen, H. J. C., Grigera, J. R. & Straatsma, T. P. The missing term in effective pair potentials. J. Phys. Chem. 91, 6269–6271 (1987).
Joung, I. S. & Cheatham, T. E. Molecular dynamics simulations of the dynamic and energetic properties of alkali and halide ions using water-model-specific ion parameters. J. Phys. Chem. B 113, 13279–13290 (2009).
Huang, Y. P. et al. SPONGE: a GPU-accelerated molecular dynamics package with enhanced sampling and AI-driven algorithms. Chin. J. Chem. 40, 160–168 (2022).
Zhang, Z. J. et al. A unified thermostat scheme for efficient configurational sampling for classical/quantum canonical ensembles via molecular dynamics. J. Chem. Phys. 147, 034109 (2017).
Miyamoto, S. & Kollman, P. A. SETTLE: an analytical version of the SHAKE and RATTLE algorithm for rigid water models. J. Comput. Chem. 13, 952–962 (1992).
Essmann, U. et al. A smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577–8593 (1995).
Acknowledgements
We thank L. Jiang, B. Song and F. Pan for inspiring discussions and the computational resources provided by the TianHe-1A, TianHe-2 supercomputer, High-Performance Computing Platform of Peking University. This work was supported by the National Key R&D Program under grants 2021YFA1400500 and 2017YFA0205003, the National Natural Science Foundation of China under grants 11888101, U22A20260, 21725302, 11935002, 21902013 and 92053202, the Strategic Priority Research Program of Chinese Academy of Sciences under grants XDB28000000 and XDB33000000, the China Postdoctoral Science Foundation under grants 2022M720003 and 2023T160011 and the Key R&D Program of Guangdong Province under grant 2020B010189001. Y.J. acknowledges the support by the New Cornerstone Science Foundation through the New Cornerstone Investigator Program and the XPLORER PRIZE.
Author information
Authors and Affiliations
Contributions
Y.J. and E.-G.W. designed and supervised the project. Y.T. and J.H. performed the STM/AFM measurements with R.M., S.Y. and D.G. Y.S., D.C., J.C. and L.-M.X. performed ab initio DFT calculations. Y.X., Y.H. and Y.Q.G. carried out the classical MD simulations. Y.S. carried out the theoretical simulations of the AFM images. M.Z.Z. and K.H.L. prepared the monolayer graphene/Cu(111) sample. Y.T., Y.S., Y.X., J.H., Y.H., J.C., C.S., Y.Q.G., E.-G.W. and Y.J. analysed the data. Y.J., Y.T., Y.S., Y.X., L.-M.X. and Y.Q.G. wrote the paper with input from all other authors. The paper reflects the contributions of all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Kai Xiao, Francois Peeters, Taesung Kim and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Sections 1–12, Figs. 1–21, Tables 1–11 and refs. 1–9.
Supplementary Video 1
Diffusion of 1D K+–water chains at 77 K on the basis of MD simulations. Three short K+–water chains were placed on the Au surface and diffused in a collective manner. The simulation time is 10 ns. Au, K, H and O atoms are denoted as yellow, purple, white and red spheres, respectively.
Supplementary Video 2
Collision of two short K+–water chains at 50 K on the basis of MD simulations. The initial equivalent kinetic energy of 347.2 meV was assigned to the two short K+–water chains at 10 ps, making them move towards collision. The initial kinetic energy serves to provide the initial motion direction, assisting in overcoming activation energy barriers for the combination between the two chains. The chain recombination dynamics is influenced by the initial conformation of the system as well as the thermal bath. The simulation time is 30 ps. The longer K+–water chain was formed at ~12 ps. Au, K, H and O atoms are denoted as yellow, purple, white and red spheres, respectively.
Supplementary Video 3
MD simulations of K+ and water molecules on Au surface at 100 K. This system contains 125 K+ ions and 375 water molecules. The simulation time is 1 ns with a time step of 1 fs. The lifetime of the chain structure is larger than the simulation duration time of 1 ns. Au, K, H and O atoms are denoted as yellow, purple, white and red spheres, respectively.
Supplementary Video 4
MD simulations of K+ and water molecules on Au surface at 300 K. This system contains 125 K+ ions and 375 water molecules. The simulation time is 1 ns with a time step of 1 fs. The lifetime of the chain structure is up to 15 ps. The chain structure can re-form during the simulation. Au, K, H and O atoms are denoted as yellow, purple, white and red spheres, respectively.
Supplementary Video 5
MD simulations of K+ and water molecules in 2D graphene channel at 300 K. This system contains 100 K+ ions and 300 water molecules. The simulation time is 1 ns with a time step of 1 fs. The top view is shown in the upper part of the video, with the graphene omitted. The bottom part shows the side view. The lifetime of the chain structure is up to 150 ps. C, K, H and O atoms are denoted as grey, purple, white and red spheres, respectively.
Source data
Source Data Fig. 1
Raw data for Fig. 1.
Source Data Fig. 2
Raw data for Fig. 2.
Source Data Fig. 3
Raw data for Fig. 3.
Source Data Fig. 4
Raw data for Fig. 4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tian, Y., Song, Y., Xia, Y. et al. Nanoscale one-dimensional close packing of interfacial alkali ions driven by water-mediated attraction. Nat. Nanotechnol. 19, 479–484 (2024). https://doi.org/10.1038/s41565-023-01550-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-023-01550-9