Abstract
Background
To determine the association of gestational age (GA) and day of life (DOL) with the circulating serum concentration of six brain injury-associated biomarkers in non-brain injured neonates born between 23 and 41 weeks’ GA.
Methods
In a multicenter prospective observational cohort study, serum CNS-insult, inflammatory and trophic proteins concentrations were measured daily in the first 7 DOL.
Results
Overall, 3232 serum samples were analyzed from 745 enrollees, median GA 32.3 weeks. BDNF increased 3.7% and IL-8 increased 8.9% each week of gestation. VEGF, IL-6, and IL-10 showed no relationship with GA. VEGF increased 10.8% and IL-8 18.9%, each DOL. IL-6 decreased by 15.8% each DOL. IL-10 decreased by 81.4% each DOL for DOL 0-3. BDNF did not change with DOL. Only 49.67% of samples had detectable GFAP and 33.15% had detectable NRGN. The odds of having detectable GFAP and NRGN increased by 53% and 11%, respectively, each week after 36 weeks’ GA. The odds of having detectable GFAP and NRGN decreased by 15% and 8%, respectively, each DOL.
Conclusions
BDNF and IL-8 serum concentrations vary with GA. VEGF and interleukin concentrations are dynamic in the first week of life, suggesting circulating levels should be adjusted for GA and DOL for clinically relevant assessment of brain injury.
Impact
-
Normative data of six brain injury-related biomarkers is being proposed.
-
When interpreting serum concentrations of brain injury biomarkers, it is key to adjust for gestational age at birth and day of life during the first week to correctly assess for clinical brain injury in neonates.
-
Variation in levels of some biomarkers may be related to gestational and postnatal age and not necessarily pathology.
Similar content being viewed by others
Introduction
Neonatal brain injury, with its two main subtypes of hypoxic-ischemic encephalopathy (HIE) in the late preterm and term infant and germinal matrix hemorrhage-intraventricular hemorrhage (GMH-IVH)/periventricular white matter injury (PVWMI) in the premature infant remains a focus of pediatric research due to its relatively high prevalence, the limited understanding and consensus on diagnostic criteria, the paucity of therapeutic options, and the serious long-term outcomes of neurological disabilities and death.1,2,3,4,5,6,7,8,9,10,11
Currently, clinical tools are limited to assess severity of brain injury, provide prognoses, and monitor intervention efficacy, promoting further research into multidimensional approaches to fill the gaps of knowledge.12,13,14 Within the multidimensional approach, blood-based biomarkers provide the potential for serial non-invasive measures to identify pathological processes based on abnormal molecular concentrations. Blood biomarkers have been extensively researched in adult traumatic brain injury and neurodegenerative diseases.15,16,17
Such studies have identified several potential biomarkers representing multiple facets of the injury continuum, including CNS-insult, inflammatory, and trophic proteins, that are now being investigated in the neonatal population. Glial fibrillary acidic protein (GFAP) and neurogranin (NRGN) are astrocyte or neuronal-specific proteins studied as potential brain-specific CNS-insult indicators. GFAP is an astrocyte cytoskeletal intermediate filament protein specific to central nervous system tissues and is released into the blood after brain injury.17 GFAP has been shown to be a significant diagnostic and potentially prognostic biomarker in infants with HIE and PVWMI.18,19,20,21,22,23 Neurogranin is a brain-specific protein kinase C substrate that plays a role in the excitatory neurons of the cortex and hippocampus and higher concentrations have been found in the circulation of patients with traumatic brain injury and neurodegeneration.15,24,25,26,27 In neonates with neonatal encephalopathy, NRGN, measured on DOL 0-1, has been associated with worse encephalopathy scores and neurodevelopmental outcomes.28 Interleukins have been investigated as potential biomarkers of neonatal brain injury among infants with neonatal encephalopathy, with elevated levels of IL-6, IL-8, and IL-10 measured within 24 h from birth associated with brain injury severity, abnormal neuroimaging findings on MRI, and impaired neurological outcomes.21,28,29,30,31 Neuronal trophic factors may play a role in neuronal cell repair and recovery after injury. Brain-derived neurotrophic factor (BDNF) is a trophic protein, shown to play a role in neuronal proliferation, differentiation, and plasticity.32,33,34,35 Decreased levels of BDNF were associated with worse encephalopathy grade and worse neurodevelopmental outcomes in neonates with encephalopathy.28,29 In addition, vascular endothelial growth factor (VEGF) is another trophic protein upregulated after tissue injury.36 In the literature, there is controversy over whether high or low VEGF is associated with encephalopathy severity.21,37,38
From the published studies, evidence shows a panel of multiple inflammatory, neuronal, and glial biomarkers measured at multiple time points would be advantageous for clinical diagnosis, prognosis, therapeutic monitoring, and identifying new therapeutic targets.39 However, a review of these biomarkers suggests they have not been studied enough to be used in routine clinical care and need validation to develop practical tests.40
The major obstacle to adopting many of these biomarkers into clinical practice is the lack of normative data in the neonatal population and the variability among gestational ages. Therefore, we aimed to determine the serum concentration of six pre-clinical brain injury-associated biomarkers which included CNS-insult (NRGN, GFAP), inflammatory (IL-6, IL-8, IL-10), and trophic (BDNF, VEGF) proteins in non-brain injured neonates from 23 to 41 weeks’ gestation.
Methods
Patients
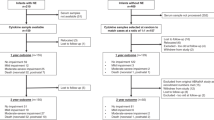
In this multicenter prospective observational cohort study, we identified and analyzed the concentrations of CNS-insult (GFAP, NRGN), inflammation (IL-6, IL-8, IL-10), and trophic-factor (BDNF, VEGF) markers from a cohort of non-brain injured neonates born between 23-41 weeks’ gestation admitted to the level 4 neonatal intensive care unit (NICU) at the 48 bed Johns Hopkins Children’s Center (2009-2019) and 97 bed Johns Hopkins All Children’s Hospital (2015–2019).2,13,14,41,42 As both units accommodate inborn and outborn patients, only infants admitted in the first 24 h of life were included. Infants with genetic syndromes, chromosomal abnormalities, and major congenital anomalies were not approached for consent. Infants with evidence of brain insult or predetermined brain injury risk factors at birth, infants who developed signs/symptoms of brain injury during the hospital stay or had neuroradiological abnormalities on any brain imaging, and infants with an abnormal neurologic exam at the time of discharge were excluded from the study (Fig. 1). The neonates in this cohort had serial serum samples collected on days of life (DOL) 0–7. The study received institutional review board approval at both hospitals, and signed parental informed consent was obtained for each participant within the infant’s first 30 days of life and prior to NICU discharge (IRB00026068).
Laboratory methods
Samples were procured from remnant serum obtained daily as part of routine patient care. Per College of American Pathologists (CAP) and clinical policy regulations, remnant serum was retained in the clinical laboratory for 48 h after all clinical testing was completed to allow for add-on testing or results validation. Once available for research use, appropriate samples for patients who provided consent as well as those potentially eligible for the study who had not yet provided consent were biobanked. Stored samples were subsequently discarded if consent was not obtained within 30 days. Biobanking entailed immediate transfer of samples into a 1.4 mL low-retention polypropylene cryovial and freezing at −80 °C. Prefreezing characterization included a description of sample quality by grading hemolysis level (none, mild, marked, or gross) using a comparison to a visual standard. All sample aliquots were exposed to 1–2 freeze/thaws prior to assaying. To minimize any effect post-collection handling may have on biomarkers levels, samples were only frozen once and thawed once and all samples were processed in the same manner. A detailed methodology describing participant engagement, consent, and the biobanking process with vulnerable populations is available.43
A custom multiplex enzyme-linked immunosorbent assay (ELISA) was developed to measure BDNF, IL-6, IL-8, IL-10, and VEGF simultaneously using robotically spotted capture antibodies on the 96-well plate format (Meso Scale Discovery [MSD], Rockville, MD) as previously reported.28 The lower limits of quantification for the BDNF, IL-6, IL-8, IL-10, and VEGF assays were 11.84, 0.15, 0.10, 0.15, and 0.41 pg/mL, respectively, with interassay coefficient percent of variation were calculated using a standardized interassay control of normal human plasma and were 24.7, 8.5, 8.5, 16.6, and 5.7, respectively. All assays were performed in the same laboratory and in a single batch utilizing 44 consecutive assay plates over 10 weeks.
A custom duplex ELISA was developed to measure GFAP and NRGN simultaneously using robotically spotted capture antibodies on the 96-well plate format (MSD, Rockville, MD) as reported previously.28 The lower limits of quantification for both the GFAP and NRGN assays were 0.009 ng/mL, with interassay percent coefficients of variation of 13.7 and 27.7, respectively.
Statistical analysis
Demographic data are presented as median and interquartile range (IQR) or percentages, as appropriate. For biomarker values that were detectable, but below the lower limit of quantification, a value was imputed by dividing the lower limit of quantification by two, noting that medians are robust to the specific choice of imputations. To investigate the relationship between each biomarker and GA, we used regression analyses adjusted for DOL. For the secreted molecules BDNF, VEGF, and interleukins, we utilized a quantile regression model to fit the median biomarker level based on the covariates, in which one individual sample per patient was selected at random with 1000 such bootstrapped iterations done to ensure that every sample had an equal chance of selection for the model—the standard error of the mean was determined based on the empirical distribution from the bootstrapped iterations. For the CNS-necrosis markers, we created a model to investigate the relationship between detectable or non-detectable GFAP and NRGN and GA. Detectable GFAP was analyzed using mixed-effects logistic regression to account for multiple measurements within the same person. Mixed-effects logistic regression did not computationally converge for NRGN; thus, we used a bootstrapping approach with logistic regression, with 1000 iterations each randomly choosing one measurement per person, similar to the discussion above. For all statistical analyses, a p-value of ≤0.05 was considered significant. Statistical analysis was performed using Stata (Version 15, StataCorp LLC, College Station, TX).
To identify sex differences in biomarkers values, we tested differences in the distribution of biomarkers between male and female infants using the rank-sum test. We accounted for within-person correlation of ranks by estimating the empirical distribution of the rank-sum statistic with 50,000 permutations of the male/female variable to determine the empirical p-value. The Monte Carlo error for the p-value ranged between 0.0008 and 0.0022 for the different biomarkers.
Results
Assay performance
The assays were performed within acceptable limits with the overall interassay percent coefficient of variation <25%. NRGN had the highest variation (27.7%), likely related to most samples being at the limit of detection. IL-10, GFAP, and NRGN were the lowest concentration analytes. It was expected that GFAP and NRGN would be at the limits of detection in a non-brain injury neonatal cohort. IL-10 was the lowest concentration analyte with a median value of 0.60 pg/mL (IQR 0.05–1.91) and below the limit of detection for many samples over all GAs.
Demographics
There were 5428 samples assessed for eligibility from 1219 neonates between 23 and 41 weeks’ gestation admitted to the JHH or All Children’s NICU. Exclusion criteria included cord pH ≤7.15, infection bacteremia within the first 7 days of life, GMH-IVH, periventricular leukomalacia, intracranial hemorrhage, stroke, seizures, abnormal brain MRI or cranial ultrasound, or therapeutic hypothermia. This resulted in 3232 serum samples to analyze from 745 enrollees. The maternal and neonatal characteristics from this cohort are presented in Table 1. The maternal population had a median gravida of 2 (IQR 1–4) and parity of 1 (IQR 0–2), with 43.0% spontaneous vaginal delivery, 20.3% with preeclampsia, and 6.4% with abruption. The distribution of GA had a median of 32.3 weeks (IQR 29–37 weeks) (Supplement Fig. 1). The neonatal cohort had a median weight of 1720 grams (IQR 1150–2790), a 5-min Apgar score of 8 (IQR 7–9), and cord pH of 7.3 (IQR 7.24–7.34). The cohort was 55.6% male, 8.7% had meconium, and 11.5% had intrauterine growth restriction.
Relationship of biomarkers to gestational age (GA)
For the cohort in aggregate, the descriptive statistics of the biomarker concentrations are shown in Table 2, including comparison by sex, which revealed no significant differences except BDNF was higher in females (p = 0.037). The relationships between the trophic and inflammatory biomarkers and GA are demonstrated in Fig. 2 and Table 3. The trophic-factor BDNF demonstrated a significant gestation-dependent increase and increased 3.7% for every week of gestation (p < 0.001). VEGF, another trophic factor, demonstrated no gestational concentration effect (p = 0.910). The inflammatory cytokines demonstrated significant gestational effects. As shown in Fig. 2, IL-8 increased 8.9% for every week of gestation (p < 0.001). IL-10 decreased during gestation but was not significant (coefficient = −0.041, p = 0.179). IL-6 also showed no relationship with GA (coefficient = 0.005, p = 0.557).
Each data point represents the biomarker concentration of a serum sample from an individual patient. The line represents the median for each gestational age and the shaded area represents the 95% confidence interval that the median is within that range. a BDNF increases by 3.7% for every gestational week (p < 0.001). b VEGF shows no relationship with gestational age (coefficient = −0.001, p = 0.910). c IL-6 shows no relationship with gestational age (coefficient = 0.005, p = 0.557). d IL-8 increases 8.9% with every gestational week (p < 0.001). e IL-10 shows no relationship with gestational age (coefficient = −0.041, p = 0.179).
The biomarker levels of the CNS-necrosis factors are shown in Fig. 3. For analysis, we evaluated the relationship between the amount of biomarker detectable and GA. Overall, 50% of samples had detectable GFAP and 33% had detectable NRGN. The odds of having detectable GFAP did not change for samples collected from infants less than 27 weeks’ gestation (p = 0.100) or between 27 and 36 weeks’ (p = 0.089) (Table 3). However, after 36 weeks’ gestation, the odds of having detectable GFAP increased by 53% for every gestational week (p = 0.002). Additionally, from 23 to 36 weeks’ gestation, the odds of having detectable NRGN decreased by 3% each week (p = 0.042). Then, after 36 weeks’ gestation, the odds of having detectable NRGN increased by 11% (p = 0.002).
Relationship of biomarkers to day of life (DOL)
The relationship between DOL and biomarker concentration is presented in Fig. 4. Unlike BDNF, VEGF, IL-6, 8, and 10 had significant daily variation. VEGF increased by 10.8% for each DOL (p < 0.001). IL-6 decreased by 15.8% for each DOL (p < 0.001). IL-8 increased each DOL by 18.9% (p < 0.001). IL-10 decreased by 81.4% for each DOL from cord blood through DOL 3 (p < 0.001). After DOL 3, IL-10 showed no relationship with DOL (p = 0.825). BDNF had no significant relationship with DOL (p = 0.152).
Each data point represents the biomarker concentration of a serum sample from an individual patient. The line represents the median for each gestational age and the shaded area represents the 95% confidence interval that the median is within that range. a BDNF shows no significant relationship with DOL (coefficient 0.021, p = 0.152). b VEGF increases by 10.8% each DOL (p < 0.001). c IL-6 decreases 15.8% each DOL (p < 0.001). d IL-8 increases 18.9% each DOL (p < 0.001). e IL-10 decreases by 81.4% each DOL less than DOL 3.
The brain necrosis markers GFAP and NRGN had significant DOL variation with the odds of having detectable GFAP decreased by 15% each DOL (p < 0.001) (Table 4). Similarly, the odds of having detectable NRGN decreased by 8% for each DOL (p = 0.008).
Clinical chorioamnionitis sensitivity analysis
In our cohort, maternal clinical chorioamnionitis was present in 20% of participants. To investigate whether the associations of either GA or DOL on the biomarkers were due to confounding by clinical chorioamnionitis, we performed a sensitivity analysis for clinical chorioamnionitis and, surprisingly, did not find any significant differences after adjustment for clinical chorioamnionitis (Tables 3 and 4). For VEGF however, after adjusting for clinical chorioamnionitis, there was a marginally significant association with GA, increasing 1.7% for each gestational week (p = 0.044).
Discussion
To our knowledge, this is the first study to report biomarker values in non-brain injured neonates across the GA spectrum and the first 7 DOL. For GA, BDNF and IL-8 increased each gestational week, while VEGF, IL-6, and IL-10 showed no relationship. For DOL, VEGF and IL-8 increased each DOL, IL-6 decreased each DOL, and IL-10 decreased each DOL for DOL 0-3 only. BDNF was not associated with DOL, and no changes were observed between days 0 and 7 of life. For CNS-insult markers, the odds of having detectable GFAP and NRGN increased each week after 36 weeks’ GA yet, the odds of having detectable GFAP and NRGN decreased with each DOL.
Biomarkers are being studied in neonates to diagnose injury, prognosticate on outcome, monitor disease progression, and potentially unveil new therapeutic interventions. In neonatal critical care, biomarkers have not yet been incorporated into clinical practice as the report pre-clinical studies are relatively small sample and oftentimes lack long-term developmental outcome data. These studies have also varied widely in the specific biomarkers or combination of biomarkers analyzed, the body fluid source, and the laboratory analysis methods. Interpretation of results has also proven to be challenging given the lack of biomarker normative data, the heterogeneity inherent in the wide GA range, the differing patterns of injury between the premature and term infant, the multifactorial disease pathophysiology, and the salient and not yet elucidated role of stress, infection, and different clinical variables in disease expression and progression.
The strengths of this study include the incorporation of two remote NICUs, a large sample size, and standardized sample collection, storage, and laboratory assays. The analysis utilized sophisticated statistical methodology to develop a biomarker model that incorporated multiple measurements from the same individual, in which we investigated both the median and mean models (Figs. 5 and 6). For some of the biomarkers, such as IL-10, the median model was more appropriate because of the larger number of undetectable data points, whereas for other biomarkers there was no difference between the mean and median model. Therefore, we decided to display the median models in the paper for consistency.
Each data point represents the biomarker concentration of a serum sample from an individual patient. The line represents the geometric mean for each gestational age and the shaded area represents the 95% confidence interval that the mean is within that range. a BDNF increases by 3.6% for every gestational week (p < 0.001). b VEGF shows no relationship with gestational age (coefficient = 0.019, p = 0.088). c IL-6 shows no relationship with gestational age (coefficient = −0.011, p = 0.243). d IL-8 increases 6.1% with every gestational week (p < 0.001). e IL-10 decreases 7.0% with every gestational week (p < 0.001).
Each data point represents the biomarker concentration of a serum sample from an individual patient. The line represents the geometric mean for each gestational age and the shaded area represents the 95% confidence interval that the mean is within that range. a BDNF increased 5.6% each DOL (p < 0.001). b VEGF increased 31.4% each DOL (p < 0.001). c IL-6 decreased 14% each DOL (p < 0.001). d IL-8 increased 17% each day (p < 0.001). e IL-10 decreased 50.3% each DOL for samples collected before DOL 3 (p < 0.001) and increased by 13.6% after DOL 3 (p < 0.001).
Positive and definite correlation between BDNF serum levels and advancing GA was found, unaffected by clinical chorioamnionitis. BDNF was the only biomarker affected by sex with values higher in females. Although significant, it is unclear if the modest increase is biologically relevant, but sex-related differences in serum BDNF have been reported in adults.44 BDNF has been shown to play a crucial modulatory role in many CNS processes, including synaptic plasticity, new synapse formation, neuronal survival, dendritic branching, and neurotransmitter excitation and inhibition.45,46,47 Similar findings were found in animal studies, where the elevation of BDNF levels resulted in an increased rate and extent of myelination and to a lesser degree, the axon caliber. This increased myelin content and caliber was maintained in adulthood.48 Even though it remains unclear as to what extent serum levels of BDNF reflect the brain concentrations,49,50,51 it is reasonable to assume that the linear increase of BDNF with GA could stem from normal brain growth.
Our findings of undetectable levels of NRGN and GFAP are also important. As these are biomarkers of CNS necrosis, they would not be expected to be elevated in non-brain injured neonates. As demonstrated, however, a number of neonates had quantifiable levels of NRGN and GFAP after birth. An explanation for our findings could be a subclinical brain injury, such has been demonstrated in neonatal and infant cardiac surgery.52,53 Additionally, numerous studies have shown long-term neurological and behavioral deficits in premature infants in the absence of grossly abnormal neuro-radiographic imaging, whether in the form of serial cranial ultrasounds or an MRI performed close to term corrected gestational age. Therefore, NRGN and GFAP are favorable candidates for biomarkers of neonatal brain injury because elevated levels would differ greatly from our study showing low levels of detection of these biomarkers in neonates without brain injuries. The mechanisms driving the temporal relationship of when GFAP and NRGN are detectable are likely gestationally regulated. Levels of GFAP and NRGN both increase in the brain with normal development.54,55 This was evident when GFAP was used in a VLBW IVH cohort where neonates with periventricular white matter injury had elevated GFAP levels, but the concentrations were lower than previously reported in full-term neonates with HIE.20,28 It is likely that the day of life changes in these molecules are related to the effect of the birth process.
We found that IL-8 increased, and IL-6 and IL-10 were unchanged with normal gestational development. Although there are numerous reports of interleukin concentrations to suggest neonatal sepsis, this is the first report to identify the normal gestational levels of IL-6, 8, and 10. In a 2016 cross-sectional study, Romero et al found that neonates born to mothers with chorioamnionitis, in the presence or absence of intra-amniotic inflammation with or without bacteria, had significantly higher median umbilical cord blood plasma concentrations of IL-6, IL-8, and IL-10.56 Similarly, Goepfert et al reported increased umbilical cord blood levels of IL-6 in preterm infants born after spontaneous preterm labor or premature rupture of membranes compared to preterm births done for maternal or fetal indications.57 In our study, we found that chorioamnionitis did not affect the association of biomarkers with GA and DOL. From our study, the progressive increase in IL-8 and nonsignificant absolute decrease in IL-10 is intriguing and possibly reflects the normal maturation of the immune system.
Furthermore, although our gestational age models are adjusted for DOL, we wanted to investigate directly how the biomarkers changed over DOL. DOL and BDNF did not have any relationship, suggesting that perhaps BDNF does not change as quickly as the other biomarkers, which all significantly changed with DOL. VEGF and IL-8 increased and IL-6 and IL-10 decreased with each DOL. Interestingly, after DOL 3, IL-10 no longer changed with DOL, suggesting a plateau in IL-10 concentration. Furthermore, these relationships were not affected by clinical chorioamnionitis. This is in agreement with previous studies that showed IL-6 decreased over the first seven days of life.58 This suggests that inflammatory biomarkers and VEGF should be adjusted for DOL in any analysis looking at biomarker concentration.
This study has several limitations. One limitation is the difficulty in identifying truly neurologically intact neonates among a NICU cohort of infants with GAs as low as 23 weeks. Our exclusion criteria were rigorous to identify infants with clinical brain injury, even among the ELBW infants, but it is well known that premature infants remain at risk for subtle neurological deficits even in the absence of grossly normal neuro-radiographic imaging. Not accounting for clinical variables of brain hypoperfusion like hypotension or symptomatic patent ductus arteriosus is also a limitation. Despite all our effort to screen for, and identify “normal, non-brain injured” neonates, the pool of patients admitted for varying NICU support does not fully represent an ideal healthy neonatal population, but fairly represents the neonatal population where these biomarkers would have clinical utility. Another limitation is that the samples used in this study were clinical remnants with delayed pre-analytic processing. However, all samples were processed in the same manner to minimize any pre-analytic variability. Lastly, when analyzing the effect of infection and inflammation, only the absence of bacteremia in the first 7 days of life and no evidence of clinical chorioamnionitis were used as variables. Another limitation is that only one of the sites documented whether clinical chorioamnionitis was associated with histological abnormalities on placenta pathology. Future biomarker studies investigating inflammatory biomarkers are needed with broader delineation of perinatal risk factors of infection/inflammation that would include the diagnosis of histologic chorioamnionitis, the presence and duration of prolonged rupture of membranes, presence, and frequency of urinary tract infections during pregnancy, as well as type and duration of maternal antibiotics treatment prior to delivery. Such future studies will also need to factor in the non-specific clinical findings in the first 7 days of life of hypotension, need for pressor support, leukocytosis, leukopenia, neutropenia, elevated serial CRP levels, the use of extended course of antibiotics, and the diagnosis of clinical sepsis, among others—all potentially suggestive of an inflammatory process.
Also, although harder to achieve, as we only sampled the first seven days of life, it is unclear if postnatal concentrations of the molecules studied, have plateaued or if there are continued developmental changes.
Conclusions
The gestational effect on serum concentrations of BDNF and IL-8 may be important biologically, suggesting that circulating levels will need to be gestationally adjusted for clinically relevant assessment of brain injury. Additionally, VEGF and the interleukins are dynamic in the immediate postnatal period, and DOL should be included in adjustments for clinically relevant assessment in neonates.
References
Fenichel, G. M. Hypoxic-ischemic encephalopathy in the newborn. Arch. Neurol. 40, 261–266 (1983).
Chin, E. M. et al. Preschool language outcomes following perinatal hypoxic-ischemic encephalopathy in the age of therapeutic hypothermia. Dev. Neurosci. 40, 627–637 (2019).
Kurinczuk, J. J., White-Koning, M. & Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic – ischaemic encephalopathy. Early Hum. Dev. 86, 329–338 (2010).
Shankaran, S. et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N. Engl. J. Med. 353, 1574–1584 (2005).
Volpe, J. J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 8, 110–124 (2009).
Bolisetty, S. et al. Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics 133, 55–62 (2014).
Chevallier, M. et al. Leading causes of preterm delivery as risk factors for intraventricular hemorrhage in very preterm infants: results of the EPIPAGE 2 cohort study. Am. J. Obstet. Gynecol. 216, 518.e1 (2017).
Leviton, A. & Paneth, N. White matter damage in preterm newborns—an epidemiologic perspective. Early Hum. Dev. 24, 1–22 (1990).
Sarnat, H. B. & Sarnat, M. S. Encephalopathy fetal distress a clinical and electroencephalographic study. Arch. Neurol. 33, 696–705 (1976).
Edwards, A. D. et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ 340, 409 (2010).
Shankaran, S. et al. Effect of depth and duration of cooling on death or disability at age 18 months among neonates with hypoxic-ischemic encephalopathy a randomized clinical trial. JAMA - J. Am. Med Assoc. 318, 57–67 (2017).
American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Neonatal encephalopathy and neurologic outcome, second edition. Obstet. Gynecol. 123, 896–901 (2014).
Gerner, G. J. et al. Correlation between white matter injury identified by neonatal diffusion tensor imaging and neurodevelopmental outcomes following term neonatal asphyxia and therapeutic hypothermia: an exploratory pilot study. J. Child Neurol. 34, 556–566 (2019).
Salas, J., Reddy, N., Carson, K. A., Northington, F. J. & Huisman, T. A. G. M. Ultrasound predicts white matter integrity after hypothermia therapy in neonatal hypoxic-ischemic injury. J. Neuroimaging 29, 743–749 (2019).
Blennow, K. A review of fluid biomarkers for Alzheimer’s disease: moving from CSF to blood. Neurol. Ther. 6, 15–24 (2017).
Pelinka, L. E. et al. GFAP versus S100B in serum after traumatic brain injury: relationship to brain damage and outcome. J. Neurotrauma 21, 1553–1561 (2004).
Missler, U., Wiesmann, M., Wittmann, G., Magerkurth, O. & Hagenström, H. Measurement of glial fibrillary acidic protein in human blood: analytical method and preliminary clinical results. Clin. Chem. 44, 138–141 (1999).
Ennen, C. S. et al. Glial fibrillary acidic protein as a biomarker for neonatal hypoxic-ischemic encephalopathy treated with whole-body cooling. Am. J. Obstet. Gynecol. 205, 251.e1–251.e7 (2011).
Blennow, M., Hagberg, H. & Rosengren, L. Glial fibrillary acidic protein in the cerebrospinal fluid: a possible indicator of prognosis in full-term asphyxiated newborn infants? Pediatr. Res 37, 260–264 (1995).
Stewart, A. et al. Glial fibrillary acidic protein as a biomarker for periventricular white matter injury. Am. J. Obstet. Gynecol. 209, 27.e1–27.e7 (2013).
Chalak, L. F. et al. Biomarkers for severity of neonatal hypoxic-ischemic encephalopathy and outcomes in newborns receiving hypothermia therapy. J. Pediatr. 164, 468–74.e1 (2014).
Massaro, A. N. et al. Serum biomarkers of MRI brain injury in neonatal hypoxic ischemic encephalopathy treated with whole-body hypothermia: a pilot study. Pediatr. Crit. Care Med. 14, 310–317 (2013).
Blennow, M., Savman, K., Ilves, P., Thoresen, M. & Rosengren, L. Brain-specific proteins in the cerebrospinal fluid of severely asphyxiated newborn infants. Acta Paediatr. 90, 1171–1175 (2001).
Yang, J., Korley, F. K., Dai, M. & Everett, A. D. Serum neurogranin measurement as a biomarker of acute traumatic brain injury. Clin. Biochem 48, 843–848 (2015).
Çevik, S. et al. NRGN, S100B and GFAP levels are significantly increased in patients with structural lesions resulting from mild traumatic brain injuries. Clin. Neurol. Neurosurg. 183, 105380 (2019).
Represa, A. et al. Neurogranin: immunocytochemical localization of a brain-specific protein kinase C substrate. J. Neurosci. 10, 3782–3792 (1990).
Guadaño-Ferraz, A., Viñuela, A., Oeding, G., Bernal, J. & Rausell, E. RC3/neurogranin is expressed in pyramidal neurons of motor and somatosensory cortex in normal and denervated monkeys. J. Comp. Neurol. 493, 554–570 (2005).
Dietrick, B. et al. Plasma and cerebrospinal fluid candidate biomarkers of neonatal encephalopathy severity and neurodevelopmental outcomes. J. Pediatr. 226, 71–79.e5 (2020).
Massaro, A. N. et al. Plasma biomarkers of brain injury in neonatal hypoxic-ischemic encephalopathy. J. Pediatr. 194, 67–75 (2018).
Çelik, Y. et al. The effects of selective head cooling versus whole-body cooling on some neural and inflammatory biomarkers: a randomized controlled pilot study. Ital. J. Pediatr. 41, 1–8 (2015).
Chaparro-Huerta, V. et al. Proinflammatory cytokines, enolase and S-100 as early biochemical indicators of hypoxic-ischemic encephalopathy following perinatal asphyxia in newborns. Pediatr. Neonatol. 58, 70–76 (2017).
Barde, Y. A., Edgar, D. & Thoenen, H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1, 549–553 (1982).
Korte, M. et al. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl Acad. Sci. USA 92, 8856–8860 (1995).
Leal, G., Comprido, D. & Duarte, C. B. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology 76, 639–656 (2014).
Park, H. & Poo, M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 14, 7–23 (2012).
Grad, S. et al. Strongly enhanced serum levels of vascular endothelial growth factor (VEGF) after polytrauma and burn. Clin. Chem. Lab Med 36, 379–383 (1998).
Lv, H. et al. Neonatal hypoxic ischemic encephalopathy-related biomarkers in serum and cerebrospinal fluid. Clin. Chim. Acta 450, 282–297 (2015).
Sweetman, D. U., Onwuneme, C., Watson, W. R., Murphy, J. F. A. & Molloy, E. J. Perinatal asphyxia and erythropoietin and VEGF: serial serum and cerebrospinal fluid responses. Neonatology 111, 253–259 (2017).
Graham, E. M., Everett, A. D., Delpech, J. C. & Northington, F. J. Blood biomarkers for evaluation of perinatal encephalopathy: state of the art. Curr. Opin. Pediatr. 30, 199–203 (2018).
Ramaswamy, V. et al. Systematic review of biomarkers of brain injury in term neonatal encephalopathy. Pediatr. Neurol. 40, 215–226 (2009).
Broni, E. K. et al. Blood biomarkers for neonatal hypoxic – ischemic encephalopathy in the presence and absence of sentinel events. J. Perinatol. https://doi.org/10.1038/s41372-020-00850-5 (2020).
Novak, C. M., Eke, A. C., Ozen, M., Burd, I. & Graham, E. M. Risk factors for neonatal hypoxic-ischemic encephalopathy in the absence of sentinel events. Am. J. Perinatol. 36, 27–33 (2019).
Schleif, W. et al. Tiny bodies, big needs: prospective biobanking of neonatal clinical remnant samples. Biopreserv. Biobank. 19, 106–110 (2021).
Shimada, H. et al. A large, cross-sectional observational study of serum BDNF, cognitive function, and mild cognitive impairment in the elderly. Front. Aging Sci. 6, 1–9 (2014).
Martínez-Moreno, A., Rivera-Olvera, A. & Escobar, M. L. BDNF induces in vivo long-lasting enhancement of synaptic transmission and structural reorganization at the hippocampal mossy fibers in a transcription and translation-independent manner. Neurobiol. Learn. Mem. 167, 107125 (2020).
Edelmann, E., Leßmann, V. & Brigadski, T. Pre- and postsynaptic twists in BDNF secretion and action in synaptic plasticity. Neuropharmacology 76, 610–627 (2014).
Panja, D. & Bramham, C. R. BDNF mechanisms in late LTP formation: a synthesis and breakdown. Neuropharmacology 76, 664–676 (2014).
Tolwani, R. J. et al. BDNF overexpression produces a long-term increase in myelin formation in the peripheral nervous system. J. Neurosci. Res. 77, 662–669 (2004).
Karege, F., Schwald, M. & Cisse, M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci. Lett. 328, 261–264 (2002).
Sartorius, A. et al. Correlations and discrepancies between serum and brain tissue levels of neurotrophins after electroconvulsive treatment in rats. Pharmacopsychiatry 42, 270–276 (2009).
Klein, A. B. et al. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J. Neuropsychopharmacol. 14, 347–353 (2011).
Graham, E. M. et al. Association of intraoperative circulating-brain injury biomarker and neurodevelopmental outcomes at 1 year among neonates who have undergone cardiac surgery. J. Thorac. Cardiovasc. Surg. 157, 1996–2002 (2019).
Easley, R. B. et al. Impaired cerebral autoregulation and elevation in plasma glial fibrillary acidic protein level during cardiopulmonary bypass surgery for CHD. Cardiol. Young. 28, 55–65 (2018).
Guo, J. H. et al. Expression pattern of NeuN and GFAP during human fetal spinal cord development. Child’s Nerv. Syst. 31, 863–872 (2015).
Nazir, F. H. et al. Expression and secretion of synaptic proteins during stem cell differentiation to cortical neurons. Neurochem. Int. 121, 38–49 (2018).
Romero, R. et al. Clinical chorioamnionitis at term V: umbilical cord plasma cytokine profile in the context of a systemic maternal inflammatory response. J. Perinat. Med. 44, 53–76 (2016).
Goepfert, A. R. et al. Umbilical cord plasma interleukin-6 concentrations in preterm infants and risk of neonatal morbidity. Am. J. Obstet. Gynecol. 191, 1375–1381 (2004).
Sood, B. G., Madan, A., Saha, S., Schendel, D. & Thorsen, P. Perinatal systemic inflammatory response syndrome and retinopathy of prematurity. Pediatr. Res. 67, 394–400 (2010).
Acknowledgements
We thank the patients and their families for their participation and contributions to this study and the Everett research group at Johns Hopkins University School of Medicine for their support and contributions and the Johns Hopkins All Children’s Pediatric Biorepository for its support. We also thank the Johns Hopkins University School of Medicine Scholarly Concentration mentor Dr. Meredith Atkinson and the Johns Hopkins University School of Medicine Dean’s Funding for their support and contributions.
Funding
This study was supported by NIH NICHD R01HD086058 [A.E., F.N., J.Y., D.V., S.B., and E.G.], NINDS KO8NS096115 [R.C.-V.], Thomas-Wilson Foundation [R.C.-V.], and JHACH Foundation Institutional Research Grant Program [S.B.].
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: S.B., B.D.F., F.N., E.G., A.T., J.Z.B.S., D.V., and A.D.E. Drafting the article or revising it critically for important intellectual content: S.B., B.D.F., F.N., E.G., A.T., V.J.B., G.G., R.C.-V., D.V., and A.D.E. Final approval of the version to be published: S.B., B.D.F., F.N., E.G., R.C.-V., D.V., and A.D.E.
Corresponding author
Ethics declarations
Competing interests
Under a license agreement between ImmunArray Ltd. and Johns Hopkins University, the University and Dr. Everett are entitled to royalties on an invention described in this study and discussed in this publication. This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies. Sandra Brooks and Barbara Dietrick wrote the first draft of the manuscript, and no honorarium, grant, or other forms of payment was given to anyone to produce the manuscript. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Brooks, S., Friedes, B.D., Northington, F. et al. Serum brain injury biomarkers are gestationally and post-natally regulated in non-brain injured neonates. Pediatr Res 93, 1943–1954 (2023). https://doi.org/10.1038/s41390-021-01906-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01906-8