Abstract
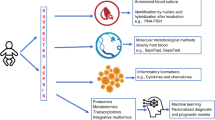
Diagnostic tests for sepsis aim to either detect the infectious agent (such as microbiological cultures) or detect host markers that commonly change in response to an infection (such as C-reactive protein). The latter category of tests has advantages compared to culture-based methods, including a quick turnaround time and in some cases lower requirements for blood samples. They also provide information on the immune response of the host, a critical determinant of clinical outcome. However, they do not always differentiate nonspecific host inflammation from true infection and can inadvertently lead to antibiotic overuse. Multiple noninfectious conditions unique to neonates in the first days after birth can lead to inflammatory marker profiles that mimic those seen among infected infants. Our goal was to review noninfectious conditions and patient characteristics that alter host inflammatory markers commonly used for the diagnosis of early-onset sepsis. Recognizing these conditions can focus the use of biomarkers on patients most likely to benefit while avoiding scenarios that promote false positives. We highlight approaches that may improve biomarker performance and emphasize the need to use patient outcomes, in addition to conventional diagnostic performance analysis, to establish clinical utility.
Similar content being viewed by others
Introduction
Sepsis comprises a systemic inflammatory response to infection with the production and release of inflammatory and immune mediators.1 Neonatal sepsis is a major cause of morbidity and mortality2 and early presentation can be nonspecific, making the diagnosis challenging. Diagnostic tests for sepsis either aim to detect the infectious agent (such as microbiological cultures) or measure the levels of markers produced by the host responding to an infection (such as C-reactive protein, CRP; procalcitonin; interleukin-6, IL-6). The latter category of “sepsis biomarkers” has many advantages compared to culture-based methods: they have a quick turnaround time, in some cases require smaller blood volumes, are less affected by antibiotic exposure, are unaffected by commensal contamination, and provide information about the host’s immune response, a critical determinant of the final outcome.3 However, host immune markers do not detect the actual pathogen or provide information on antimicrobial sensitivities. Importantly, they do not always distinguish between inflammation secondary to infection, and a sterile inflammatory state which will not benefit from antibiotic treatment. The performance of a biomarker also depends on its kinetics relative to the stage of the disease4 and has been incorporated in the implementation of procalcitonin use.5 CRP rises later in the disease course, suggesting a limited role for CRP in antibiotic initiation decisions.4 Despite this, single CRP values continue to be used in practice and drive both initiation and continuation of antibiotic therapy.6,7 When used in the absence of microbiologically confirmed infection and/or appropriate clinical context, these tests can increase antibiotic overuse,8,9 contribute to the adverse effects of antibiotic exposure,10,11 and increase microbial resistance.12
Noninfectious inflammatory conditions associated with abnormal host inflammation markers such as malignancies and rheumatologic diseases are rare in neonates, while infection is relatively common. This may contribute to the difficulty in formulating an alternate explanation for an abnormal marker. However, multiple noninfectious conditions unique to the neonatal population can lead to inflammatory marker profiles that mimic those seen among infected infants, particularly in the first few days after birth.13 Birth and early postnatal period are marked by physiological stress and complex adaptations in the newborn.14 It is also the period when the most common indication for antibiotic administration in the neonatal intensive care unit (NICU) occurs—empiric antibiotic administration for suspected early-onset sepsis (EOS).15 Our goal was to review noninfectious conditions and patient characteristics that alter host inflammatory markers commonly used for EOS diagnosis. To focus the scope of the review, we have limited the discussion predominantly to CRP, one of the most widely available sepsis biomarkers in clinical use, and included information on other biomarkers briefly, and where available.
EOS evaluation
Sepsis biomarkers, specifically CRP along with hematological markers, have been used for decades to assist with various aspects of EOS evaluation.16 CRP is also part of some national guidelines for EOS evaluation, recommended for use in diagnosis, and in the determination of the length of treatment.17 However, routine use of CRP in EOS evaluations has several challenges: the reported sensitivity of CRP in EOS evaluations is variable;18 among infants with maternal perinatal risk factors CRP use is associated with increased procedures and antibiotic use with limited information on improved patient outcomes;8,9,19,20 and its incremental contribution to decision making, beyond available clinical information, is debatable.6 Many of these challenges stem from noninfectious conditions that can cause elevated CRP. Procalcitonin and IL-6 are promising but less widely used sepsis biomarkers. Although these biomarkers may perform better than CRP,5 they are also influenced by noninfectious conditions and may involve similar challenges when implemented for routine use. Tables 1 and 2 summarize the noninfectious perinatal factors, patient characteristics, and conditions that are can influence common sepsis biomarkers used in EOS evaluation.
Perinatal factors and patient characteristics
Birth injury
Injury during the process of birth occurs at a rate of 1.9 per 1000 live births.21 While birth injury is reported to increase CRP levels, many reports that highlight these associations do so without formal statistical analysis, making the attribution anecdotal rather than a true association.18,22,23,24 Other studies have refuted such associations after more formal analysis.25,26 In particular, Kääpä et al. reported results from a prospective study of 238 mother–infant dyads where they measured CRP levels at 24 and 72 h after birth.26 Signs of tissue trauma including cephalhematoma, fractured clavicle, and severity of bruising were documented. They noted a significant association of vaginal delivery (VD) and vacuum extraction with elevated CRP at 24 h. However, they found no association between the presence or severity of tissue trauma and the level of CRP.
Delivery mode and labor
Multiple studies report higher mean/median CRP levels among infants delivered by VD and emergency cesarean delivery (CD) compared to elective CD.27,28,29,30 The association is attributed to fetal “stress” with labor,31 and potentially with differences in mother-to-child colonization patterns.32 Instrumental vaginal deliveries are also associated with a higher maximum CRP value and are attributed to labor duration, rather than tissue traumatization.26,27 Labor duration and VD are associated with higher total neutrophil levels, higher leukocyte count, and immature neutrophils that resolve after the first 24 h.33,34,35 Duration of labor is also associated with higher IL-6 levels.36,37,38,39 Notably, the delivery mode is not associated with differences in immature-to-total (IT) neutrophil count,34,35 or procalcitonin levels.29,30
Variation in peak levels by delivery mode can impact the proportion of infants who meet a set cutoff threshold for the diagnosis of sepsis. Overall, the proportion of uninfected infants with the highest CRP value above the 10 mg/L cut-off varies from 3 to 25% of the overall cohort depending on the study13,19,27,29 and occurs more frequently in infants delivered by VD.28,30 In a cohort study of infants admitted to the NICU and ultimately managed as uninfected, the 75th quartile for the highest CRP value at 36 h after birth was 13.7 mg/L compared to 2.74 mg/L among those delivered by CD.30 Similarly, the 95th centile for CRP at 48 h after birth in a cohort of uninfected term infants was 12.1 and 11.3 mg/L for infants delivered by VD and emergency CD, respectively, compared to 6 mg/L for infants delivered by CD.28
Perinatal risk factors for infection
Perinatal factors associated with the risk of EOS occur at a substantially higher incidence than actual invasive infection40 and can alter the inflammatory response in infants independently of actual infection. In a study of serial CRP values obtained among term infants ultimately diagnosed as uninfected, almost one-fourth of infants with perinatal risk factors had a CRP value above the 10 mg/L cut-off.19 Similarly, among 554 infants born to mothers with a diagnosis of chorioamnionitis, 22% were found to have a CRP value >10 mg/L and 28% had either an abnormal IT ratio or an elevated CRP at 12 h of age.9 An elevated 12 h CRP led to both prolonged antibiotics administration and increased frequency of lumbar punctures regardless of culture results. The lack of specificity when defining chorioamnionitis41 and variation in the use of perinatal risk factors to identify at-risk infants42 can result in the variable performance of inflammatory biomarkers.
Studies have described multiple perinatal factors that affect biomarker levels. Chiesa et al. enrolled infants without evidence of maternal intra-amniotic infection and with an unremarkable early postnatal course to establish reference levels among uninfected infants.29 After adjusting for gestational age, age at sampling, and infant sex, they noted a significant association of mean CRP value with a duration of active labor (14.5% increase per hour), membrane rupture (0.4% increase per hour), and intrapartum antibiotic prophylaxis (increase by 28%) compared to infants without these factors. These factors were not associated with changes in procalcitonin level in this study; however, prolonged membrane rupture has been associated with higher procalcitonin levels in other studies.43,44 Other perinatal variables associated with elevated CRP levels, often reported as unadjusted associations include maternal fever,45 antenatal steroid administration,29 maternal diagnosis of hypertension,46 and low Apgar scores.46 The level of procalcitonin has been variably associated with maternal antibiotic exposure with significantly lower values reported in one study47 and no change in another study.43 Maternal fever was associated with elevated maternal IL-6 levels and with elevated IL-6 in cord blood.48
Many of these factors are interrelated and only a few studies perform multivariable models to identify independent contributions. Study design and analytical approaches further limit the interpretation of results. For example, while maternal antibiotic exposure was found to be associated with elevated CRP levels,29 in a different study when the analysis was restricted to patients with an indication for intrapartum prophylaxis association was found only with incomplete prophylaxis.28 Despite limitations, these studies confirm that multiple perinatal factors known to be associated with increased risk of infection are also associated with abnormal markers among uninfected infants, confounding the diagnostic performance of these biomarkers. Finally, the association of perinatal risk factors for infection with abnormal sepsis biomarkers cannot simply be attributed to undetected infection,22 as many of these studies ensured an unremarkable course for study infants for multiple weeks after birth.27,28,29,49
Gestational age
Preterm gestation is associated with lower CRP,13,19,27,29,50 and higher procalcitonin29,47 and IL-6 values.46 The lower baseline level of CRP in preterm infants may alter diagnostic performance. In a cohort of 179 terms and 353 preterm infants, CRP had a lower sensitivity of 53% among preterm infants compared to 86% in term infants for diagnosing culture-confirmed EOS.13 After birth, the median CRP value increased for all gestational ages, with an earlier (27–36 h) but lower peak value in preterm infants compared to term infants (56–70 h).13 Procalcitonin levels (adjusted for gender and age at sampling) among uninfected infants were negatively associated with gestational age: term infants had an estimated peak value of 2.9 ng/mL (0.4–18.7) at 24 h while preterm infants (<37 weeks) had a peak value of 6.5 ng/mL (0.9–48.4) at 21–22 h.29 Neutrophil levels also vary by gestation age with higher peak values in term infants compared to moderately and late preterm infants and earlier peak levels compared to extremely preterm infants.33
Chronological age
Many sepsis biomarkers increase immediately after birth, followed by a decline over 72–96 h. This rise and fall is seen with CRP,19,29,30,50 procalcitonin,29,43,44 IL-6,51 and neutrophil counts.33,52 Accounting for age improves the diagnostic performance of hematologic markers,53 and has been used with procalcitonin,5 but has not been widely incorporated in the interpretation of CRP. Instead, serial values are used to trend changes that incorporate information from both kinetics of the marker in response to disease progression, if present, and physiological changes that occur in the first days after birth.16 Such an approach is often aimed at discontinuing antibiotics rather than initiation.17 However, single values are frequently used in practice despite poor performance.6
In light of the many perinatal and patient-related factors that affect baseline CRP levels, a single common threshold for all newborns may be inadequate. A dynamic threshold that accounts for gestational age and chronological age has been proposed as a means to improve performance, but is not yet widely available.18
Hypoxic–ischemic encephalopathy
Neonates diagnosed with hypoxic–ischemic encephalopathy (HIE) are also at a higher risk for EOS. The incidence of culture-confirmed infection among infants enrolled in therapeutic hypothermia (TH) trials ranges from 3 to over 12%54 and in database studies from 1 to 2%.55,56 No difference in the incidence of EOS is reported with the use of TH.54 Sending cultures and initiating empiric antibiotics is therefore common among infants with HIE.56 Stopping antibiotics once cultures are sterile is, however, more variable. In a study of more than 1500 HIE cases, 16% of infants were treated as culture-negative infections with 8 median days of antibiotics.56 The hesitation to stop antibiotics when cultures are negative persists among neonatal providers57 and is heightened when managing children who are critically ill. Part of this hesitation comes from concerns about the reliability of blood cultures and part from reliance on abnormal sepsis biomarkers instead.58,59 However, the reliability of biomarkers can also be affected by many factors in HIE, not related to infection, that result in systemic inflammation.
The pathophysiology of HIE, regardless of concomitant infection, includes an inflammatory cascade with microglial activation and migration, infiltration of peripheral macrophages into the brain, and the release of cytotoxic and pro-inflammatory cytokines such as IL-6 and IL-8 both locally in the neural circulation and in the systemic circulation.60,61 TH, alternately, is known to reduce neuroinflammation and can alter the dynamics of common sepsis biomarkers in the absence of proven infection.62,63,64 An elevated CRP level >10 mg/L is reported in 55–68% of infants with HIE in the absence of culture-confirmed infection and is associated with the severity of HIE.65,66,67 Besides higher values, CRP levels were slower to peak and remained high for a longer duration among patients undergoing TH compared to normothermic historic controls with HIE, independent of perinatal infection risk factors, the severity of HIE, and concomitant meconium aspiration.58,59,67 Higher levels of procalcitonin and IL-6 levels are also reported in neonates and adults receiving TH.63,64,68 The cut-off values proposed for IL-6 levels in sepsis diagnosis vary from <10 to over 300 pg/mL.69 Among 22 infants with HIE and without infection, IL-6 median levels were 198.2 pg/mL (interquartile range (IQR): 49.8–358.3) and 101.9 pg/mL (IQR: 24.9–562.9) on days 1 and 2, respectively.63 These values suggest that reliance on abnormal inflammatory markers in infants with HIE will promote prolonged antibiotic administration in the majority. An appropriately obtained blood culture has a high detection rate for the vast majority of organisms between ≥4 colony-forming unit/mL,70,71 and it is unlikely that two-thirds of cases of HIE cases have actual bacteremia that was not detected in the blood culture. In general, the wide center variation in prolonged antibiotic administration to infants suggests antibiotic overuse,56 while there is no evidence to suggest that prolonged antibiotics based on inflammatory markers improve outcomes. In addition, infants with HIE have an increased risk of drug toxicity that can be compounded by unnecessary antibiotic exposure.72
In summary, the use of sepsis biomarkers to guide antibiotic therapy in infants with HIE infants is fraught with challenges. Studies are urgently needed to establish the best antibiotic use practices in this population.
Meconium aspiration syndrome
Meconium-stained amniotic fluid complicates approximately 10–20% of all pregnancies and meconium aspiration syndrome (MAS) occurs in 5–10% of these newborns.73,74,75,76 The pathogenesis of MAS is multifactorial and involves airway obstruction, surfactant dysfunction, pulmonary hypertension, and alveolar inflammation with resultant hypoxia and hypercapnia.77 Inflammation plays a central role in the pathogenesis of MAS, affecting both surfactant production and function, and pulmonary perfusion leading to local and ultimately systemic manifestations.78 Pro-inflammatory cytokines (IL-8, IL-1B, IL-6, and tumor necrosis factor-α) are found in tracheal aspirates of patients affected by MAS as early as 6 h after exposure and recapitulated in animal studies.79,80,81,82,83,84 IL-8, found in meconium, plays a role in attracting neutrophils from the system circulation into alveoli spaces.82 Inflammatory markers have been proposed as markers of disease severity and therapeutic response. Among 239 infants without culture-confirmed infection, severe MAS (requiring mechanical ventilation) in the first 2 days after birth was associated with a significantly higher CRP and IT neutrophil ratio and a lower white blood cell and absolute neutrophil count, compared to infants with less severe MAS (no mechanical ventilation required).85 Conversely, a decrease in inflammatory markers 96 h after birth was associated with an improvement in respiratory parameters.79
The diagnosis of early-onset culture-negative infection is highly variable between units2 and is often based on a combination of inflammatory markers and clinical symptoms.7 The elevated inflammatory markers observed in MAS and the need for critical care in these infants can potentially lead to prolonged use. However, there is no established benefit from such use of antibiotics. Three trials conducted in low- to middle-income settings have compared antibiotic administration in infants with MAS: two trials compared 7 days of antibiotics and one compared 3 days of empiric antibiotics with no antibiotics.86,87,88 Individually and in a meta-analysis, no differences were observed in the incidence of proven infection, mortality, or duration of hospital admission.89,90
Although no difference in the incidence of bacteremia is reported between infants born with and without meconium-stained amniotic fluid, the baseline rate of infection in some of these studies is high.91 Therefore, we focus on sending appropriate microbiological tests and stopping antibiotics when they are negative rather than not initiating antibiotics at all. The association between inflammatory indices in the early phase of the disease and the severity of MAS raises the question of whether the inflammatory markers should be more rigorously evaluated as predictors of the disease course, which can inform therapeutic planning, such as the decision to transport to a center with extracorporeal membrane oxygenation facilities.
Surgical conditions affecting early life biomarker levels
Surgery is associated with metabolic stress and cytokine release that can raise many common inflammatory biomarkers.92,93 Surgical interventions soon after birth may involve major procedures such as correction of congenital anomalies or minor elective procedures such as circumcision. Inflammatory biomarker levels in response to circumcision during birth hospitalization have not been well described. In a study of 115 children aged 1–13 years undergoing circumcision with different modes of anesthesia, there was no difference in CRP levels before and after the procedure.94 It is unclear whether CRP and other inflammatory biomarkers are equally unaffected during neonatal circumcision.
In clinical presentations that require major surgery soon after birth, sepsis biomarkers can be used both in the evaluation for EOS and to monitor for, the ongoing risk of infection. A surgical condition where sepsis biomarkers are especially difficult to interpret is gastroschisis. Elevated CRP, I:T ratio, and IL-6 levels are reported both before and after surgery in infants with gastroschisis compared to control infants and infants with other anomalies such as omphalocele.95,96 These high levels are thought to reflect inflammation that is intrinsic to gastroschisis and is not considered a reliable marker for EOS.96,97 Nevertheless, clinical reports show that when obtained in gastroschisis patients, abnormal biomarker levels result in more invasive procedures such as lumbar punctures95 and longer courses of antibiotics.97 Another use of inflammatory markers is to assess for postoperative infection. Studies vary in detecting changes in biomarker levels after neonatal surgery.96,98 The ability of a particular test to discriminate a postsurgical inflammatory response from infection is also variable.99,100,101 In most cases higher cut-offs and close attention to the temporal relation with surgery are required for optimal test performance98,99,101 As with many of the other conditions triggering sepsis evaluation after birth, it is unclear whether the use of biomarkers aids decision making and improves patient outcomes.
Discussion
In summary, multiple noninfectious factors present among infants admitted to the NICU affect the median values of common sepsis biomarkers and the proportion of infants who meet the threshold for a “positive” test. Beyond EOS evaluation, other investigators have also highlighted sensitivity issues when using CRP in late-onset infection evaluations. In a recent meta-analysis of 22 studies using a preset cut-off of 5–10 mg/L, the pooled sensitivity was 0.62 (95% confidence interval, 0.50–0.72).102 The authors concluded that CRP would not contribute to improving sepsis diagnosis and may increase unnecessary medicalization. Along these lines, the deimplementation of routine CRP use in EOS evaluation significantly reduced antibiotic use without reported adverse effects in a single-center study.103 One may say, at this point, why don’t we just cut our losses with CRP and move on? And while that may be one approach, there remain unresolved issues. First, there is a gap in sepsis diagnosis that could be improved with a fast turnaround test. Second, the use of CRP is still prevalent.74 Finally, many of the issues identified for CRP and other common biomarkers may also be relevant for the next novel inflammatory biomarker that is introduced into practice.
There are several ways to improve test performance. One is to recognize the noninfectious conditions that influence test results and incorporate them into the interpretation of sepsis biomarker results. For example, leukopenia in a growth-restricted infant delivered to a mother with preeclampsia prior to the onset of labor or rupture is likely not related to EOS and its use for antibiotic initiation after birth could be avoided.104 Another characteristic infrequently addressed is the changing pretest probability of infection in different clinical scenarios. A biomarker is expected to be valuable when the pretest probability of infection in the patient being tested is neither too high (almost all clinicians would treat regardless of test results) nor too low (no clinician would treat regardless of test results). Studies that include patients with extreme pre-test probabilities that are not a decision conundrum to clinicians, do not capture the information a clinician desires in real-world practice. A similar idea can be extended to the pretest probability of inflammation in the absence of infection. Testing should be avoided in scenarios where the probability of sterile inflammation is too high for a biomarker to discriminate additional infection.
Another approach could be to incorporate adjusted receiver-operating characteristic curves105,106 that account for covariates, such as gestational or chronological age, associated with the distribution of a biomarker value among uninfected infants. The traditional approach of using biomarkers for the diagnosis of sepsis with one cut-off point, regardless of patient age, characteristics, or prior probability of infection of the patient, is potentially inadequate. Covariate adjustment, a cornerstone in bias reduction in association studies, is infrequently considered in the analysis of neonatal sepsis biomarker performance.18 Variables that are associated with both biomarker value and the risk of infection, such as duration of membrane rupture and labor, qualify as confounders. Not accounting for these variables overestimates the performance of diagnostic markers.105 In contrast, accounting for variables that are associated with the marker but not necessarily with EOS, such as delivery mode or sampling time, can improve the accuracy of the marker. While combining biomarkers may improve performance, accounting for covariate distribution in uninfected infants that influence individual markers and/or outcomes will still be required.
The endpoint for sepsis biomarker studies is often time-to-infection diagnosis and improved identification of infection cases.107 Given the many concerns with the culture-based gold standard of infection diagnosis, it may be more relevant to focus on measures of patient outcomes, such as mortality, and measures of morbidity, such as length of hospitalization and readmissions. Rigorous analysis is needed to understand which sepsis biomarkers change decision making beyond that provided by history and clinical exam, and whether incorporating the test into existing risk prediction models, such as the sepsis risk calculator, has incremental value with demonstrable health benefits.
A final thought is whether we have used all information that these common tests provide us with. Host inflammation is recognized as a key mediator of multiple adverse outcomes among infants admitted to the NICU. Yet, the clinical focus when using common inflammatory biomarkers like CRP is primarily on antibiotic administration. A CRP value of >100 mg/L was reported in 4% of NICU admissions (258 infants); of the 106 infants identified in the first 3 days, most were of term gestation and 81% were considered not infected.108 One wonders if such a heightened inflammatory response is not relevant to the health of these infants. Are there practices in neonatal care beyond antibiotics that can be informed with these values? Does evidence of early inflammation impact ultimate outcomes and, if so, can that be manipulated in favor of the patient? Sepsis biomarkers provide information about the state of the host. Whether there is an infection or not, an abnormal value may have health implications, as has been demonstrated in other settings.109,110,111 Using these widely available markers to further our understanding of the newborn’s immunological state may provide important avenues for improving outcomes.
References
Singer, M. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315, 801–810 (2016).
Mukhopadhyay, S. et al. Impact of early-onset sepsis and antibiotic use on death or survival with neurodevelopmental impairment at 2 years of age among extremely preterm infants. J. Pediatr. 221, 39–46.e5 (2020).
Wynn, J., Cornell, T. T., Wong, H. R., Shanley, T. P. & Wheeler, D. S. The host response to sepsis and developmental impact. Pediatrics 125, 1031–1041 (2010).
Eschborn, S. & Weitkamp, J. H. Procalcitonin versus C-reactive protein: review of kinetics and performance for diagnosis of neonatal sepsis. J. Perinatol. 39, 893–903 (2019).
Stocker, M. et al. Procalcitonin-guided decision making for duration of antibiotic therapy in neonates with suspected early-onset sepsis: a multicentre, randomised controlled trial (NeoPIns). Lancet 390, 871–881 (2017).
Nabulsi, M., Hani, A. & Karam, M. Impact of C-reactive protein test results on evidence-based decision-making in cases of bacterial infection. BMC Pediatr. 12, 140–140 (2012).
Ayrapetyan, M., Carola, D., Lakshminrusimha, S., Bhandari, V. & Aghai, Z. H. Infants born to mothers with clinical chorioamnionitis: a cross-sectional survey on the use of early-onset sepsis risk calculator and prolonged use of antibiotics. Am. J. Perinatol. 36, 428 (2019).
Mukherjee, A., Davidson, L., Anguvaa, L., Duffy, D. A. & Kennea, N. NICE neonatal early onset sepsis guidance: greater consistency, but more investigations, and greater length of stay. Arch. Dis. Child Fetal Neonatal Ed. 100, 248 (2015).
Kiser, C., Nawab, U., McKenna, K. & Aghai, Z. H. Role of guidelines on length of therapy in chorioamnionitis and neonatal sepsis. Pediatrics 133, 992–998 (2014).
Ting, J. Y. & Roberts, A. Association of early life antibiotics and health outcomes: evidence from clinical studies. Semin. Perinatol. 44, 151322 (2020).
Wang, T. et al. Early life antibiotic exposure and host health: Role of the microbiota-immune interaction. Semin. Perinatol. 44, 151323 (2020).
Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Healthcare Quality Promotion (DHQP). Biggest threats and data: 2019 AR threats report. https://www.cdc.gov/drugresistance/biggest-threats.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fdrugresistance%2Fbiggest_threats.html (2019).
Hofer, N., Müller, W. & Resch, B. Non-infectious conditions and gestational age influence C-reactive protein values in newborns during the first 3 days of life. Clin. Chem. Lab Med. 49, 297–302 (2011).
Hillman, N. H., Kallapur, S. G. & Jobe, A. H. Physiology of transition from intrauterine to extrauterine life. Clin. Perinatol. 39, 769–783 (2012).
Cantey, J. B., Wozniak, P. S. & Sánchez, P. J. Prospective surveillance of antibiotic use in the neonatal intensive care unit: results from the SCOUT study. Pediatr. Infect. Dis. J. 34, 267–272 (2015).
Benitz, W. E. Adjunct laboratory tests in the diagnosis of early-onset neonatal sepsis. Clin. Perinatol. 37, 421–438 (2010).
National Institute for Health and Care Excellence. Neonatal infection: antibiotics for prevention and treatment (NG195). https://www.nice.org.uk/guidance/ng195 (2021).
Hofer, N., Zacharias, E., Müller, W. & Resch, B. An update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology 102, 25–36 (2012).
Macallister, K., Smith-Collins, A., Gillet, H., Hamilton, L. & Davis, J. Serial C-reactive protein measurements in newborn infants without evidence of early-onset infection. Neonatology 116, 85–91 (2019).
Sturgeon, J. P., Zanetti, B. & Lindo, D. C-reactive protein (CRP) levels in neonatal meningitis in England: an analysis of national variations in CRP cut-offs for lumbar puncture. BMC Pediatr. 18, 380–x (2018).
Dumpa, V. & Kamity, R. Birth Trauma. [Updated 2021 Sep 6]. In StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK539831/ (Treasure Island (FL), StatPearls Publishing, 2021).
Emmerson, A. J. C-reactive protein and the newborn infant. Arch. Dis. Child Educ. Pract. Ed. 96, e1 (2011).
Berger, C., Uehlinger, J., Ghelfi, D., Blau, N. & Fanconi, S. Comparison of C-reactive protein and white blood cell count with differential in neonates at risk for septicaemia. Eur. J. Pediatr. 154, 138–144 (1995).
Pourcyrous, M., Bada, H. S., Korones, S. B., Baselski, V. & Wong, S. P. Significance of serial C-reactive protein responses in neonatal infection and other disorders. Pediatrics 92, 431–435 (1993).
Wagle, S., Grauaug, A., Kohan, R. & Evans, S. F. C-reactive protein as a diagnostic tool of sepsis in very immature babies. J. Paediatr. Child Health 30, 40–44 (1994).
Kääpä, P. & Koistinen, E. Maternal and neonatal C-reactive protein after interventions during delivery. Acta Obstet. Gynecol. Scand. 72, 543–546 (1993).
Mjelle, A. B., Guthe, H. J. T., Reigstad, H., Bjørke-Monsen, A. L. & Markestad, T. Serum concentrations of C-reactive protein in healthy term-born Norwegian infants 48-72h after birth. Acta Paediatr. 108, 849–854 (2019).
Perrone, S. et al. C reactive protein in healthy term newborns during the first 48h of life. Arch. Dis. Child Fetal Neonatal Ed. 103, F163–F166 (2018).
Chiesa, C. et al. C reactive protein and procalcitonin: reference intervals for preterm and term newborns during the early neonatal period. Clin. Chim. Acta 412, 1053–1059 (2011).
Ishibashi, M., Takemura, Y., Ishida, H., Watanabe, K. & Kawai, T. C-reactive protein kinetics in newborns: application of a high-sensitivity analytic method in its determination. Clin. Chem. 48, 1103–1106 (2002).
Bellieni, C. V. et al. C-reactive protein: a marker of neonatal stress? J. Matern. Fetal Neonatal Med. 27, 612–615 (2014).
Thompson, A. L., Houck, K. M. & Jahnke, J. R. Pathways linking caesarean delivery to early health in a dual burden context: immune development and the gut microbiome in infants and children from Galápagos, Ecuador. Am. J. Hum. Biol. e23219 (2019).
Schmutz, N., Henry, E., Jopling, J. & Christensen, R. D. Expected ranges for blood neutrophil concentrations of neonates: the Manroe and Mouzinho charts revisited. J. Perinatol. 28, 275–281 (2008).
Hasan, R., Inoue, S. & Banerjee, A. Higher white blood cell counts and band forms in newborns delivered vaginally compared with those delivered by cesarean section. Am. J. Clin. Pathol. 100, 116–118 (1993).
Chirico, G., Gasparoni, A., Ciardelli, L., Martinotti, L. & Rondini, G. Leukocyte counts in relation to the method of delivery during the first five days of life. Biol. Neonate 75, 294–299 (1999).
Chan, C. J., Summers, K. L., Chan, N. G., Hardy, D. B. & Richardson, B. S. Cytokines in umbilical cord blood and the impact of labor events in low-risk term pregnancies. Early Hum. Dev. 89, 1005–1010 (2013).
Barug, D. et al. Reference values for interleukin-6 and interleukin-8 in cord blood of healthy term neonates and their association with stress-related perinatal factors. PLoS ONE 9, e114109 (2014).
Jokic, M. et al. Fetal distress increases interleukin-6 and interleukin-8 and decreases tumour necrosis factor-alpha cord blood levels in noninfected full-term neonates. BJOG 107, 420–425 (2000).
Malamitsi-Puchner, A. et al. The influence of the mode of delivery on circulating cytokine concentrations in the perinatal period. Early Hum. Dev. 81, 387–392 (2005).
Mukhopadhyay, S., Eichenwald, E. C. & Puopolo, K. M. Neonatal early-onset sepsis evaluations among well-appearing infants: projected impact of changes in CDC GBS guidelines. J. Perinatol. 33, 198–205 (2012).
Higgins, R. D. et al. Evaluation and management of women and newborns with a maternal diagnosis of chorioamnionitis: summary of a workshop. Obstet. Gynecol. 127, 426–436 (2016).
Mukhopadhyay, S. et al. Variation in sepsis evaluation across a national network of nurseries. Pediatrics 139, https://doi.org/10.1542/peds.2016-2845 (2017).
Turner, D. et al. Procalcitonin in preterm infants during the first few days of life: introducing an age related nomogram. Arch. Dis. Child Fetal Neonatal Ed. 91, 283 (2006).
Assumma, M. et al. Serum procalcitonin concentrations in term delivering mothers and their healthy offspring: a longitudinal study. Clin. Chem. 46, 1583–1587 (2000).
Mathai, E. et al. Is C-reactive protein level useful in differentiating infected from uninfected neonates among those at risk of infection? Indian Pediatr. 41, 895–900 (2004).
Chiesa, C. et al. C-reactive protein, interleukin-6, and procalcitonin in the immediate postnatal period: influence of illness severity, risk status, antenatal and perinatal complications, and infection. Clin. Chem. 49, 60–68 (2003).
Lee, J., Bang, Y. H., Lee, E. H., Choi, B. M. & Hong, Y. S. The influencing factors on procalcitonin values in newborns with noninfectious conditions during the first week of life. Korean J. Pediatr. 60, 10–16 (2017).
Goetzl, L., Evans, T., Rivers, J., Suresh, M. S. & Lieberman, E. Elevated maternal and fetal serum interleukin-6 levels are associated with epidural fever. Am. J. Obstet. Gynecol. 187, 834–838 (2002).
Chiesa, C. et al. Serial measurements of C-reactive protein and interleukin-6 in the immediate postnatal period: reference intervals and analysis of maternal and perinatal confounders. Clin. Chem. 47, 1016–1022 (2001).
Rallis, D. et al. C-reactive protein in infants with no evidence of early-onset sepsis. J. Matern. Fetal Neonatal Med. 1–8. https://doi.org/10.1080/14767058.2021.1888921 (2021). [Epub ahead of print].
Panero, A. et al. Interleukin 6 in neonates with early and late onset infection. Pediatr. Infect. Dis. J. 16, 370–375 (1997).
Henry, E. & Christensen, R. D. Reference intervals in neonatal hematology. Clin. Perinatol. 42, 483–497 (2015).
Newman, T. B., Puopolo, K. M., Wi, S., Draper, D. & Escobar, G. J. Interpreting complete blood counts soon after birth in newborns at risk for sepsis. Pediatrics 126, 903–909 (2010).
Jacobs, S. E. et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 2013, CD003311 (2013).
Hakobyan, M. et al. Outcome of infants with therapeutic hypothermia after perinatal asphyxia and early-onset sepsis. Neonatology 115, 127–133 (2019).
Rao, R. et al. Antimicrobial therapy utilization in neonates with hypoxic-ischemic encephalopathy (HIE): a report from the Children’s Hospital Neonatal Database (CHND). J. Perinatol. 40, 70–78 (2020).
Cantey, J. B. & Baird, S. D. Ending the culture of culture-negative sepsis in the neonatal ICU. Pediatrics 140, https://doi.org/10.1542/peds.2017-0044 (2017).
Okumuş, N. et al. Effect of therapeutic hypothermia on C-reactive protein levels in patients with perinatal asphyxia. Am. J. Perinatol. 32, 667–674 (2015).
Chakkarapani, E., Davis, J. & Thoresen, M. Therapeutic hypothermia delays the C-reactive protein response and suppresses white blood cell and platelet count in infants with neonatal encephalopathy. Arch. Dis. Child Fetal Neonatal Ed. 99, 458 (2014).
Martín-Ancel, A. et al. Interleukin-6 in the cerebrospinal fluid after perinatal asphyxia is related to early and late neurological manifestations. Pediatrics 100, 789–794 (1997).
Jenkins, D. D. et al. Serum cytokines in a clinical trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J. Cereb. Blood Flow. Metab. 32, 1888–1896 (2012).
Polderman, K. H. Mechanisms of action, physiological effects, and complications of hypothermia. Crit. Care Med. 37, 186 (2009).
Saito, J. et al. Temporal relationship between serum levels of interleukin-6 and c-reactive protein in therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy. Am. J. Perinatol. 33, 1401–1406 (2016).
Schuetz, P. et al. Serum procalcitonin, C-reactive protein and white blood cell levels following hypothermia after cardiac arrest: a retrospective cohort study. Eur. J. Clin. Invest. 40, 376–381 (2010).
Rath, S., Narasimhan, R. & Lumsden, C. C-reactive protein (CRP) responses in neonates with hypoxic ischaemic encephalopathy. Arch. Dis. Child Fetal Neonatal Ed. 99, F172 (2014).
Cilla, A. et al. Effect of hypothermia and severity of hypoxic-ischemic encephalopathy in the levels of C-reactive protein during the first 120h of life. Am. J. Perinatol. 37, 722–730 (2020).
Muniraman, H. et al. Biomarkers of hepatic injury and function in neonatal hypoxic ischemic encephalopathy and with therapeutic hypothermia. Eur. J. Pediatr. 176, 1295–1303 (2017).
Munteanu, A. I., Manea, A. M., Jinca, C. M. & Boia, M. Basic biochemical and hematological parameters in perinatal asphyxia and their correlation with hypoxic ischemic encephalopathy. Exp. Ther. Med. 21, 259 (2021).
Sun, B. et al. A meta-analysis of interleukin-6 as a valid and accurate index in diagnosing early neonatal sepsis. Int. Wound J. 16, 527–533 (2019).
Schelonka, R. L. et al. Volume of blood required to detect common neonatal pathogens. J. Pediatr. 129, 275–278 (1996).
Lancaster, D. P., Friedman, D. F., Chiotos, K. & Sullivan, K. V. Blood volume required for detection of low levels and ultralow levels of organisms responsible for neonatal bacteremia by use of bactec peds plus/F, plus aerobic/F medium, and the BD Bactec FX System: an in vitro study. J. Clin. Microbiol. 53, 3609–3613 (2015).
Frymoyer, A., Meng, L., Bonifacio, S. L., Verotta, D. & Guglielmo, B. J. Gentamicin pharmacokinetics and dosing in neonates with hypoxic ischemic encephalopathy receiving hypothermia. Pharmacotherapy 33, 718–726 (2013).
Tran, S. H., Caughey, A. B. & Musci, T. J. Meconium-stained amniotic fluid is associated with puerperal infections. Am. J. Obstet. Gynecol. 189, 746–750 (2003).
Oyelese, Y. et al. Meconium-stained amniotic fluid across gestation and neonatal acid-base status. Obstet. Gynecol. 108, 345–349 (2006).
Ward, C. & Caughey, A. B. The risk of meconium aspiration syndrome (MAS) increases with gestational age at term. J. Matern. Fetal Neonatal Med. 1–6 https://doi.org/10.1080/14767058.2020.1713744 (2020). [Epub ahead of print].
Lee, J. et al. Meconium aspiration syndrome: a role for fetal systemic inflammation. Am. J. Obstet. Gynecol. 214, 366.e1–366.e9 (2016).
Tyler, D. C., Murphy, J. & Cheney, F. W. Mechanical and chemical damage to lung tissue caused by meconium aspiration. Pediatrics 62, 454–459 (1978).
Jones, C. A. et al. Undetectable interleukin (IL)-10 and persistent IL-8 expression early in hyaline membrane disease: a possible developmental basis for the predisposition to chronic lung inflammation in preterm newborns. Pediatr. Res. 39, 966–975 (1996).
Cayabyab, R. G., Kwong, K., Jones, C., Minoo, P. & Durand, M. Lung inflammation and pulmonary function in infants with meconium aspiration syndrome. Pediatr. Pulmonol. 42, 898–905 (2007).
Davey, A. M., Becker, J. D. & Davis, J. M. Meconium aspiration syndrome: physiological and inflammatory changes in a newborn piglet model. Pediatr. Pulmonol. 16, 101–108 (1993).
de Beaufort, A. J. et al. Meconium is a source of pro-inflammatory substances and can induce cytokine production in cultured A549 epithelial cells. Pediatr. Res. 54, 491–495 (2003).
de Beaufort, A. J., Pelikan, D. M., Elferink, J. G. & Berger, H. M. Effect of interleukin 8 in meconium on in-vitro neutrophil chemotaxis. Lancet 352, 102–105 (1998).
Ochi, F. et al. Procalcitonin as a marker of respiratory disorder in neonates. Pediatr. Int. 57, 263–268 (2015).
Okazaki, K. et al. Serum cytokine and chemokine profiles in neonates with meconium aspiration syndrome. Pediatrics 121, 748 (2008).
Hofer, N., Jank, K., Strenger, V., Pansy, J. & Resch, B. Inflammatory indices in meconium aspiration syndrome. Pediatr. Pulmonol. 51, 601–606 (2016).
Basu, S., Kumar, A. & Bhatia, B. D. Role of antibiotics in meconium aspiration syndrome. Ann. Trop. Paediatr. 27, 107–113 (2007).
Lin, H. C., Su, B. H., Tsai, C. H., Lin, T. W. & Yeh, T. F. Role of antibiotics in management of non-ventilated cases of meconium aspiration syndrome without risk factors for infection. Biol. Neonate 87, 51–55 (2005).
Shankar, V., Paul, V. K., Deorari, A. K. & Singh, M. Do neonates with meconium aspiration syndrome require antibiotics? Indian J. Pediatr. 62, 327–331 (1995).
Kelly, L. E., Shivananda, S., Murthy, P., Srinivasjois, R. & Shah, P. S. Antibiotics for neonates born through meconium-stained amniotic fluid. Cochrane Database Syst. Rev. 6, CD006183 (2017).
Natarajan, C. K., Sankar, M. J., Jain, K., Agarwal, R. & Paul, V. K. Surfactant therapy and antibiotics in neonates with meconium aspiration syndrome: a systematic review and meta-analysis. J. Perinatol. 36(Suppl. 1), 49 (2016).
Wiswell, T. E. & Henley, M. A. Intratracheal suctioning, systemic infection, and the meconium aspiration syndrome. Pediatrics 89, 203–206 (1992).
Caglayan, F., Caglayan, O., Gunel, E. & Sahin, T. K. Monitoring the metabolic response to major surgery in neonates. Int J. Surg. Investig. 2, 309–312 (2000).
Günel, E., Cağlayan, O., Cağlayan, F. & Sahin, T. K. Acute-phase changes in children recovering from minor surgery. Pediatr. Surg. Int. 14, 199–201 (1998).
Buyukkocak, U. et al. Anaesthesia and the acute phase protein response in children undergoing circumcision. Mediat. Inflamm. 2005, 312–315 (2005).
Ramadan, G., Rex, D., Okoye, B. & Kennea, N. L. Early high C-reactive protein in infants with open abdominal wall defects does not predict sepsis or adverse outcome. Acta Paediatr. 99, 126–130 (2010).
Bölke, E. et al. Different acute-phase response in newborns and infants undergoing surgery. Pediatr. Res. 51, 333–338 (2002).
Williams, S. L. et al. Evaluation of early onset sepsis, complete blood count, and antibiotic use in gastroschisis. Am. J. Perinatol. 35, 385–389 (2018).
Pavcnik-Arnol, M., Bonac, B., Groselj-Grenc, M. & Derganc, M. Changes in serum procalcitonin, interleukin 6, interleukin 8 and C-reactive protein in neonates after surgery. Eur. J. Pediatr. Surg. 20, 262–266 (2010).
Aryafar, A. et al. Procalcitonin concentration measured within the first days of cardiac surgery is predictive of postoperative infections in neonates: a case-control study. Pediatr. Cardiol. 40, 1289–1295 (2019).
Arkader, R. et al. Procalcitonin does discriminate between sepsis and systemic inflammatory response syndrome. Arch. Dis. Child. 91, 117–120 (2006).
Neunhoeffer, F. et al. Serum concentrations of interleukin-6, procalcitonin, and c-reactive protein: discrimination of septical complications and systemic inflammatory response syndrome after pediatric surgery. Eur. J. Pediatr. Surg. 26, 180–185 (2016).
Brown, J. V. E., Meader, N., Wright, K., Cleminson, J. & McGuire, W. Assessment of C-reactive protein diagnostic test accuracy for late-onset infection in newborn infants: a systematic review and meta-analysis. JAMA Pediatr. 174, 260–268 (2020).
Singh, N. & Gray, J. E. Antibiotic stewardship in NICU: De-implementing routine CRP to reduce antibiotic usage in neonates at risk for early-onset sepsis. J. Perinatol. 41, 2488–2494 (2021).
Garber, S. J. et al. Delivery-based criteria for empiric antibiotic administration among preterm infants. J. Perinatol. 41, 255–262 (2021).
Janes, H. & Pepe, M. S. Adjusting for covariates in studies of diagnostic, screening, or prognostic markers: an old concept in a new setting. Am. J. Epidemiol. 168, 89–97 (2008).
Liu, D. & Zhou, X. H. ROC analysis in biomarker combination with covariate adjustment. Acad. Radiol. 20, 874–882 (2013).
Reinhart, K., Meisner, M. & Brunkhorst, F. M. Markers for sepsis diagnosis: what is useful? Crit. Care Clin. 22, 503–x (2006).
Keane, M., Fallon, R., Riordan, A. & Shaw, B. Markedly raised levels of C-reactive protein are associated with culture-proven sepsis or necrotising enterocolitis in extremely preterm neonates. Acta Paediatr. 104, 289 (2015).
Emerging Risk Factors Collaboration. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N. Engl J. Med. 367, 1310–1320 (2012).
Thompson, A. L. Caesarean delivery, immune function and inflammation in early life among Ecuadorian infants and young children. J. Dev. Orig. Health Dis. 10, 555–562 (2019).
Jiang, N. M. et al. Early life inflammation and neurodevelopmental outcome in bangladeshi infants growing up in adversity. Am. J. Trop. Med. Hyg. 97, 974–979 (2017).
Funding
S.M. receives funding from Eunice Kennedy Shriver National Institute of Child Health and Human Development from the National Institutes of Health grant (K23HD088753).
Author information
Authors and Affiliations
Contributions
C.T. conducted a literature review, wrote the first draft of the manuscript, and reviewed, revised, and approved the final manuscript. S.M. conceptualized the review, conducted a literature review, edited, revised, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tiozzo, C., Mukhopadhyay, S. Noninfectious influencers of early-onset sepsis biomarkers. Pediatr Res 91, 425–431 (2022). https://doi.org/10.1038/s41390-021-01861-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01861-4
This article is cited by
-
Paediatric and neonatal sepsis and inflammation
Pediatric Research (2022)