Abstract
LBW infants are at risk of iron deficiency (ID), which is associated with impaired nervous system development and may lead to prolonged auditory brainstem response (ABR) latencies. We hypothesized that iron supplementation shortens ABR latencies in marginally LBW (MLBW, 2000-2500 g) infants. In a randomized, controlled trial, 285 healthy MLBW infants received 0, 1, or 2 mg iron/kg/d of iron supplements from 6 wk to 6 mo of age. ABR absolute wave V latencies and central conduction time (CCT) were measured at the endpoint. There were no significant differences between groups in ABR wave V latencies (n = 218). Furthermore, there were no significantly prolonged ABR latencies in infants with ID (n = 32). CCT was significantly higher in the 2 mg group than in the placebo group (n = 126). However, there were no significant correlations between CCT and iron intake or any iron status variable, suggesting that differences in CCT were not caused by iron. We conclude that iron supplements did not improve ABR latencies, and iron-deficient MLBW infants did not have impaired ABR latencies at 6 mo, suggesting that ABR is not a sensitive measure of impaired neurological development or that mild/moderate ID causes no such impairment in MLBW infants.
Similar content being viewed by others
Main
Iron deficiency (ID) is the most common single nutrient deficiency worldwide, and infants are at particular risk (1). Iron plays an important role in the development of the CNS and is essential to neural myelination and neurotransmitter function (2). Iron deficiency anemia (IDA) during infancy is associated with poor neurological development (3,4), even though causality has not been proven in randomized trials.
Auditory brainstem response (ABR) is a noninvasive, objective, neurophysiologic method, for assessing the development of the CNS. ABR latencies measure conduction speed in the auditory system from the cochlea to the inferior colliculus in the upper brainstem. These latencies decrease rapidly from late gestation through the first 3–6 mo of life and continue to decrease at a slower rate during the first 3–5 y of life, reflecting CNS myelination in preterm and term infants during infancy. Wave I, III, and V latency and I-V interpeak latency [central conduction time (CCT)] have been suggested as useful measures of this process, because they are the most easily identifiable and reproducible (5). The maturation of these latencies is closely correlated to GA (6–8) and not affected by late preterm birth (9). Roncagliolo et al. (10) tested the hypothesis that prolonged ABR latencies could be an early indicator of CNS impairment caused by IDA and showed an association between IDA at 6 mo and prolonged wave V latencies and CCT at 6, 12, and 18 mo of age. In a follow-up trial at 4 y of age, the differences in ABR latencies remained, particularly in absolute wave V latencies, suggesting that IDA may contribute to long-lasting impairment of nervous system development (11).
The use of iron supplementation for prevention or treatment of IDA in infants has been evaluated in many trials. In addition to decreasing the risk of anemia, it has been suggested to improve cognitive and behavioral development in iron deficient infants (4,12). However, humans have no active mechanism for iron excretion, and the risk of iron overload has to be considered. It has been reported by several investigators, that iron supplementation of iron replete infants may have adverse effects, e.g. increased risk of infections and impaired growth (12–14).
LBW (<2500 g) infants are generally considered a risk group for ID during the first 6 mo of life because of low iron stores at birth and rapid growth, and the World Health Organization recommend iron supplements for all LBW infants (15). However, >60% of LBW infants are born with marginally LBW (MLBW, 2000–2500 g), and it has not been determined in clinical trials, whether this group benefits from early iron supplementation (16–18). MLBW infants include both small for GA (SGA) term infants and preterm (GA <37 wk) infants. The risks and benefits of iron supplementation could theoretically be different between those two groups because they are at slightly different stages of brain maturation.
This is a part of a randomized trial investigating the effect of iron supplementation in MLBW infants. Laboratory, growth, and morbidity outcomes are reported elsewhere. In brief, we found a high prevalence of ID and IDA in unsupplemented MLBW infants at 6 mo (36 and 10%, respectively), a prevalence that was effectively reduced in infants supplemented with 2 mg/kg/d from 6 wk to 6 mo of age (4 and 0%, respectively) (19). The aims of the present study were to investigate the short-term neurophysiologic effects of iron supplementation, and test the hypothesis that iron supplements, compared with placebo, improve (shorten) the absolute wave V latencies and the CCT in both preterm and term MLBW infants.
METHODS
Study design and participants.
In a randomized, controlled, double blinded intervention trial, we examined the effect of iron supplementation given to MLBW infants from 6 wk to 6 mo of age. The study was conducted between March 2004 and June 2007 at two Swedish centers: Umeå University Hospital, Umeå and Karolinska University Hospital, Stockholm. The inclusion criteria were birth weight, 2000-2500 g; no diseases; and no previous blood transfusion or iron supplementation. Exclusion criteria were anemia (Hb <90 g/L) or other hematological disorder diagnosed at 6 wk. Eligible infants were identified from delivery records, and parents were contacted at the hospital or at home and given written and oral information. Parents accepting participation gave written informed consent. The study was approved by the Ethical Review Boards at Umeå University and the Karolinska Institute and registered with ClinicalTrials.gov, number NCT00558454.
Intervention.
Included infants were stratified for sex and study center and randomized into three groups receiving different doses of iron supplements: 0 mg/kg/d (placebo), 1 mg/kg/d, or 2 mg/kg/d. The iron supplement was ferrous succinate mixture (Ferromyn S; Astra Zeneca, Södertälje, Sweden) containing 3.7 mg/mL of iron, and the individual doses of iron supplements/placebo were adjusted for infant weight at 12 and 19 wk. The placebo mixture was prepared by Apoteket Production & Laboratories, Stockholm, and had similar taste and color. Investigators and parents were blinded to the treatment assignment using randomization codes, as described in detail elsewhere (19). To monitor compliance, parents were asked to register all doses given in a daily calendar and bottles were weighed before and after use. Poor compliance was defined as <70% of doses given.
Data collection.
Infants visited the study center at 6, 12, 19 wk, and 6 mo postnatal age. The following anthropometric data were collected at each visit: naked weight using a digital baby scale, length using a wooden measuring board, head circumference using a plastic measuring tape, and knee-heel length using a knemometer.
Venous blood samples were collected at 6 wk, 12 wk, and 6 mo, and analyzed for Hb, mean cell volume (MCV), ferritin, transferrin saturation (TS), and transferrin receptor concentration (TfR) as described elsewhere (19).
Anemia was defined as Hb <90 g/L at 6 wk, Hb <95 g/L at 12 wk, and Hb <105 g/L at 6 mo (20,21). ID at 6 mo was defined when at least two of four of the following indicators of iron status were outside the following cutoffs: ferritin <12 μg/L, MCV <71fl, TS <10%, and TfR >11 μg/L (20–23). IDA was defined as the combination of anemia and ID. Infants with confirmed anemia at 12 wk were referred to a pediatrician for further evaluation and treatment. Anemic infants at 12 wk, who were prescribed iron due to suspected IDA, discontinued the intervention but continued data collection and the results were included in the analyses according to intention to treat.
Auditory brainstem response.
At 6 mo, ABR latencies were measured. The tests were performed using Interacoustics EP25 ABR system (Interacoustics A/S, Assens, Denmark) in Stockholm and Nicolet Viking II (Nicolet Biomedical, Inc, Madison) in Umeå. All children were first tested by otoacoustic emissions (OAE ILO88; Otodynamiscs Ltd, Hatfield, United Kingdom) to ensure middle and inner ear function. The examinations were performed without sedatives and with the children awake or spontaneously at sleep. Signals were recorded using ipsilateral stimuli of 1250 square wave clicks at 80 dB and 19.8 Hz. To ensure reproducibility, at least two curves were registered, and the one with the best signal quality was used (Fig. 1). The absolute wave I and V latencies were registered, and the CCT was calculated as the difference between them. Both the right and left ear were tested and mean values were used. If only a unilateral measurement was obtained, this was used.
Sample size and statistical analysis.
All analyses were performed on an intention to treat basis. In a priori power calculations, a minimum sample size of 64 in each randomization group was estimated to detect a difference at 6 mo of 0.1 ms in mean CCT, with an expected mean (SD) of 4.55 (0.2) ms (10), using a power of 80% and a significance level of 0.05. Assuming a dropout rate of 20% and an additional poor compliance rate of 15%, a group size of 95 in each group was chosen as target for enrollment.
SD scores (SDS) for anthropometric data were calculated, corrected for GA at birth, using a Swedish growth reference (24). Statistical analyses were performed using PASW statistics for Windows 17.0 (March 11, 2009; SPSS, Inc.,Chicago). Two factor ANOVA was used when comparing means. To explore possible confounders, baseline and background variables were tested using univariate regression analyses. When comparing groups, a multivariate model using analysis of covariance (ANCOVA) was constructed, correcting for significant confounding background and baseline variables. Extreme outliers (outside ± 3 SD) for measurements of wave I and V latencies were considered erroneous and excluded from the analyses.
RESULTS
Out of 285 included infants, 18 were excluded (16 because of anemia at 6 wk, 1 because of beta thalassemia, and 1 because of AB0 immunization at birth) and 24 (8%) dropped out of the study. Nine infants (3%) were given unblinded iron at 12 wk because of suspected IDA. Poor compliance was observed in 62 infants (23%). As reported elsewhere, there were no significant differences between the groups in any background or baseline characteristic or in compliance (19). Of included infants, 148 (55%) were preterm and GAs at birth ranged from 31 to 40 completed weeks.
Wave V latencies were successfully measured in 223 infants, 182 in Stockholm and 41 in Umeå. Four of those were excluded as extreme outliers (two from the placebo group and two from the 2 mg/kg/d group). Unfortunately, because of movement artifacts in these nonsedated infants, a measurement of wave I latency, necessary for the calculation of CCT, was only obtained in 131 cases, whereof 5 were excluded as extreme outliers (2 from the placebo group, 2 from the 1 mg/kg/d group, and 1 from the 2 mg/kg/d group). The mean (SD) postnatal age at ABR analysis was 188 (18) d. Background and baseline characteristics for the 223 infants with ABR measures are described in Table 1. There were no significant differences between the groups in any of those variables, neither in those with a successful CCT measure (data not shown).
Mean wave I and V latencies differed significantly between the two study centers. Mean (SD) wave V latency was 6.44 (0.44) ms in Umeå and 6.01 (0.23) ms in Stockholm (p < 0.001). Mean (SD) wave I latency was 1.93 (0.14) ms in Umeå and 1.40 (0.14) ms in Stockholm (p < 0.001). To exclude differences in curve evaluation as a reason for this, the ABR curves from both study centers were all evaluated by one of us for a second opinion. All curve evaluations were considered correct and the differences were thus explained by the fact that different devices were used. In univariate regression analyses of possible confounders, sex (r = +0.16; p = 0.015) and head circumference at 6 mo (r = +0.22; p = 0.001) were significantly correlated to wave V latencies. In a bivariate regression analysis including both these confounders, the effect of head circumference remained significant (r = +0.18; p = 0.016) but not the effect of sex (p = 0.272). No other possible confounders tested were found significantly correlated to wave V latency in univariate analyses, including postmenstrual age at examination (p = 0.303), baseline ferritin (p = 0.370), and being SGA at birth SGA (p = 0.604). A multivariate model was constructed including sex, head circumference at 6 mo, and study center and used for the analyses of group effects. In addition, postmenstrual age at examination was included in the model, because it has been reported to be associated with ABR latencies in other trials (6–8).
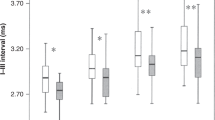
Multivariate analyses of the overall group effect on ABR latencies and stratified analyses for preterm and term infants separately are summarized in Table 2 and Table 3. There were no significant differences or trend toward differences between the groups in absolute wave I or wave V latencies. The group effect for infants born at term was similar to those born preterm. All analyses were repeated, controlling for baseline ferritin without changing the results (data not shown). Unexpectedly, CCT was significantly increased in a dose-dependent fashion for infants receiving iron drops, prompting further multivariate analyses. In those, we found no significant correlation between CCT and any of the four iron status variables (ferritin, MCV, TS, and TfR), Hb at 6 mo, or baseline ferritin, using the multivariate model described above, and analyzing all groups together. Nor did we find a significant correlation between CCT and total actual iron intake or actual iron intake from supplements, calculated as previously described (19). In the subgroup with complete CCT registrations (n = 126), the same dose-dependent effect in iron supplemented infants was found on wave V latency (p = 0.017), and an opposite, nonsignificant trend was observed in the subgroup where no CCT registration had been obtained (n = 91). However, we found no significant baseline differences between these two subgroups. Furthermore, we performed similar analyses per protocol, excluding infants with poor compliance (n = 60) and infants given unblinded supplements from 12 wk (n = 9). In the per protocol analyses, there was no longer a significant difference in CCT between groups. Mean CCT was 4.49 ms in the placebo group and 4.60 ms in the 2 mg group (n = 92; p = 0.206). Finally, to explore possible adverse effects of iron supplements on ABR latencies in infants with higher compared with lower iron stores at baseline, stratified analyses were performed based on baseline ferritin. Infants with baseline ferritin below the 25th and above the 75th percentile, respectively, were compared (Table 4). There was no significant interaction and not even a tendency to a negative effect of iron on CCT in the group with higher baseline ferritin.
In a similar multivariate model, the ABR latencies in the subgroups developing ID and IDA at 6 mo were compared with those not developing ID and IDA, respectively (Table 5). There was no significant group difference in CCT or absolute wave I or V latencies.
DISCUSSION
This is the first randomized trial investigating the effect of preventive iron supplementation of MLBW infants. In this report, we focus on short-term neurophysiologic effects and evaluate the use of ABR as an early indicator of impaired neurologic development.
Roncagliolo et al. compared 29 infants with IDA at 6 mo with 26 nonanemic controls (mean Hb 95 versus 122 g/L). They observed a nonsignificant trend toward a prolonged CCT at 6 mo among the anemic infants (CCT 4.65 versus 4.55 ms), a difference which became significant at 12 and 18 mo in spite of iron treatment. Results for wave V latencies were very similar as for CCT. Roncagliolo et al. (10) suggested that the prolonged latencies were caused by delayed CNS myelination in infants with early IDA. Similar results have been reported by Cankaya et al. (25), who recorded ABR at 12 mo and found significantly prolonged CCT in 13 infants with IDA compared with 15 controls (4.58 versus 4.20 ms; p = 0.002).
In the present study, we found no significant differences in absolute ABR latencies between groups, even though the iron supplementation resulted in a significant difference in prevalence of ID and IDA (19). The trial was powered to detect a group difference of 0.1 ms, which was the difference in wave V latencies and CCT, presented by Roncagliolo et al. at 6 mo. However, even though the difference in prevalence of ID was large in this trial (35% in placebo versus 4% in the 2 mg-group) still almost two-thirds of infants in the placebo group did not develop ID, and similarly even a larger proportion did not develop IDA. Theoretically this would imply that the trial was underpowered, if only the infants with diagnosed ID would have an impaired ABR maturation. However, even when we specifically compared those infants in our trial who had IDA (or ID), we did not observe prolonged latencies compared with non-IDA infants, and a post study power calculation using the actual number of IDA and non-IDA infants shows that a difference of 0.2 ms in wave V latency would have been detected with a power of 80%. This and the fact that there was not even a trend of prolonged latencies in the placebo group suggests that the reason for negative findings with regard to absolute latencies was not that the study was underpowered, but rather that there was no impairment of ABR latencies in these unsupplemented MLBW infants at 6 mo. For this, there are several possible explanations. First, it may suggest that the severity or duration of ID in our infants was not of enough magnitude to result in prolonged ABR latencies. Second, the ABR maturation of these infants may not have been in a vulnerable phase when ID occurred. The exact period of vulnerability with regard to effects of iron on brain development in infants remains to be determined. Because ABR maturation follows GA, independent of the time of birth, the preterm infants of this trial are at a different stage of maturation during the intervention, compared with term infants. However, we did not observe any trend toward prolonged ABR latencies in unsupplemented term or preterm infants. Finally, there might possibly be a delayed effect of ID on ABR latencies, occurring after the actual exposure. This was suggested by Roncagliolo et al. (10) who observed group differences in ABR latencies that increased and became significant only 6–12 mo after treatment of IDA. However, there is no known mechanism that would explain such a delayed effect. Furthermore, in contrast to Roncagliolo et al., we did not even find a trend at 6 mo. Therefore, it must be considered that our negative results also could suggest that there is no true association between IDA and prolonged ABR latencies. Such an association has never been shown in randomized controlled trials and, similar to us, others have found negative results. Kürekçi et al. (26) found no significant differences in ABR latencies between 25 infants with IDA, 24 with ID, and 44 controls (mean Hb 81, 119, and 125 g/L, respectively). Moreover, Sarici et al. (27) found no difference in ABR latencies between 20 IDA infants (Hb 88 g/L) and 20 controls (Hb 119 g/L) at 6–24 mo.
Instead, in contrast to the hypothesis, we unexpectedly found longer CCTs in the 2 mg group. This could be interpreted as an adverse effect of iron supplements. However, there are several reasons to reject that interpretation. First, if the CCT was truly increased, the wave V latency would be equally prolonged. We observed no significant effect on wave V latencies, for which the number of observations was much higher. Second, if iron supplements would cause prolonged CCT because of excessive iron load, we would expect to find a significant positive association between CCT and iron status at 6 mo, which was not the case. Third, if prolonged CCT was an adverse effect of iron supplements, we would expect to see an even more pronounced effect when the analysis was performed per protocol as opposed to intention to treat. However, the per protocol analyses showed a smaller and nonsignificant group difference in CCT. Furthermore, there was no significant association between CCT and actual iron intake or iron intake from supplements. Finally, if iron supplements caused prolonged CCT because of excessive iron intake, this effect would be more pronounced in infants with initially higher iron stores. However, there was no association between CCT and baseline ferritin and in the stratified analyses the effect on CCT was not increased but rather less pronounced in infants with baseline ferritin above the 75th percentile. Thus, we find no evidence that the group difference in CCT was related to iron and the lack of a difference in wave V latency suggests that the group difference in CCT is most likely a type I error.
It is well known that preterm infants have prolonged ABR latencies, decreasing with GA (8). It has been shown in several studies that the maturation rate of ABR latencies in infants is relatively rapid up to 6 mo postterm and closely correlated to postmenstrual age. The ABR latencies decrease most rapidly prenatally and during the first months of life but continue to decrease until 3–5 y of age (6). Furthermore, it has been shown that the event of late preterm birth does not affect the maturation rate, because after correcting for GA, latencies are similar between late preterm infants and those born at term (9). The infants of this trial include both preterm and term infants, ranging from 31 to 40 complete weeks of gestation and a corresponding range of 57 to 74 postmenstrual weeks at the time for ABR. Theoretically, preterm and term infants could have responded differently to the intervention because they are at different stages of auditory nerve maturation and myelinization. They could be more or less vulnerable to an insult (ID or iron overload), depending on the postmenstrual age during the intervention. Furthermore, depending on the reason for LBW, the SGA infants might be more vulnerable for adverse outcomes if insults during late pregnancy had compromised CNS development. However, we found no differences in outcomes between term SGA and preterm infants. The effects were similar in both term and preterm infants for all outcomes. Even though the unexpected finding with regard to CCT was only significant in the term group, there was a similar trend in preterm infants and there was no interaction (p = 0.544).
An important observation was the positive correlation between ABR outcomes and head circumference at 6 mo, i.e. the larger the head, the longer the latencies. The finding was independent of sex and supports previous observations by others (28). In previous studies of ABR latencies in IDA infants, head circumference has generally not been considered. We suggest that this confounding factor should be measured and carefully considered in future studies of ABR latencies in iron deficient infants.
There are some limitations of this trial. First, the number of infants with measurable CCT was fewer than planned, making it difficult to interpret the CCT findings. Second, mean ABR latencies differed significantly between the two study sites, using two different devices. We believe that his was because of differences in calibration, even though both devices were calibrated according to the manufacturers' instructions. However, the main results of the study remained when statistically controlling for study site and also when analyzing each study site separately (data not shown). Third, ABR was not performed at baseline. Theoretically, some infants could have impaired ABR latencies already at the study start, i.e. caused by prenatal or perinatal damage such as asphyxia or prenatal ID. Such baseline confounders, if distributed unequally between the randomization groups could possibly have affected the results. However, baseline and background data were very similar between the groups, also in the subgroup with measurable CCT. Furthermore, no infants were diagnosed with asphyxia and as a part of the study design; all infants with anemia at baseline were excluded. Together, this suggests that baseline ABR latencies, even though not measured, are not likely to have confounded our results.
In summary, we found no positive or adverse effects of iron supplementation on absolute wave I or V latencies at 6 mo in MLBW infants, even though they are at risk for ID and IDA. Prolonged latencies were also not observed in infants developing ID or IDA. Our results could suggest that ABR is not a sensitive measure of impaired neurological development in MLBW infants or that mild/moderate ID causes no such impairment at 6 mo.
Abbreviations
- ABR:
-
auditory brainstem response
- ANCOVA:
-
analysis of covariance
- CCT:
-
central conduction time
- ID:
-
iron deficiency
- IDA:
-
iron deficiency anemia
- MCV:
-
mean cell volume
- MLBW:
-
marginally LBW (2000–2500 g)
- TfR:
-
transferrin receptor concentration
- TS:
-
transferrin saturation
References
World Health Organization 2001 Iron deficiency anaemia: assessment, prevention and control. A guide for programme managers. Available at: http://www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf. Accessed May 28, 2009
Beard JL 2008 Why iron deficiency is important in infant development. J Nutr 138: 2534–2536
Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T 2006 Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev 64: S34–S43
Sachdev H, Gera T, Nestel P 2005 Effect of iron supplementation on mental and motor development in children: systematic review of randomised controlled trials. Public Health Nutr 8: 117–132
Wilkinson AR, Jiang ZD 2006 Brainstem auditory evoked response in neonatal neurology. Semin Fetal Neonatal Med 11: 444–451
Eggermont JJ 1992 Development of auditory evoked potentials. Acta Otolaryngol 112: 197–200
Eggermont JJ, Salamy A 1988 Development of ABR parameters in a preterm and a term born population. Ear Hear 9: 283–289
Starr A, Amlie RN, Martin WH, Sanders S 1977 Development of auditory function in newborn infants revealed by auditory brainstem potentials. Pediatrics 60: 831–839
Jiang ZD 1995 Maturation of the auditory brainstem in low risk-preterm infants: a comparison with age-matched full term infants up to 6 years. Early Hum Dev 42: 49–65
Roncagliolo M, Garrido M, Walter T, Peirano P, Lozoff B 1998 Evidence of altered central nervous system development in infants with iron deficiency anemia at 6 mo: delayed maturation of auditory brainstem responses. Am J Clin Nutr 68: 683–690
Algarín C, Peirano P, Garrido M, Pizarro F, Lozoff B 2003 Iron deficiency anemia in infancy: long-lasting effects on auditory and visual system functioning. Pediatr Res 53: 217–223
Iannotti LL, Tielsch JM, Black MM, Black RE 2006 Iron supplementation in early childhood: health benefits and risks. Am J Clin Nutr 84: 1261–1276
Dewey KG, Domellof M, Cohen RJ, Landa Rivera L, Hernell O, Lonnerdal B 2002 Iron supplementation affects growth and morbidity of breast-fed infants: results of a randomized trial in Sweden and Honduras. J Nutr 132: 3249–3255
Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, Dhingra U, Kabole I, Deb S, Othman MK, Kabole FM 2006 Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet 367: 133–143
Edmond K, Bahl R 2006 Optimal feeding of low-birth-weight infants: technical review. World Health Organization, Geneva. Available at: http://whqlibdoc.who.int/publications/2006/9789241595094_eng.pdf. Accessed November 20, 2008
Office for National Statistics 2008 Birth Statistics: Review of the National Statistician on Births and Patterns of Family Building in England and Wales, 2007. Office for National Statistics, Newport. Series FM1 No. 36. Available at: http://www.statistics.gov.uk/downloads/theme_population/FM1_36/FM1-No36.pdf. Accessed April 4, 2009
United Nations Children's Fund (UNICEF) 2008 The State of the World's Children 2009: Maternal and Newborn Health. United Nations Children's Fund, New York. Available at: http://www.unicef.org/sowc09/docs/SOWC09-FullReport-EN.pdf. Accessed May 28, 2009
Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, Munson ML, Centers for Disease Control and Prevention National Center for Health Statistics National Vital Statistics System 2007 Births: final data for 2005. Natl Vital Stat Rep 56: 1–103
Berglund S, Westrup B, Domellof M 2010 Iron supplements reduce the risk of iron deficiency anemia in marginally low birth weight infants. Pediatrics 126: e874–e883
Domellöf M, Dewey KG, Lönnerdal B, Cohen RJ, Hernell O 2002 The diagnostic criteria for iron deficiency in infants should be reevaluated. J Nutr 132: 3680–3686
Saarinen UM, Siimes MA 1978 Developmental changes in red blood cell counts and indices of infants after exclusion of iron deficiency by laboratory criteria and continuous iron supplementation. J Pediatr 92: 412–416
Saarinen UM, Siimes MA 1977 Developmental changes in serum iron, total iron-binding capacity, and transferrin saturation in infancy. J Pediatr 91: 875–877
Saarinen UM, Siimes MA 1978 Serum ferritin in assessment of iron nutrition in healthy infants. Acta Paediatr Scand 67: 745–751
Niklasson A, Albertsson-Wikland K 2008 Continuous growth reference from 24th week of gestation to 24 months by gender. BMC Pediatr 8: 8
Cankaya H, Oner AF, Egeli E, Caksen H, Uner A, Akcay G 2003 Auditory brainstem response in children with iron deficiency anemia. Acta Paediatr Taiwan 44: 21–24
Kürekçi AE, Sarici SU, Karaoglu A, Ulas UH, Atay AA, Serdar MA, Akin R, Ozcan O 2006 Effects of iron deficiency versus iron deficiency anemia on brainstem auditory evoked potentials in infancy. Turk J Pediatr 48: 334–339
Sarici SU, Serdar MA, Dundaroz MR, Unay B, Akin R, Deda G, Gokcay E 2001 Brainstem auditory-evoked potentials in iron-deficiency anemia. Pediatr Neurol 24: 205–208
Ponton CW, Eggermont JJ, Coupland SG, Winkelaar R 1993 The relation between head size and auditory brain-stem response interpeak latency maturation. J Acoust Soc Am 94: 2149–2158
Acknowledgements
We thank the following staff for dedicated field work and data collection: Kerstin Andersson in Stockholm, Ruth-Gerd Larsson, Åsa Sundström, Margareta Bäckman, and Astrid Pettersson in Umeå. We also thank Olle Hernell for scientific advice and support and Bo Lönnerdal for help with laboratory analyses, Hugo Lagercrantz for support of the Stockholm part of the study and Hans Stenlund for statistical advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by grants from the Swedish Research Council Formas 222-2005-1894, Västerbotten County Council (ALF), the Jerring foundation, the Oskar foundation, and the Medical Faculty, Umeå University. The iron drops were provided from Astra Zeneca, Sweden.
The authors report no conflicts of interest.
Rights and permissions
About this article
Cite this article
Berglund, S., Westrup, B., Haraldsson, E. et al. Effects of Iron Supplementation on Auditory Brainstem Response in Marginally LBW Infants. Pediatr Res 70, 601–606 (2011). https://doi.org/10.1203/PDR.0b013e3182320cd0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e3182320cd0
This article is cited by
-
Reference intervals for reticulocyte hemoglobin content in healthy infants
Pediatric Research (2018)