Abstract
We investigated whether supplementation of regular formula (RF) with cholesterol (Ch) (RF+Ch) influenced circulating Ch levels and de novo synthesis compared with their breast-fed (BF) counterparts in 4-mo-old infants. The incorporation rate of deuterium in body water into erythrocyte membrane-free Ch over 48 h was used as an index of cholesterogenesis. Plasma total-Ch and LDL-Ch concentrations were highest(p < 0.02) in BF infants, compared with infants in the RF-fed groups. Infants in the RF+Ch groups showed an intermediate response; their plasma total-Ch and LDL-Ch concentrations were not significantly different from the BF or the RF-fed groups. Plasma total/HDL-Ch and LDL/HDL-Ch ratios were higher (p < 0.05) in BF, and higher in RF+Ch-fed infants, compared with those fed RF, whereas not different between BF and RF+Ch-fed infants. At 4 mo of age, Ch FSR was 4-fold lower(p < 0.0001) in BF versus other groups, but not significantly different between RF- and RF+Ch-fed infants. Thus, despite addition of Ch to the concentration found in breast milk, FSR remained elevated compared with that of the group fed breast milk, with an intermediate response in circulating Ch levels. It is speculated that factors other than Ch intake account for the differential Ch metabolism between formula-fed and BF infants.
Similar content being viewed by others
Main
It has been well documented that infants fed human milk have higher plasma total- and LDL-Ch concentrations than those fed formula(1–8). This difference has been attributed to the greater Ch content of human milk compared with commercial formulas(8–10). Human milk contains significant quantities of Ch (0.26-0.28 mmol/L, 10-11 mg/dL). In contrast, cow's milk-based formulas possess lesser amounts (0.08-0.13 mmol/L, 3-5 mg/dL) of Ch, whereas soya-based formulations have none. In infants, a low Ch intake (0.09 mmol/L, 3.5 mg/dL) appears to up-regulate endogenous Ch synthesis(5,6). Infants fed human milk, with typically 3-fold higher Ch content compared with cow's milk-based commercial formulas currently available, appear to down-regulate cholesterogenesis(5,6).
The long-term implications of manipulating Ch synthesis and Ch transported by lipoproteins in plasma, in developing human infants, have not been established. Barker's retrospective studies(11–13) of men born in Hertfordshire, England, to identify environmental risk factors for cardiovascular disease, suggest a relationship(11) between type and duration of infant feeding, adult serum Ch concentration, and mortality from ischemic heart disease. Some authors(14,15) suggest that differences in plasma Ch concentrations in BF and formula-fed infants are transient in nature and do not manifest themselves past the age of 12 mo. However, others have reported lower serum Ch levels at ages 7-12 y(16) in children previously fed a low Ch formula as infants, and conversely, lower concentrations in women 30-50 y of age(17) previously BF as infants. In theory, smaller central pools of Ch later in life may contribute to reduced LDL-Ch concentrations, thereby potentially reducing cardiovascular disease risk.
Several studies of Ch supplementation of infant formulas, but using lower amounts of Ch than that found in human milk have reported higher plasma Ch concentrations(5–7,18–20) and lower cholesterogenesis(5,6) rates than their unsupplemented counterparts. One study from Finland(21) reported that infants fed formula supplemented with taurine + Ch had lower serum Ch concentrations when compared with those fed unsupplemented formula or BF, even though the formula contained as much Ch as did human milk. However, Ch supplementation of infant formula alone, in amounts identical to that found in human breast milk, have not been previously studied in terms of its effect on cholesterogenesis. Whether the differences in Ch content between breast milk and cow's milk-based formula are responsible for the dissimilar plasma lipid profile and FSR observed between BF and formula-fed infants have not been established.
We therefore conducted a prospective partially randomized controlled study evaluating the effects of Ch fortification of cow's milk-based infant formula, to human milk levels, on circulating Ch concentration and Ch synthesis rates to determine whether Ch synthesis rates and circulating Ch concentrations are influenced by dietary Ch intake when fatty acid composition is identical. We hypothesized that, at 4 mo of age, endogenous Ch FSR of infants fed RF+Ch, modified to contain Ch similar to amounts found in human milk, will be similar to the FSR of infants who are BF and will be less than the FSR of infants fed RF [schematically (FSR) BF ≈ RF+Ch < RF]. As an ancillary hypothesis, we theorized that, at 4 mo of age, plasma Ch concentrations of infants fed RF+Ch or BF will be similar, and both will be greater than those of infants fed RF.
METHODS
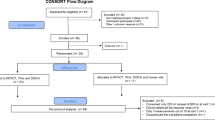
Study population and protocol. Thirty-seven healthy infants(19 male, 18 female) who were full-term, appropriate for gestational age, and had no parental history of hypercholesterolemia or hypertriglyceridemia were recruited from the normal newborn nurseries of the University Hospital of Cincinnati and other area hospitals. Fourteen infants exclusively BF for the first 4 mo of life comprised the human milk-fed group. Twenty-three were randomized according to a computer-generated random numbers table to receive RF (SMA® ready to serve iron fortified, 0.85 mmol Ch/L, 33 mg Ch/L; Wyeth-Ayerst Laboratories, Philadelphia, PA) (n = 11) or RF+Ch(SMA® ready to serve iron fortified, 3.44 mmol Ch/L, 133 mg/L Ch; Wyeth-Ayerst Laboratories) (n = 12). Crystalline-free Ch powder was dissolved in components oils, forming an emulsion, before combining macronutrient ingredients. Analysis of a random batch of RF+Ch was made before distribution to the infants to assure that the Ch contents were within 13 ± 2 mg/dL. The nutrient compositions of formulas are summarized in Table 1. All infants received their formula within the first 3-7 d of life. Formula was provided free to the formula-fed infants for the entire duration of the study to ensure compliance. The study was approved by the Institutional Review Boards of all involved hospitals, and informed consent was obtained from parents before enrollment of the infants. Thirty-two infants completed the study. There was insufficient blood drawn to determine FSR in one infant from the BF group, two from the RF group, and two from the RF+C group.
Mothers from the three groups kept 3-d diet diaries for each month until the testing period at 4 mo. Mothers recorded information regarding the frequency of breast-feeding, the volume of supplemental RF per day, and the volume of formula per day in the formula groups. The diets were analyzed by a registered dietitian experienced in the analysis of these diaries, to determine whether any significant disparity occurred among the formula groups that may have confounded the outcome variable of FSR. By design, other forms of nutrition such as cereal were not allowed, except for multivitamins.
Plasma lipid analysis. Determinations of serum Ch and lipid profiles were performed at the Medical Research Laboratories in Cincinnati, OH, with enzymatic techniques validated by the National Institutes of Health Lipid Research Clinics. In the presence of Mn2+ and heparin, chylomicrons, VLDL, and LDL were selectively precipitated, leaving only HDL in solution. The precipitated lipoproteins were sedimented by centrifugation, leaving the clear supernate containing HDL-Ch that was then analyzed enzymatically. Triglycerides were also determined enzymatically. LDL-Ch concentrations were calculated from serum total Ch concentrations with the equation formulated by Friedewald et al.(22), used previously by Cruz et al.(6) with infants.
Cholesterol biosynthesis measurements. Ch synthesis rates were determined at 4 mo of age. This age was chosen because infants in the first 4 mo of life generally receive exclusively human milk or formula(23), so little potential existed for interference from other diets. Thus, differences in FSR and lipid profiles could be specifically attributed to the type of milk consumed. Measurements were performed over 48 h. On d 1, 8 mL of blood were obtained to determine baseline body water deuterium enrichment. Infants were then orally given 500 mg/kg body weight of deuterium oxide (D2O, 99.96% deuterium; Isotec Inc., Miamisburg, OH). On d 2, 8 mL of blood were obtained for deuterium enrichment measurement, after which 50 mg/kg body weight of D2O was given orally to maintain constant body water deuterium enrichment. On d 3, a final 8-mL blood sample was obtained. All blood samples were drawn between 0900 and 1100 h.
Ch FSRs were determined as the rate of incorporation of deuterium into the red blood cell membrane(24). Erythrocyte lipids were extracted by organic solvent and dried under nitrogen. Free Ch was isolated by thin-layer chromatography, then eluted from thin-layer chromatography silica scrapings and quantitatively transferred to Pyrex combustion tubes containing CuO and a silver wire. Tubes were flame-sealed under vacuum, and Ch was combusted completely at 520°C to CO2 and water. Combustion water was cryogenically separated from CO2 by vacuum distillation into Pyrex tubes containing 50 mg of zinc. These were flame-sealed under vacuum, and the water was reduced to H2 at 520°C. Deuterium enrichment of the resultant gas was measured by dual inlet isotope ratio mass spectrometry (VG Isogas 903D). Plasma-water enrichment was measured after dilution of 24- and 48-h plasma samples with water of known isotopic abundance to bring the enrichment into the working range of the International Atomic Energy Agency (Vienna) mass spectrometer calibration standards.
Erythrocyte Ch deuterium enrichment values at 24 and 48 h were expressed relative to the corresponding mean plasma water sample enrichment at each time point, after correction for the deuterium-protium ratio in Ch, to yield FSRs (pool/day) for the free Ch pool. The FSR index represents that fraction of the free portion of the rapidly turning over central Ch pool that is synthesized in 24 h as per the formula(24): FSR(%/day) = (δch/δplasma) × 0.478 × 100 where δ refers to deuterium enrichment above baseline over 24 h. The factor of 0.5 is used to yield a daily FSR from each respective 48-h time period.
Statistical analysis. One-way ANOVA was used to test differences among groups(25). The Tukey-Kramer method for multiple comparisons was used to determine differences between pairs of groups(25). General linear models were used to determine correlations between outcome variable (FSR) and the independent variables. Statistical significance was considered for p values less than 0.05. Results are presented as mean ± SEM.
RESULTS
Demographic data are summarized in Table 2. There were no differences in birth weight and length, weight, and ponderal index at 4 mo across treatment groups. Nor were differences observed in volume of milk intake among the formula groups as determined from dietary records.
ANOVA indicated that plasma total-Ch (p < 0.02) and LDL-Ch concentrations were higher (p < 0.04) in BF (4.31 ± 0.21, 2.27 ± 0.23 mmol/L), compared with RF-fed (3.39 ± 0.23, 1.21 ± 0.18 mmol/L) groups (Fig. 1; Table 3). There was an intermediate response in plasma total-Ch and LDL-Ch concentrations (3.83 ± 0.60, 1.78 ± 0.22 mmol/L) for infants fed RF+Ch. The difference between the RF+Ch-fed and BF, or RF-fed groups did not reach significance. Total/HDL-Ch ratios were higher(p < 0.02) in infants BF (3.79 ± 0.24) and higher(p < 0.05) in infants fed RF+Ch (3.52 ± 0.24), compared with those fed RF (2.83 ± 0.23) (Fig. 2); whereas not significantly different between BF and RF+Ch-fed infants. Similarly, LDL/HDL-Ch ratios were higher (p < 0.002) in infants BF (1.99 ± 0.23), and higher (p < 0.03) in infants fed RF+Ch (1.63 ± 0.24), compared with those fed RF (1.02 ± 0.23)(Fig. 3); whereas not significantly different between BF and RF+Ch-fed infants. No differences were observed in serum HDL-Ch and triglyceride concentrations among groups.
Deuterium enrichments of body water and erythrocyte Ch are shown in Figure 4. Deuterium enrichment in body water was similar across treatment groups. Erythrocyte Ch deuterium enrichment was consistently lower in BF compared with formula-fed groups (Fig. 4). No difference in erythrocyte Ch enrichment was observed between RF and RF+Ch-fed groups.
Deuterium enrichments of plasma water(outset) (BF infants, ○ RF-fed infants, ♦; RF+Ch-fed infants,▴;) and erythrocyte Ch (inset) (BF infants, ○; RF-fed infants,♦; RF+Ch-fed infants, ▴). Dotted line as determined previously by Jones et al.(24).
Ch FSR of RF (8.58 ± 0.27%/d), and RF+Ch (8.29 ± 0.37%/d), were elevated (p < 0.0001) compared with BF infants (2.19± 0.29%/d), whereas FSR did not differ significantly between RF and RF+Ch groups (Fig. 5).
DISCUSSION
The main objective of the present work was to examine the effects of Ch fortification of cow's milk-based formula, in amounts similar to that found in human milk, upon circulating Ch concentrations and in vivo synthesis during early infancy. We demonstrated increased cholesterogenesis and a modest response in circulating plasma Ch concentrations in formula-fed infants compared with BF infants, as a result of dietary Ch fortification to levels found in breast milk.
The issue of whether dietary Ch in human milk is beneficial, benign, or detrimental to infants has been a topic of speculation for several decades(1–21). Progress in this area has been hindered by the difficulty in separating the effects of dietary Ch feeding from those of dietary fatty acids when a BF group is used as a control group. Because individual fatty acids may have significant direct effects on Ch metabolism(27,28), and the fatty acid composition of formula cannot be made identical to the fatty acid composition of human milk, no direct inferences with regard to the effect of fatty acids on Ch metabolism can be made in the present study.
Feedback inhibition of Ch synthesis mediated by changes in the activity of the rate-limiting step of Ch biosynthesis, hepatic hydroxymethylglutaryl CoA reductase, has been demonstrated by dietary Ch feeding in guinea pigs, hamsters, and pigs(29–31). In humans, the inhibitory effect of dietary Ch feeding on cholesterogenesis has been variable, with studies reporting both alterations(32–34) or no effect(35–37). The variable results of these former studies may be attributed to several factors, including the type and amount of dietary Ch and fat included in the diet and the efficacy of the methodologies used.
To our knowledge, only two previous studies examining Ch synthesis as a function of dietary Ch intake have been reported in infants(5,6). Using deuterium incorporation techniques, previous investigators(5,6) have estimated that endogenous Ch synthesis ranges from 2 to 11%/d in human infants BF or fed formula with varying concentrations of Ch and disparate fatty acid profiles. Both research groups attributed the differences in FSR to quantity of dietary Ch, reporting quantity of Ch intake to be significantly and negatively associated with FSR (p > 0.0001), although acknowledging the role that fatty acids, phytosterols, phytoesterogens, and hormones may have in mediating serum lipid concentration and endogenous Ch metabolism. These previous studies(5,6) did not specifically supplement Ch intake in formula-fed infants to the level found in breast milk. We conducted the study in a population of 4-mo-old infants fed identical formula, with 0.85 mmol Ch/L (33 mg Chl/L) or 3.44 mmol Ch/L (133 mg Ch/L). The effect of dietary Ch on endogenous Ch synthesis rates was specifically determined by eliminating confounding dietary variables; energy, protein, lactose, fat, and in particular linoleic acid consumption were not different between the formula groups.
In the present study, similar plasma total- and LDL-Ch concentrations and higher FSRs occurred in formula-fed infants, regardless of dietary Ch fortification, compared with BF infants. Wong et al.(5) and Cruz et al.(6) found lower serum total- and LDL-C concentrations and higher FSRs in formula-fed infants, that was dietary Ch dose-dependent, compared with BF infants. Moreover, infants fed soy formula supplemented with Ch to the level found in cow's milk-based formula had lower FSRs than those fed unsupplemented soy formula(6), suggesting the Ch component did suppress endogenous Ch synthesis. In the present study, infants fed cow's milk-based formula supplemented with Ch to the level found in human milk had FSRs that were not different from those fed regular cow's milk-based formula, suggesting that other factors may explain the differences between cholesterogenesis in breast- and formula-fed infants. Moreover it appears, from the plasma lipid results reported, that compared with infants BF or RF-fed, infants fed RF+Ch have an intermediate response to Ch fortification, suggestive of a modest effect.
In the present study small blood volumes precluded more extensive kinetic analysis, such as absorption, inter- and intra-pool transfer rates, absolute synthetic rates, and catabolic rates. Correspondingly, as FSR was the main outcome variable in the present study, fecal Ch excretion was not measured. However, increased Ch efflux appears as the most likely mechanistic hypothesis to explain the insignificant rise in plasma Ch despite increased dietary Ch in the RF+Ch-fed group. Fecal Ch excretion was measured in preterm infants(7) who received standard preterm formula with 30 mg of Ch/dL, compared with those fed the same formula (5.5 mg/dL Ch) or fortified breast milk (mean Ch content 15.3 mg/dL). The group fed the higher Ch formula had higher Ch excretion and Ch balance (35.5 mmol kg-1 d-1; +21.8 mg kg-1 d-1) than in the groups fed breast milk (20.1 mmol kg-1 d-1; +8.6) or the standard formula (18.2 mmol kg-1 d-1; 7.7 mg kg-1 day-1). The process enabling preterm infants to regulate a higher Ch intake than during breast feeding by increasing fecal Ch excretion, as suggested by Boehm et al.(7), may be operating similarly in the RF+Ch-fed infants in the present study, explaining the similar rates of cholesterogenesis observed compared with those RF-fed.
Our results and those previously reported(6) may also be explained in part by differences in quantity of Ch supplementation and Ch solubility in the respective formulations based on fatty acid profile. Differential intestinal absorption of Ch based on dietary fatty acid type and position in triacylglycerol for fats ingested concomitantly has been observed in rats(38–41), hamsters(42), and baboons(43) with no effect in rabbits(44), whereas similar work in humans is lacking. From work in baboons(43), it is has been suggested that Ch absorption and hepatic Ch concentration regulate plasma Ch responses to diet, but by different mechanisms. Furthermore, dietary lipid induced changes in intestinal morphology and nutrient uptake may or may not be reversible, affecting the ability of the intestine to adapt to an altered nutrient intake in later life(41). Similar to previous work(6) we controlled for the effects of fatty acids on Ch biosynthesis by feeding identical formula and varying only the concentrations of Ch.
The possibility that Ch added to the formula in our study was not absorbed cannot be ruled out. Absorption of supplemental Ch in the form administered in the diet may have direct bearing on the rates of cholesterogenesis reported in this study. Plasma Ch concentration comprises less than 9% of total body Ch(45). Ch absorption from the gastrointestinal tract is an integral component of whole body homeostasis. Enterohepatic recirculation of endogenous Ch readily mixes with dietary Ch to form a single pool of intestinal Ch(46). Gastrointestinal absorption of dietary Ch by adults is about 45-50%(47–50). Samuel and McNamara(48), comparing the absorption of endogenous and exogenous Ch in adults, found endogenous Ch absorption to be 46 ± 15%, and exogenous 34 ± 8%. Similar to those investigations, the current study used only nonesterified Ch. It should be noted that endogenous(biliary) Ch is entirely non-esterified Ch. So far, data describing Ch absorption in human infants have not been reported.
Nonesterified microcrystalline Ch added to commercial formula is ingested as a component of an emulsion, whereas dietary Ch in breast milk is unique, being ingested as a physiologic structural component. Thus, different transport medium for dietary Ch may have some bearing on bioavailability. Furthermore, Van Lier et al.(51) cautions that Ch will oxidize rather rapidly if an aqueous phase is present and that the stability of Ch in an emulsion may be suspect. The majority of Ch in human milk is nonesterified; 80% of this Ch is found in the outer milk fat globule membrane, which is formed predominantly from the plasma membrane of epithelial cells in the breast(52). The central core of the milk fat globule membrane contains 15-20% of total Ch as esters(53). It is necessary for Ch esters to be hydrolyzed before absorption, and only nonesterified Ch is absorbed, whereas the intact esters are not. Pancreatic Ch esterase catalyzes the hydrolysis. It does not seem reasonable that a Ch ester would be better absorbed than would nonesterified Ch, unless the exogenous Ch exceeds the solubility limit of the dietary oil. It is unlikely that 15-20% of total Ch in breast milk as esters would explain the Ch synthesis and plasma lipoprotein Ch differences in the current study.
The Ch content of breast milk during infancy is relatively constant across populations when age of development is considered(52). Similar to FSRs that were previously reported, 2.1%/d by Wong et al.(5), 2.62%/d by Cruz et al.(6) for BF infants at 4 mo of age, the FSR of BF infants in our study was 2.19%/d. Lipoprotein Ch concentrations we report are also similar to those previously reported(5,6) in BF infants. Composition of breast milk varies with stage of lactation and among mothers. Breast milk Ch concentration was 2.82-4.52 mmol/L (n = 6) for the Wong et al. report(5) and 2.59-3.88 mmol/L (n = 13) for the Cruz et al.(6) study. These similar results in two different groups of BF infants, coupled with our results in infants fed identical formula supplemented with Ch in amounts similar to that in breast milk, provide strong evidence that dietary Ch appears to be a factor responsible for the differences in circulating Ch concentrations, but does not fully explain the variations in cholesterogenesis observed(5,6) when infants are fed formula. The results of our study appear not to support the theory that adaptive regulatory mechanisms in early infancy enable human infants to respond to changes in Ch intakes within the physiologic range. The teleologic argument, that such homeostatic mechanisms would prevent excess Ch accumulation during breast-feeding or, conversely, provide for an increase in Ch availability during instances of lower or negligible intake, during formula feeding, is not wholly consistent with the results observed during our study. No evidence to date would disprove the assumption that breast milk is the gold standard for infant nutrition, and that values for FSR in BF infants are considered to be the norm. The apparent need for Ch in early infancy, a period rapid of growth, is reflected by the almost 4-fold difference in FSR for supplemented and unsupplemented infants compared with BF infants. This increase, however, appears to be irrespective of direct Ch supplementation. A possible interpretation would be that crystalline-free, food grade Ch supplementation of infant formula has a modest effect on circulating lipoprotein concentrations, and no effect on Ch synthesis in human infants at 4 mo of age. This interpretation assumes no differences in Ch absorption among the three feeding groups.
Methods for endogenous Ch synthesis determinations based on deuterium incorporation have been well established(24,54–56), offering the advantage of being direct, short-term, and noninvasive over intake balance methods(57–60) or isotopic kinetic decay analyses(45,61–63). The efficacy of using erythrocyte Ch deuterium enrichment to study human lipid metabolism was first reported by London and Schwartz(64). Subsequently, use of deuterium incorporation into newly synthesized erythrocyte membrane free Ch, based on the tritiated water uptake methodology developed in animals(65), was further refined and developed for measurement of short-term Ch synthesis in humans(24,66). The interpretation of results depends upon three primary assumptions that have been well described previously by Jones(24,66). The first is that a constant fraction of deuterium atoms in free Ch synthesized de novo originates from plasma water. Because water freely and rapidly diffuses across cell membranes, plasma water deuterium concentration provides a measure of precursor enrichment, and deuterium enrichment of the newly synthesized Ch molecules corresponds to enrichment of the central pool, which includes the liver, plasma, and intestine. A second assumption is that, within the central pool, rapid movement of newly synthesized Ch from the liver to plasma lipoproteins and then to cell membranes occurs, and therefore the deuterium content of erythrocyte membranes reflects newly synthesized Ch. The third assumption is that deuterium uptake by cell membranes represents synthesis from the central pool only, and it does not represent influx of newly formed Ch from other pools or synthesis by erythrocytes.
Recently Ch synthesis was quantified using two independent methods. Daily whole body Ch balance average over several days agreed with incorporation of deuterium oxide into newly synthesized Ch over a 24-h period(67). The correlation between the two methods was r = 0.745 (p < 0.001). Deuterium incorporation into erythrocyte membrane Ch is predicted to provide a reasonable measure of FSR, predicated upon extensive simulations of the free Ch central pool defined by other investigators(62,68).
Previous approaches to determining Ch FSR used both linear(54,69) and monoexponential(28,55,70) constrained-fit models. An assumption of these models is that, at constant precursor enrichment, as seen in Figure 4, body-Ch deuterium-enrichment plateaus at about one-half of the body-water deuterium-enrichment concentration(71). To avoid complications of recycled Ch and mixing between the rapidly miscible and the other two slower turning over pools, enrichment data from earlier linear phase time points provide the most reliable indexes of uptake for FSR calculation. Enrichment patterns are linear over the initial portion, 0-48 h, of this curve(5,6,70) as evidenced by our erythrocyte deuterium-enrichment data in Figure 4. Similar infant feeding studies(5,6) show a relatively linear erythrocyte deuterium-enrichment pattern with an asymptote in some subjects after 40 h.
The lower FSR in the BF group could potentially be viewed as an artifact resulting from a dilution effect. The higher dietary Ch intakes and plasma Ch concentrations of BF infants might possibly cause a higher Ch content in the central pool, generating a dilution effect on calculations of synthesis rates. This is unlikely, given that the central pool of BF infants is probably 15-20% larger compared with formula-fed infants, based on previous piglet studies(72). Moreover, as demonstrated in adults(55,70) and in infants fed either human milk or formula(73,74), erythrocyte membrane Ch is fairly constant regardless of diet. The 15-20% difference in the central pool between BF and formula-fed groups and the 15% difference in plasma Ch concentrations are therefore neither sufficient to explain nor proportionate to the almost 4-fold difference in FSR rates among groups. It has been theorized that approximately half the difference in FSR between the BF and formula-fed groups can be explained by an expanded central pool(5). The remaining difference in FSR might be due to down-regulation of hepatic hydroxymethylglutaryl-CoA reductase and Ch synthesis. It has also been suggested that expansion of the central pool is probably due to the increased absorption of dietary Ch in the BF infants coupled with a down-regulation of LDL-receptor activity in the liver(75,76). Our results showing no difference in FSR and circulating Ch concentrations between RF and RF+Ch formula-fed infants do not support the contention of an expanded central pool attributable to dietary Ch intake.
In summary, we have examined for the first time the in vivo endogenous Ch synthesis rates of human infants fed identical formula with and without crystalline food grade free Ch in amounts similar to human milk, and have established that Ch synthesis rates are not affected by this form of Ch supplementation.
We conclude that crystalline food grade-free Ch supplementation of infant formula affects lipid profiles modestly, but not Ch synthesis rates. These findings support the view that dietary Ch is not the sole component of human breast milk causing feedback inhibition of Ch biosynthesis in human infants. From these findings we speculate also that Ch added to cow's milk formula may not be well absorbed.
Acknowledgments. The authors thank Kathryn Pramuk, M.S., R.D., Wyeth-Ayerst, for aid in this study.
Abbreviations
- RF :
-
regular formula
- Ch :
-
cholesterol
- BF :
-
breast-fed
- FSR :
-
fractional synthetic rate
References
Carlson SE 1991 Plasma cholesterol and lipoprotein levels during fetal development and infancy. Ann NY Acad Sci 623: 81–89.
Jooste PL, Rossouw LJ, Steenkamp HJ, Rossouw JE, Swanepoel ASP, Charlton DO 1991 Effect of breast-feeding on the plasma cholesterol and growth of infants. J Pediatr Gastroenterol Nutr 13: 139–142.
Innis SM, Hamilton JJ 1992 Effects of developmental changes and early nutrition on cholesterol metabolism in infancy. J Am Col Nutr 11: 63S–68S.
Kali MJT, Salmenpera L, Siimes MA, Perheentupa J, Miettinen TA 1992 Exclusive breast-feeding and weaning: effect on serum cholesterol and lipoprotein concentration in infants during the first year of life. Pediatrics 89: 663–666.
Wong WW, Hachey DL, Insull W, Opeckun AR, Klein PD 1993 Effect of dietary cholesterol on cholesterol synthesis in breast-fed and formula-fed infants. J Lipid Res 34: 1403–1411.
Cruz MLA, Wong WW, Mimouni F, Hachey DL, Setchell KDR, Klein PD, Tsang R 1994 Effects of infant nutrition on cholesterol synthesis rates. Pediatr Res 35: 135–140.
Boehm G, Moro G, Muller DM, Muller H, Raffler G, Minol I 1995 Fecal cholesterol excretion in preterm infants fed breast milk of formula with different cholesterol contents. Acta Pediatr 84: 240–244.
Mize CE, Uauy R, Kramer R, Benser M, Allen S, Grundy SM 1995 Lipoprotein-cholesterol responses in healthy infants fed defined diets from ages 1 to 12 months: comparison of diets predominant in oleic acidversus linoleic acid, with parallel observations in infants fed a human milk-based diet. J Lipid Res 36: 1178–1187.
Friedman G, Goldberg SJ 1975 Concurrent and subsequent serum cholesterols of breast- and formula-fed infants. Am J Clin Nutr 28: 42–45.
Huttenen JK, Saarinen UM, Kostiainen E, Siimens MA 1983 Fat composition of the infant diet does not influence subsequent serum lipid levels in man. Atherosclerosis 46: 87–94.
Fall CH, Barker DJ, Osmond C, Winter PD, Clark PM, Hales CN 1992 Relation of infant feeding to adult serum cholesterol concentration and death from ischaemic heart disease. BMJ 304: 801–805.
Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME 1989 Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 298: 564–567.
Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ 1989 Weight in infancy and death from ischaemic heart disease. Lancet 2: 577–580.
Fomon SJ, Rogers RR, Ziegler EE, Nelson SE, Thomas LN 1984 Indices of fatness and serum cholesterol at eight years in relation to feeding and growth during early infancy. Pediatr Res 18: 1233–1238.
Wagner V, von Stockhausen HB 1988 The effect of feeding human milk and adapted milk formulae on serum lipids and lipoprotein levels in young infants. Eur J Pediatr 147: 292–295.
Hodgson PA 1976 Comparison of serum cholesterol in children fed high, moderate, or low cholesterol milk diets during the neonatal period. Metabolism 25: 739–746.
Marmont MG, Page CM, Atkins E, Douglas JWB 1980 Effect of breast-feeding on plasma cholesterol and weight in young adults. J Epidemiol Community Health 34: 164–167.
Watkins JB, Jarvenpaa A-L, Szczepanik-Van Leeuwen PA, Klein RD, Rassin DK, Gaull G, Raiha, NCR 1983 Feeding the low birth weight infant. V. Effects of taurine, whole sterol, and human milk on bile acid kinetics. Gastroenterology 85: 793–800.
Jarvenpaa AL, Raiha NCR, Rassin DK, Gaull GE 1983 Feeding the low-birth-weight infant. I. Taurine and cholesterol supplementation of formula does not affect growth and metabolism. Pediatrics 71: 171–178.
Van Biervliet J-P, Vinaimont N, Vercaemst R, Rosseneu M 1992 Serum cholesterol, cholesteryl ester, and high-density lipoprotein development in newborn infants: response to formula supplemented with cholesterol and gamma-linoleic acid. J Pediatr 120: S101–S108.
Rassin DK, Gaull GE, Jarvenpaa A-L, Raiha NC 1983 Feeding of the low-birth-weight infants. II. Effect of taurine and cholesterol supplementation on amino acids and cholesterol. Pediatrics 71: 179–186.
Friedewald RT, Levy RI, Frederickson P 1972 Estimation of the concentration of low density lipoprotein concentration without use of preparative ultracentrifuge. Clin Chem 18: 499–502.
Purvis GA, Bartholmey S 1988 Infant feeding practices commercially prepared baby foods. In: Tsang RC, Nichols BL (eds) Nutrition during Infancy. Hanley Belfus, Philadelphia, 399–417.
Jones PJH, Leitch CA, Li ZC, Connor WE 1993 Human cholesterol synthesis measurement using deuterated water: theoretical and procedural considerations. Arterioscler Thromb 13: 247–253.
Zar BH 1984 Biostatistical Analysis, 2nd Ed. Prentice-Hall, Englewood Cliffs, NJ, pp
Lubchenco LO, Hansman C, Boyd E 1966 Intrauterine growth in length and head circumference. Pediatrics 37: 403–408.
Grundy SM, Ma D 1990 Dietary influences on serum lipids and lipoproteins. J Lipid Res 31: 1149–1172.
Mazier MJ, Jones PJH 1997 Diet fat saturation and feeding state modulate rates of cholesterol synthesis in normo-lipidemic men. J Nutr 127: 332–340.
Turley SD, West CE 1976 Effect of cholesterol and cholestryamine feeding and of fasting on sterol synthesis in the liver, ileum, and lung of the guinea pig. J Lipids 11: 571–577.
Shady DK, Deutsche JM 1988 Interaction of dietary cholesterol and triglyceride in the regulation of hepatic low density lipoprotein transport in the hamster. J Clin Invest 81: 300–309.
Jones PJH, Hrboticky N, Hahn P, Innis SM 1990 Comparison of breast-feeding and formula-feeding on intestinal and hepatic cholesterol metabolism in neonatal pigs Am J Clin N. utr 51: 979–984.
Nestel PJ, Poyser A 1976 Changes in cholesterol synthesis and excretion when cholesterol intake is increased. Metabolism 25: 1591–1599.
McNamara DJ, Kolb R, Parker TS, Batwin H, Samuel P, Brown CD, Ahrens EH Jr 1987 Heterogeneity of cholesterol homeostasis in man: response to changes in dietary fat quality and cholesterol quantity. J Clin Invest 79: 1729–1739.
Jones PJH, Pappu AS, Hatcher L, Li ZC, Illingsworth DR, Connor WE 1996 Dietary cholesterol feeding suppresses human cholesterol synthesis measured by deuterium incorporation and urinary mevalonic acid levels. Arterioscler Thromb Vasc Biol 16: 10–16.
Wilson JD, Lindsey CA 1965 Studies on the influence of dietary cholesterol on cholesterol metabolism in steady state in man. J Clin Invest 44: 1805–1814.
Grundy SM, Ahrens EH, Davignon J 1969 The interaction of absorption and cholesterol synthesis in man. J Lipid Res 10: 304–315.
Jones PJH, Lichtenstein AH, Schaefer EJ 1994 Interaction of dietary fat saturation and cholesterol level on cholesterol synthesis measured using deuterium incorporation. J Lipid Res 35: 1093–1101.
Chen IS, Hotta SS, Ikeda I, Cassidy MM, Shepard AJ, Vahouny GV 1987 Digestion, absorption and effects on cholesterol absorption of menhaden oil, fish oil concentrate and corn oil by rats. J Nutr 117: 676–680.
Kamei M, Ohgaki S, Kanbe T, Niiya I, Mizutani H, Matsui-Yuasa I, Otani S, Morita S 1995 Effects of highly hydrogenated soybean oil and cholesterol on plasma, liver cholesterol, and fecal steroids in rats. Lipids 30: 533–539.
Thomson AB, Keelan M, Llam T, Rajotte RV, Garg ML, Clandinin MT 1993 Fish oil modifies effects of high cholesterol diet on intestinal absorption in diabetic rats. Diabetes Res 22: 171–183.
Thomson AB, Keelan M, Cheng T, Clandinin MT 1993 Delayed effects of early nutrition with cholesterol plus saturated or polyunsaturated fatty acids on intestinal morphology and transport function in the rat. Biochim Biophys Acta 1170: 80–91.
Berr F, Goetz A, Schreiber E, Paumgartner G 1993 Effects of dietary n-3 versus n-6 polyunsaturated fatty acids on hepatic excretion of cholesterol in the hamster. J Lipid Res 34: 1275–1284.
Kushwaha, RS, Rice KS, Lewis DS, McGill HC Jr, Carey KD 1993 The role of cholesterol absorption and hepatic cholesterol content in high and low responses to dietary cholesterol and fat in pedigreed baboons. Metabolism 42: 714–722.
Meijer, GW, Lemmens AG, Versluis A, Van Zutphen LF, Beynen AC 1991 The hyper-cholesterolemic effect of dietary coconut fatversus corn oil in hypo- or hyperresponsive rabbits is not exerted through influencing cholesterol absorption. J Lipid Res 1980: 699–713.
Goodman DS, Smith FR, Seplowitz AH, Ramakrishnan R, Dell RB 1980 Prediction of the parameters of whole body cholesterol metabolism in humans. J Lipid Res 21: 699–713.
Grundy, SM 1983 Absorption and metabolism of dietary cholesterol. Ann Rev Nutr 983: 71–76.
Zilversmit DB 1973 A proposal linking atherogenesis to the interaction of endothelial lipoprotein lipase with triglyceride-rich lipoproteins. Circ Res 33: 633–638.
Samuel P, McNamara DJ 1983 Differential absorption of exogenous and endogenous cholesterol in man. J Lipid Res 24: 265–276.
Miettinen TA, Kesaniemi YA 1989 Cholesterol absorption: regulation of cholesterol synthesis and elimination and within-population variations of serum cholesterol levels. Am J Clin Nutr 49: 629–635.
Jandacek RJ, Ramirez MM, Crouse JR 1990 Effects of partial replacement of dietary fat by olestra on dietary cholesterol absorption in man. Metabolism 39: 848–852.
Van Lier JE, Smith LL 1969 Chromatography of some cholesterol autoxidation products on Sephadex. J Chromatogr 41: 37–42.
Jensen RG 1989 The Lipids of Human Milk. CRC Press, Boca Raton, FL, 187–192.
Bitman J, Wood DL, Mehta NR, Hamosh P, Hamosh M 1986 Comparison of the cholesteryl ester composition of human milk from preterm and term mothers. J Pediatr Gastroenterol Nutr 5: 780–786.
Jones PJH, Scanu AM, Schoeller DA 1988 Plasma cholesterol synthesis using deuterated water in humans: effect of short-term food restriction. J Clin Lab Med 111: 627–633.
Wong WW, Hachey DL, Feste A, Leggit J, Clarke LL, Pond WG, Klein PD 1991 Measurement of in vivo cholesterol synthesis form 2H2O: a rapid procedure for the isolation, combustion, and isotopic assay of erythrocyte cholesterol. J Lipid Res 32: 1049–1056.
Jones PJH, Lichtenstein, AH, Schaefer EJ 1994 Interaction of dietary fat saturation and cholesterol level on cholesterol synthesis measured using deuterium incorporation. J Lipid Res 35: 1093–1101.
Gamble JL, Blackfan K 1920 Evidence indicating a synthesis of cholesterol by infants. J Biol Chem 1920: 42:4401-409
Huang CTL, Rodriguez JT, Woodward WE, Nichols B 1976 Comparison of patterns of fecal bile acid and neutral sterols between children and adults. Am J Clin Nutr 29: 1196–1203.
Potter JM, Nestel P 1976 Greater bile acid excretion with soy bean than with cow milk in infants. Am J Clin Nutr 29: 546–551.
Nestel PJH, Poser A, Bolton T 1979 Changes in cholesterol metabolism in infants in response to dietary cholesterol and fat. Am J Clin Nutr 32: 2177–2182.
Ferezou J, Rautueau J, Coste T, Gouffier E, Chevalier F 1983 Cholesterol turnover in human plasma lipoproteins: studies with stable and radioactive isotopes. Am J Clin Nutr 36: 235–244.
Dell RB, Ramakrishnan R, Palmer RH, Goodman DS 1985 A convenient six-point blood sampling schedule for determining whole body cholesterol kinetics in humans. J Lipid Res 26: 575–582.
Schwartz CC, Zech LA, VandenBroek JM, Cooper PS 1993 Cholesterol kinetics in subjects with bile fistula: positive relationship between size of the bile acid precursor pool and bile acid synthetic rate. J Clin Invest 91: 923–938.
London IM, Schwartz H 1953 Erythrocyte cholesterol: the metabolic behaviour of the cholesterol of human erythrocytes. J Clin Invest 32: 1248–1252.
Dietschy JM, Spady DK 1984 Measurement of rates of cholesterol synthesis in vivo using tritiated water. J Lipid Res 25: 1469–1476.
Jones PJH 1990 Use of deuterated water for measurement of short-term cholesterol synthesis in humans. Can J Physiol Pharmacol 68: 955–959.
Jones PJH, Ausman LM, Croll DH, Feng JY, Schaefer EA, Lichtenstein AH 1998 Validation of deuterium incorporation against sterol balance for measurement of human cholesterol biosynthesis. J Lipid Res (in press)
Schwartz CC, Vlahcevic ZR, Berman M, Meadows J 1965 Central role of high density lipoprotein in plasma free cholesterol metabolism. J Clin Invest 70: 105–116.
Jones PJH, Schoeller DA 1990 Evidence for diurnal periodicity in human cholesterol synthesis. J Lipid Res 31: 667–673.
Leitch CA, Jones PJH 1991 Measurement of triglyceride synthesis in humans using deuterium oxide and isotope ratio mass spectrometer. Biol Mass Spectrom 20: 392–396.
Jones PJH, Leitch CA, Pederson RA 1993 Meal-frequency effects on plasma hormone concentrations and cholesterol synthesis in humans. Am J Clin Nutr 57: 868–874.
Hachey DL, Wong WW, Pond W, Clark L, Llaurador M, Klein P 1992 Cholesterol synthesis in genetically lean and obese piglets on either low or high cholesterol diets. FASEB J 6: 1091
DeLucchi C, Pita ML, Periago JL, Gil, A 1988 Influences of diet and postnatal age on the lipid composition of red blood cell membrane in newborn infants. Ann Nutr Metab. 32: 231–239.
Putnam JC, Carlson SE, DeVoe PW, Barness, L 1983 The effect of variations in dietary fatty acids on the fatty acid composition of erythrocyte phosphatidylcholine and phosphatidylethanolamine in human infants. Am J Clin Nutr 36: 106–114.
Cai H-J, Xie C-L, Chen Q, Chen X-Y, Chen Y-H 1991 The relationship between hepatic low-density lipoprotein receptor activity and serum cholesterol level in the human fetus. Hepatology 13: 852–857.
Uauy, R, Mize C, Grundy S 1989 Effect of early diet on plasma lipoproteins (L) and LDL receptor activity at 1 year of age. Pediatr Res 25: 126A
Author information
Authors and Affiliations
Additional information
Supported by the American Heart and Stroke Foundation, the Cincinnati Children's Hospital Clinical Research Center, and Wyeth-Ayerst Laboratories.
Rights and permissions
About this article
Cite this article
Bayley, T., Alasmi, M., Thorkelson, T. et al. Influence of Formula versus Breast Milk on Cholesterol Synthesis Rates in Four-Month-Old Infants. Pediatr Res 44, 60–67 (1998). https://doi.org/10.1203/00006450-199807000-00010
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199807000-00010