Introduction
Disorders of the oral and pharyngeal phases of swallowing are very disabling and may place those affected at risk for death from asphyxiation owing to upper airway obstruction by large food bolus or aspiration pneumonia. Inability to swallow food also results in rapid dehydration and starvation acutely and weight loss and malnutrition in the long term. Even when deranged oral and pharyngeal motility does not constitute a threat to life, it may severely affect the quality of life.
Epidemiology
The current knowledge of the epidemiology of deglutition disorders resulting from oral and pharyngeal dysmotility is limited because of difficulties in documenting this condition in the medical records or surveys. These documentation difficulties occur in several ways: (1) the underlying disease may be recorded but not the swallowing disturbance; (2) nonspecific symptoms (e.g., cough) are recorded; and (3) some subjects have unrecognized, and thus unrecorded, swallowing problems. Among hospitalized United States military veterans in 1983, 1% received a discharge diagnosis of dysphagia. The prevalence of dysphagia among hospitalized patients is increasing over time. Oropharyngeal dysphagia is a particular problem in the elderly. Community surveys suggest that 15% to 20% of geriatric patients have dysphagia. Patients over 85 years old were 18 times more likely to have a discharge diagnosis of dysphagia than those under the age of 25 years. About 33% of residents of skilled nursing facilities exhibit impaired ability to eat. Among patients with acute stroke, about half are initially found to have symptoms or evidence of oropharyngeal dysfunction. However, in many of these patients dysphagia resolves over time.
On the individual level, the burden of having a disorder of deglutition is considerable. In a study of predominately elderly patients with swallowing disorders, about half reported having to alter their diet, eating less, finding eating less enjoyable, being embarrassed about their eating, avoiding eating with others, and suffering weight loss. The overall medical costs for the care of those afflicted with swallowing disability are large. In 1992 in the United States, the Medicare program covered enteral feedings for over 200,000 patients at a cost of $505 million. These costs are only a small fraction of all costs associated with the care of patients with oral and pharyngeal dysphagia. These can only be expected to increase as the average age of the population increases.
Classification of Oral and Pharyngeal Motor Disorders
There are two major conceptual frameworks for classifying oral and pharyngeal motor disorders. The first employs a functional perspective, which seeks to classify the effects of the underlying neuromuscular disorder on the ability of the deglutitive apparatus to maintain normal bolus handling and transport. The second framework uses a pathophysiology perspective to classify disturbances in oropharyngeal motility based on the etiology and pathogenesis of the underlying neuromuscular disorders. The information obtained from both frameworks is necessary to assess and treat the patient.
Functional Classification
The functional classification of oropharyngeal dysmotility is important because at the time of presentation the etiopathogenesis is often obscure. Indeed, the underlying cause may remain undiagnosed or prove to be poorly treatable, so that specific therapy cannot be applied. Thus, an understanding of the functional disturbance is necessary to determine the safety of oral feeding and necessary modifications in diet. Often treatments can improve the function even if the underlying etiology cannot be corrected. Sometimes the presence of a specific functional deficit provides clues to the underlying etiology, although most functional disturbances may be manifestations of a variety of disorders. Patients with oropharyngeal dysphagia may have multiple functional defects, and symptoms may not be related specifically to any one defect. Functional disorders can be categorized by the phase of swallowing in which they occur and the specific deficits identified. Schematic examples of functional disturbances in bolus transport are illustrated in Figure 1.
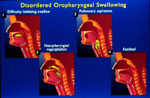
Figure 1: Illustrations of disordered oropharyngeal swallowing.
Panel 1 shows bolus of barium in the oral cavity due to patient not being able to initiate a swallow. Panel 2 shows nasopharyngeal regurgitation due to failure of velopharyngeal closure. Panel 3 shows aspiration of barium into the larynx and trachea. Panel 4 shows residual barium in the vallecula (space between the posterior aspect of the tongue and the epiglottis). (Source: From the AGA Clinical Teaching Project, with permission from American Gastroenterology Association).
Functional Disturbances in the Oral Phase
Functional problems in the oral phase of deglutition include disruption of the preparatory phase, poor bolus control, difficulty in initiating a swallow, and impaired bolus transport.
Disruption of Preparatory Phase
Functional disturbances in the preparatory phase are manifested by an inability to reduce and mix the bolus and position it appropriately on the dorsum of the tongue in anticipation of the swallow. The facial, masticatory, and tongue (intrinsic/extrinsic) muscle groups are most likely to be affected. Muscle weakness or spasticity can result in failure to position boluses over grinding surfaces and weak grinding forces. Sensory deficits cause failure to recognize inconsistent bolus reduction or placement. Abnormalities in higher brain centers can result in failure to initiate or maintain mastication.
Abnormal bolus control Abnormal bolus control is manifested by a failure to maintain the bolus within the oral cavity before swallow initiation. This can result in spilling the bolus from the lips antegrade or into the pharynx retrograde. The muscle groups potentially involved in this muscle disturbance are the same as those in the preparatory phase, with the addition of the palatal muscle group. Sensory deficits may prevent the patient from recognizing the spill or impending spill.
Premature spill is demonstrated in Video 1. (Figure 2).
Video 1: Videofluoroscopic example of premature spill.
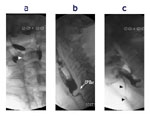
The videoclip is from a patient with severe oropharyngeal dysphagia. The orientation is anterior-posterior. Before the swallow onset, retained material is seen in the valleculae and piriform sinuses (the oval density is an external marker). Before the swallow onset premature spill (white arrow) is observed on the right. The speed of the videoclip has been reduced slightly from real time to aid comprehension.
Impaired swallow initiation Difficulty in initiating the oral phase of swallowing results from dysfunction of the brainstem collection of neurons that form the swallow pattern generator or from failure of sensory or cortical input to activate the swallow sequence at this level. The functional result is excessive oral manipulation of the bolus, accumulation of bolus in the oral cavity, or movement of bolus into the pharynx. The latter action would normally trigger a pharyngeal or reflexive swallow, but this is often impaired as well.
Abnormal bolus transport Abnormal bolus transport in the oral phase is primarily a result of dysfunction of the intrinsic and extrinsic muscles of the tongue. This is usually manifested by failure to clear the bolus from the oral cavity with a swallow, at times resulting in repetitive swallows in an attempt to clear retained bolus material.
Functional Disturbances in the Pharyngeal Phase
Functional disturbances in the pharyngeal phase usually manifest as abnormalities in clearance of swallowed boluses from the pharynx. These can be a result of propulsive failure, outflow obstruction, or failure to maintain luminal closure at entrance and exit points to the pharynx. Sensory disturbances can prevent modification of muscle function in response to different transport demands or prevent detection of failed bolus transport through the pharynx.
Muscle weakness and incoordination in the pharyngeal phase Although the tongue provides most of the force for moving the bolus into the esophagus, muscles in the pharyngeal group contract in an aboral sequence to clear the trailing portion of the bolus from the pharynx into the esophagus. Weakness or incoordination in this pharyngeal contraction sequence results in portions of the bolus remaining in the pharynx at the end of the swallow. Weakness in the pharyngeal elevators results in a longer pathway for bolus transport and may contribute to impaired bolus clearance to a lesser degree. Weakness in the palatal muscles results in a failure to seal the pharynx superiorly, allowing for regurgitation of the bolus into the nasopharynx. Weakness in the basal tone or reflex responses of the upper esophageal sphincter (UES) could contribute to esophagopharyngeal regurgitation, although the evidence that this is of clinical importance is limited. Conversely, failure of the UES to relax in response to esophageal distention, as seen in achalasia, may rarely result in a distended esophagus that obstructs the larynx.
Pharyngeal outflow obstruction Passage of a bolus from the pharynx into the esophagus requires opening of the UES. Normal opening relies on several factors: (1) relaxation of the UES, (2) anterior traction on the UES by the action of selected suprahyoid and infrahyoid muscles as the larynx moves forward, (3) pulsion forces transmitted by the oncoming bolus, and (4) distensibility of the UES resulting from its elastic tissue properties. Abnormalities of one or more of these factors can cause impaired opening of the UES and abnormal bolus clearance from the pharynx. Impaired UES opening is often seen as a cricopharyngeal bar on lateral view videofluoroscopy (Video 2). This can result from abnormalities in magnitude or timing or UES relaxation, paradoxical UES contraction, or abnormal UES distensibility. Complete bolus clearance from the pharynx may still occur despite the presence of factors that impair opening, such as diminished traction, relaxation, or compliance, so long as adequate pulsion force exists. However, this necessitates an elevation in the intrabolus pressure to overcome the higher resistance to bolus flow. This higher intrabolus pressure may activate pressure receptors in the mucosa to trigger a symptom of dysphagia. Such higher pressures may also contribute to the development of pulsion (Zenker's) diverticulum.
Video 2: Videofluoroscopic example of cricopharyngeal bar.
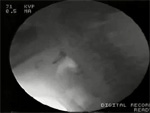
The videoclip is of a lateral view on videofluoroscopy in a patient with a cricopharyngeal bar, which is seen impinging on the barium column posteriorly (arrow). Note that after completion of the initial swallow there is slight retention of residue in the valleculae and piriform sinuses. This is mostly cleared on a subsequent dry swallow. The speed of the videoclip has been reduced slightly from real time to aid comprehension.
Functional Disturbances in Airway Protection
Aspiration of food can occur before, during, or after the act of swallowing (Video 3). Pre-deglutitive aspiration of food occurs due to premature spilling of food into the oropharynx and its subsequent entry into the unprotected laryngeal opening before the oral phase of swallowing is triggered. Abnormalities in protective laryngeal closure reflexes may contribute to the aspiration. Inter-deglutitive aspiration occurs when protective laryngeal closure fails owing to weakness or incoordination of muscles in the laryngeal group. Post-deglutitive aspiration occurs when abnormal bolus clearance from the hypopharynx leaves bolus residue in the vicinity of the laryngeal entrance. Once deglutition apnea ceases and the airway opens, the retained bolus can spill into the larynx, especially if respiration resumes in the inspiratory phase.
Video 3: Videofluoroscopic example of aspiration.
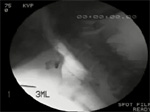
The subject is the same as in Video 1. A marker disk is present anteriorly. During the swallow there is poor opening of the UES and substantial retention of bolus within the hypopharynx. During a subsequent dry swallow, some of this material is seen to enter the airway (dark arrowheads). The speed of the videoclip has been reduced slightly from real time to aid comprehension.
Pathophysiologic Classification
Disorders arising at all levels of the nervous system may produce oropharyngeal dysphagia, which may be caused by a wide range of neuromuscular and systemic diseases. Recognition of the etiology and pathophysiology of the disorders causing oropharyngeal dysmotility can help provide specific treatment. To some extent, these disorders can be classified by the location of the lesion or deficit, although some disorders may produce deficits at multiple levels. Specific examples of some disorders follow.
Lesions Above the Brainstem
A variety of lesions above the brainstem can cause dysphagia (Table 1). Lesions of the cerebral cortex may produce a variety of different neurologic manifestations that accompany the dysphagic symptoms. Cerebrovascular accidents (strokes) are by far the most common causes of acute oropharyngeal dysphagia. Uncommonly, dysphagia can be the presenting or only symptom of stroke. Moreover, patients with stroke are often unaware of their swallowing problem and fail to adjust their oral intake to compensate for their deficits. Unilateral strokes involving the right hemisphere are more likely to result in aspiration than those of the left hemisphere. Stroke involving both hemispheres carries a higher risk of subsequent aspiration. Although both cortical hemispheres are involved in swallowing, there is usually lateralization of dominance that varies among individuals. Indirect evidence suggests that dysphagia is more likely to result when the dominant hemisphere is affected by stroke. Recovery of swallow function following unilateral stroke appears to require reorganization of the uninvolved side.
The recognition that the deglutition disorder results from stroke is important for several reasons. First, in the acute setting, treatment can be given to try to reverse the ischemic insult or prevent its enlargement. Second, workup can begin to identify the underlying etiology for the stroke, such as critical stenosis of intra- and extracranial arteries or cardiac embolic sources. Finally, steps can be taken to prevent recurrence of stroke, such as control of hypertension and antiplatelet therapy.
Other manifestations of stroke depend on the extent and location of the lesion. There may be sensorimotor dysfunction in parts of the body, movement disorders, seizure activity, cognitive deficits, and alterations in personality and affect, and decline in the level of consciousness.
Cortical lesions often exhibit greater effects on the oral phase of swallowing, which has the most potential for conscious modification. For instance, patients with operculum syndrome have difficulties in chewing and initiating oral swallowing, whereas reflexive pharyngeal swallowing may be normal. Patients with higher-center dysfunction often exhibit inefficient, tremulous, repetitive, and dyskinetic tongue motion, as well as poor bolus control with the tongue, resulting in premature spill. Exaggerated reflexive and emotional muscle responses can be observed with suprabulbar palsy, wherein there is loss of the inhibitory influence from descending pyramidal tracts. There can also be dissociation between the loss of the voluntary control of muscle groups and preservation of emotional or automatic function, and vice versa. In addition, patients with cortical dysfunction are often unaware of their disordered oropharyngeal motility and fail to make compensations for their dysfunction.
A wide variety of other neurologic disorders can produce subacute and progressive dysphagia. These include Whipple's disease, and various bacterial, fungal, spirochete (neurosyphilis), and viral encephalopathies.
Lesions of the Extrapyramidal System
Parkinson's disease is an important cause of oropharyngeal dysphagia and is usually manifest late in its course. Patients are often unaware of their swallow deficits. Abnormalities can be observed in both the oral (drooling, repetitive tongue pumping, piecemeal deglutition, premature spill, oral residue) and pharyngeal (pharyngeal residue, poor relaxation of the upper esophageal sphincter, aspiration) phases of deglutition.
Lesions of the Brainstem
Many lesions of the brainstem may cause oropharyngeal dysphagia (Table 2). Ischemic or hemorrhagic vascular events are also common causes of lesions in the brainstem. They may be associated with underlying atherosclerotic disease, sources for embolic events, hypercoagulable states, or vasculitis. Because of the crowding of a number of critical nuclei in the brainstem, more than one function is often affected by brainstem lesions. For example, lateral medullary infarction (Wallenberg's syndrome) from the occlusion of the posterior inferior cerebellar artery can produce not only dysphagia and dysarthria (as a consequence of destruction of the nucleus ambiguus and connections to the nucleus tractus solitarius), but also vertigo, nystagmus, nausea, and vomiting; ipsilateral limb ataxia; ipsilateral loss of facial sensation (pain and temperature); and contralateral loss of sensation (pain and temperature) to the trunk and extremities. This constellation of deficits is helpful in localizing the lesion.
Other brainstem lesions that cause oropharyngeal dysphagia are central pontine myelinolysis, multiple sclerosis, and olivopontocerebellar atrophy expanding neoplasms. Central pontine myelinolysis may result acutely from psychogenic polydypsia and hyponatremia. Multiple sclerosis usually shows lesions elsewhere in the neuraxis and presents with a waxing and waning course. Olivopontocerebellar atrophy may progress over years. Expanding neoplasms can show progression over a matter of days. Such progression may manifest as both worsening dysphagia as well as evidence of involvement of other neighboring structures.
Lesions of the Peripheral Motor Nerves
Lesions of the peripheral motor nerves that may affect deglutition are listed in Table 3. Lesions of the efferent fibers in peripheral nerves cause flaccid paralysis of the denervated muscles. Lesions of the afferent fibers may result in pain, loss of sensation, or disruption of reflexes. Knowledge of neural pathways by which different structures in the swallowing apparatus are innervated can help identify the site of the lesion. Cranial and peripheral nerves may be involved in such disorders as amyotrophic lateral sclerosis (ALS, motor neuron disease), poliomyelitis, multiple sclerosis, chronic inflammatory demyelinating polyradiculoneuropathy, herpes zoster (Ramsay Hunt syndrome), Guillain-Barré syndrome, paraneoplastic neuropathy (Eaton-Lambert syndrome), diabetes mellitus, B12 deficiency, and neurofibromatosis. Fasciculation of the tongue are seen with lower motor neuron degeneration in ALS. Lesions of these nerves derange oral phase swallowing. A waxing and waning course of the illness is suggestive of multiple sclerosis or chronic inflammatory demyelinating polyradiculoneuropathy. Rapid development of dysfunction over hours to days is indicative of an infectious or postinfectious process, such as herpes zoster (Ramsay Hunt syndrome) or Guillain-Barré syndrome. A more gradual progression of symptoms is typical of degenerative processes, such as ALS or postpolio syndrome. Recent history of acute diarrhea, such as Campylobacter enteritis, increases the likelihood of Guillain-Barré syndrome. The presence of café-au-lait spots suggests underlying neurofibromatosis. A vesicular rash may precede neurologic manifestations in Ramsay Hunt syndrome, although in some cases the neurologic symptoms occur first.
Diseases of the Neuromuscular Junction and Muscles
Many diseases of the neuromuscular junction and muscles may cause dysphagia (Table 4). Neuromuscular transmission may be impaired, leading to severe muscle weakness of the oropharyngeal and laryngeal muscles. Impaired neuromuscular transmission in myasthenia gravis is due an autoimmune response mediated by specific antiacetylcholine receptor antibodies. These antibodies are immunoglobulin G and T-cell dependent and may be associated with thymoma and thyroid disease. Eaton-Lambert syndrome is also an autoimmune disorder due to paraneoplastic antibodies against presynaptic calcium channels. Certain drugs and toxins can also block neuromuscular transmission, and there are also a number of rare congenital myasthenia syndromes that are not autoimmune in nature.
Muscle disorders such as myositis and metabolic and degenerative myopathies can involve muscles involved in swallowing. Myositis that involves oropharyngeal muscles may occur in association with polymyositis, dermatomyositis, and inclusion body myositis. Polymyositis and dermatomyositis are frequently associated with autoimmune disorders and may be presentations of a paraneoplastic syndrome. Metabolic and endocrine myopathies, such as hypo- or hyperthyroidism, are infrequent but treatable causes of oropharyngeal dysphagia. Degenerative myopathies such as myotonic dystrophy and oculopharyngeal myopathy can be diagnosed by careful family history and characteristic physical findings. The combination of gradual onset of ptosis with oropharyngeal dysphagia after age 40 is classic for oculopharyngeal muscular dystrophy. This primarily autosomal dominant disorder has an increased prevalence in French-Canadians, but the condition has been observed throughout the world.
Other Causes and Associated Conditions
A variety of medical and surgical treatments can have adverse consequences for deglutition. Disturbances at either end of life arising from developmental disorders or senescence can affect oropharyngeal function.
Iatrogenic causes of oropharyngeal dysphagia Oropharyngeal dysphagia may occur as a complication of head and neck surgery for cancer, thyroid disease, or cervical spine disorders. Mechanisms include resection of critical neuromuscular structures, injury during traction and manipulation of the pharyngeal plexus, compression by tissue swelling or hematoma, and reduction in mobility owing to adhesions or foreign-body implants. Laryngotracheal intubation and creation of a tracheostomy are associated with subsequent swallowing disturbance and aspiration. This may be related to decreased laryngeal elevation with deglutition, owing to tethering of the larynx by the tube. Defects in laryngeal sensation and coordination of deglutitive apnea have also been reported. Radiation injury, usually for cancer treatment, may cause nerve injury and muscle fibrosis, as well as mucosal inflammation and xerostomia. Reduced sensation occurs months after therapy. Mucosal inflammation may cause painful swallowing. Xerostomia may make it difficult to prepare and lubricate a bolus, exacerbating the oral phase dysphagia.
Medications can have adverse effects on swallowing by diverse mechanisms. Agents that are dopamine antagonists, such as many neuroleptics and antiemetics, can produce a tardive dyskinesia; this movement disorder can produce a significant disruption in the normal pattern of deglutition. Neuroleptics have also been reported to retard the swallow reflex in patients with dementia. Sedatives such as benzodiazepines and the 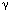 -aminobutyric acid (GABA)-B receptor agonist baclofen may inhibit swallowing at the brainstem level. Corticosteroids can cause a myopathy that produces muscle weakness. Botulinum toxin, used to treat movement disorders of the head and neck by blocking acetylcholine release, can spread from the muscle in which it is injected to produce pharyngeal muscle weakness. In patients with already compromised deglutitive function, cholinergic agonists and antagonists may result in excess and insufficient saliva production, respectively.
-aminobutyric acid (GABA)-B receptor agonist baclofen may inhibit swallowing at the brainstem level. Corticosteroids can cause a myopathy that produces muscle weakness. Botulinum toxin, used to treat movement disorders of the head and neck by blocking acetylcholine release, can spread from the muscle in which it is injected to produce pharyngeal muscle weakness. In patients with already compromised deglutitive function, cholinergic agonists and antagonists may result in excess and insufficient saliva production, respectively.
Developmental disorders Cerebral palsy is a common disorder during neonatal development that affects swallowing. These patients exhibit motor spasticity and altered eating and swallowing behavior. Dysphagic patients can manifest a variety of oral and pharyngeal phase abnormalities, including silent aspiration. As these patients are often unable to describe their symptoms, they may present with behavior changes, such as refusal to feed, or with hypoxia during feeding. Infants, who are unable to be fed orally during early life fail to develop normal sensorimotor and affective responses to oral and pharyngeal stimulation. This can result in long-lasting aversion to oral feeding.
Aging and oropharyngeal motility With aging, sensory responses and tissue compliance show senescent decline, whereas the muscle strength necessary to transport the bolus remains largely preserved. Studies have reported the following findings occurring with aging: (1) Sensory thresholds are elevated, so that greater strength of stimulus is necessary for perception. This also results in higher thresholds for elicitation of various reflexes that serve to protect the airway, such as the pharyngoglottal closure reflex, esophagoglottal closure reflex, laryngo-UES contractile reflex, pharyngo-UES contractile reflex, and reflexive swallow. Once the response is triggered, however, the magnitude of the motor response is largely preserved. (2) Basal UES pressure is reduced, although contraction pressure during deglutition is preserved. (3) Decreased traction on the UES with deglutition results in reduced opening and elevated upstream intrabolus pressure and a prolongation of bolus transit times. (4) Pharyngeal contractile strength is preserved or even augmented. (5) Coordination of muscle activity during deglutition remains normal.
Diagnosis and Treatment of Oral and Pharyngeal Motility Disorders
The Swallow Team
It is now clear that the evaluation and management of the patient with oropharyngeal dysmotility fall outside the purview of any one medical specialty or subspecialty. The expertise of speech-language pathologists, gastroenterologists, otolaryngologists, neurologists, radiologists, pulmonologists, dentists, oncologists, and psychiatrists is often required. It is important that these different experts function together as a team for the benefit of the patient. Communication among the team is critical to avoid unnecessary testing, diagnostic delay, and iatrogenic complications.
Approach to Patients
Patients with oropharyngeal dysmotility present in one of four broad clinical scenarios: (1) The patient has symptoms suggestive of oral or pharyngeal dysmotility (Table 5). (2) The patient has a finding, such as recurrent pneumonia, that may be due to oral or pharyngeal dysmotility, but other diagnoses are also possible. (3) The patient has a neuromuscular disorder (e.g., stroke) that increases the risk for an associated oropharyngeal motility disorder that is at present silent clinically (i.e., without current history of overt symptoms or complications). (4) Symptoms progress in an established case of oropharyngeal dysmotility.
The approach to the patient with suspected oropharyngeal dysmotility involves the following tasks: (1) Identify the functional defects and assess their severity. Specifically determine whether the deficits place the patient at risk for malnutrition, asphyxiation, or aspiration pneumonia. (2) Determine the underlying etiology of the motility disorder. (3) Exclude other conditions that may mimic the clinical presentation of oropharyngeal dysmotility. (4) Institute therapeutic measures directed both toward the underlying etiology as well as the disturbances in deglutitive physiology. (5) Implement necessary dietary and lifestyle modifications so that the patient can maintain oral feeding status. (6) Develop a plan for nonoral feeding or airway protection that can be accepted by the patient, when oral feeding cannot be achieved.
The above tasks do not necessarily proceed sequentially. Certain maneuvers can have both diagnostic and therapeutic benefit. The tools to achieve the above tasks are (1) the patient interview, (2) the physical examination, (3) tests of oropharyngeal function, (4) tests to resolve the differential diagnosis and establish an underlying etiology, and (5) therapeutic maneuvers.
Conditions that Mimic Oropharyngeal Motor Disorders
Some mechanical lesions can give rise to symptoms of dysphagia resembling those of oropharyngeal dysmotility. Common lesions that fall into this category include neoplasms, webs and strictures due to radiation, gastroesophageal reflux disease (GERD), or pill injury in the oropharynx or the cervical esophagus. Even distal esophageal obstructions may have symptoms referred to the neck.
Psychogenic dysphagia is rare and should be a diagnosis of exclusion, as the majority of the patients who are thought initially to have psychogenic dysphagia are found subsequently to have an organic explanation for their symptoms. Patients with psychogenic dysphagia may be unable or unwilling to cooperate with diagnostic testing due to swallow anxiety or bolus aversion. Patients with psychogenic dysphagia usually exhibit hesitancy on initiating a swallow, but once a swallow is triggered deglutition proceeds normally. Having the patient take a series of swallows in rapid succession, such as draining a cup of water or barium, obviates the possibility of consciously modifying the approach to each individual swallow, and the sequence of swallows continues without difficulty. Patients with psychogenic dysphagia have a higher degree of anxiety but do not meet the criteria for an eating disorder. Some patients with psychogenic dysphagia have phagophobia or fear of eating.
Other lesions that may mimic oropharyngeal dysmotility include tracheoesophageal fistula, postnasal drip, and gastroesophagopharyngeal reflux with or without concomitant asthma.
Identifying Functional Disturbances
A careful history and physical examination often provide important clues as to the nature of the functional disturbances in deglutition. The examination at the bedside can be enhanced by observing directly the patient's ability to swallow different types of boluses. Apart from clinical history, results of several bedside tests, such as reduced ability of water instilled into the pharynx to elicit a swallow, decreased cough response to inhaled acidic aerosols, and reduced oxygen saturation on pulse oxymetry during swallowing indicate increased risk for aspiration or pneumonia. Because bedside studies have some limitations, particularly in patients with "silent" aspiration, additional functional testing is usually necessary.
Barium swallow and videofluorography When the etiology of the swallow disturbance is uncertain, the first diagnostic test in most cases should be a barium pharyngoesophagram. In addition to providing some information of functional disturbances, it can identify more distal lesions in the esophagus that have symptoms referable to the neck. The test is incomplete without a solid bolus challenge to detect obscure stenoses.
Videofluorography involves the fluoroscopic study of the patient during deglutition of a wide range of bolus consistencies. Videofluorography allows the assessment of bolus preparation and transport through the oral cavity and pharynx into the proximal esophagus as well as any misdirection and aspiration of boluses. Videofluorography also offers the possibility of detecting other important structural abnormalities, such as diverticula, webs, and tumors. It can be used to monitor the responses to swallow therapy.
Videoendoscopy Videoendoscopy involves the transnasal placement of a small-caliber fiberoptic or digital endoscope to observe the larynx and pharynx during deglutition. Videoendoscopy can detect nasopharyngeal regurgitation, premature spill, and aspiration.
The utility of both videofluorography and videoendoscopy are enhanced by the videotaping of the studies for subsequent review, including slow-motion playback. The contrasting strengths and limitations of these two studies are listed in Table 6. The limitations of each at times can make them complementary studies in evaluating oropharyngeal functional disturbances.
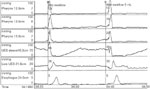
Manometry Manometry involves the placement of pressure-sensing catheters in the lumen of the oropharynx. The recorded pressure changes reflect the magnitude and coordination of contractions or relaxations of the adjacent musculature. The greatest utility for manometry in the oropharynx is the determination of deglutitive relaxation of the UES. (Figure 3). Symptomatic failure of UES relaxation may be present despite the observation of UES opening on videofluorography. Manometry can also assess weakness of the pharyngeal musculature (Figure 4). The asymmetry and deglutitive movement of oropharyngeal structures make for significant challenges in the accurate recording and interpretation of manometric data.
Figure 3: Manometric example of upper esophageal sphincter dysfunction.
Manometric tracing is from a patient with cervical dysphagia for solids and cough. Upper esophageal sphincter (UES) basal pressure is being recorded with a sleeve device. During the dry swallow, the UES is seen to fail to relax and, in fact, starts to contract slightly before the arrival of the pharyngeal pressure wave at that level. The subsequent low pressure seen after passage of the peristaltic wave is an artifact related to emptying of the sleeve by the contraction, with subsequent slow refilling. Note that the tracing below the sleeve, located in the distal part of the UES, shows a spurious relaxation. This is the result of the temporary displacement of the UES above this point sensor (the UES remains over the sleeve). The 5 mL water swallow shows a slight relaxation, but this is incomplete and of short duration.
Figure 4: Manometric example of pharyngeal muscle weakness.
This manometric study shows a dry swallow a patient with oculopharyngeal muscular dystrophy who has cervical dysphagia for solids. Note the low amplitudes of the pharyngeal pressure waves. The UES basal pressure is not reduced, and the peristaltic wave in the proximal esophagus is normal.
Determination of the Etiology of Oral and Pharyngeal Dysmotility
Again, information obtained by a careful history and physical examination can help make the diagnosis or formulate the differential diagnosis. The majority of neuromuscular disorders affecting oropharyngeal motility can also affect other regions of the body, often before clinical manifestations are observed in the oropharynx. Recognition of the pattern of involvement elsewhere may facilitate the diagnosis of the underlying oropharyngeal motility disorder. However, disorders of mastication and swallowing may be the initial or the sole presentation of these disorders. Such presentations have been reported with myasthenia gravis, inclusion body myositis, thyrotoxicosis, and stroke. A wide variety of imaging studies, electromyography, and other laboratory tests are available to determine the neurologic defect underlying the oropharyngeal dysmotility. Focal oropharyngeal involvement by inflammatory muscle disorders can make diagnosis difficult, because standard tests for inflammatory myopathies, such as serum creatine kinase and peripheral muscle electromyography, may be normal. At times, biopsy of the affected muscle for histopathologic analysis is necessary to make the diagnosis.
Treatment of Oropharyngeal Dysmotility Disorders
Treatment of the underlying condition, when possible, is of the highest priority. Examples for which specific treatments are available include thyroid disease, myasthenia gravis, and inflammatory myopathies. Drugs causing or exacerbating dysphagia, such as neuroleptics and anticholinergics should be discontinued if possible. However, most of the patients with oropharyngeal dysmotility will not have a curable or treatable etiology.
When treatment of the underlying disease is not possible, prevention of aspiration and maintaining oral feeding are the main goals of therapy. Many patients with irreversible swallow dysfunction can maintain oral feeding with appropriate dietary modifications and other interventions.
Diet Modification and Physical Therapy
Some functional deficits can be overcome to some extent by appropriate changes in the diet. Patients with poor bolus control can be helped by thickening of liquids. Patients with weakness or pharyngeal outflow obstruction will be helped by softer diets and conversion of pills to liquid form. Certain body positions and active maneuvers may facilitate bolus passage and prevent aspiration during deglutition. Depending on the defects, a trained swallow therapist can teach the patient such maneuvers as chin tuck, head tilt, effortful swallow, supraglottic swallow, sustained swallow (Mendelsohn maneuver), and isometric/isotonic head-raising (Shaker) exercises. Uncontrolled studies in patients with neurogenic dysphagia requiring tube feeding indicate that many can return to oral feeding status following such swallow therapy.
Medical Therapy
Medical therapy for functional disturbances in oropharyngeal motility is limited. Patients with excessive drooling or coughing of aspirated saliva may be helped by reducing salivary secretion through anticholinergic drugs or the injection of the salivary glands with botulinum toxin. Botulinum toxin injection into the UES may be of benefit in impaired deglutitive UES relaxation, although repeat treatments are usually necessary and the paralytic effect can adversely spread to adjacent muscles.
Relief of Pharyngeal Outflow Obstruction
In patients with outflow obstruction from the pharynx due to a failure of deglutitive UES relaxation, myotomy of the cricopharyngeus muscle has been reported to relieve symptoms and improve bolus transit. The studies to date are uncontrolled, and there are no clear predictors of success. Similarly, dilation of the UES with large-caliber bougies or balloons (up to 20-mm diameter) has been reported in uncontrolled studies to benefit patients felt to have UES dysfunction contributing to their dysphagia. The mechanism for the improvement is unknown, and the predictors of success are uncertain, as is the duration of the response. Dilation therapy had the advantage of a rapid clinical response and avoids the potential complications of a myotomy.
Procedures to Prevent Aspiration
Several surgical procedures may help prevent aspiration. These include vocal cord medialization procedures and laryngeal suspension. For more severe cases of intractable aspiration, operations that separate the airway from the path of the bolus, such as laryngotracheal separation, may be considered. These have the disadvantage of requiring a permanent tracheostomy, but have the advantage of allowing some patients with even major swallowing disturbances to resume oral feeding.
Nonoral Feeding
Nonoral feeding is considered when a patient cannot maintain adequate hydration and nutrition despite dietary and other interventions. Usually, this is best accomplished over the long term by a tube placed through the abdominal wall into the stomach (G-tube). This can be performed endoscopically, radiologically, or surgically. Patients with feeding tube placement after stroke should have follow-up assessment of swallowing ability, as they may recover function even months later. It is also important to recognize that feeding tubes do not prevent the development of aspiration pneumonia in this population, nor does early institution of tube feedings improve long-term outcome.