Abstract
The escalating threat of bone-related diseases poses a significant challenge to human health. Mesenchymal stem cell (MSC)-derived extracellular vesicles (MSC-EVs), as inherent cell-secreted natural products, have emerged as promising treatments for bone-related diseases. Leveraging outstanding features such as high biocompatibility, low immunogenicity, superior biological barrier penetration, and extended circulating half-life, MSC-EVs serve as potent carriers for microRNAs (miRNAs), long no-code RNAs (lncRNAs), and other biomolecules. These cargo molecules play pivotal roles in orchestrating bone metabolism and vascularity through diverse mechanisms, thereby contributing to the amelioration of bone diseases. Additionally, engineering modifications enhance the bone-targeting ability of MSC-EVs, mitigating systemic side effects and bolstering their clinical translational potential. This review comprehensively explores the mechanisms through which MSC-EVs regulate bone-related disease progression. It delves into the therapeutic potential of MSC-EVs as adept drug carriers, augmented by engineered modification strategies tailored for osteoarthritis (OA), rheumatoid arthritis (RA), osteoporosis, and osteosarcoma. In conclusion, the exceptional promise exhibited by MSC-EVs positions them as an excellent solution with considerable translational applications in clinical orthopedics.
Similar content being viewed by others
Facts
-
MSC-EVs-loaded non-coding RNAs can regulate bone disease progression.
-
MSC-EVs can be used as drug carriers for bone diseases.
-
Targeting of MSC-EVs to bone tissue can be increased by engineered modifications.
Open questions
-
How to increase the yield and purity of EVs secreted by MSC?
-
Which engineering modification techniques can further improve bone targeting in MSC-EVs?
-
How the drug loading efficiency of MSC-EVs can be further improved?
Introduction
Bones, integral in maintaining body posture, serve not only to protect internal organs and support hematopoiesis but also actively regulate the body’s mineral balance, calcium and phosphorus metabolism, and maintain normal physiological functions [1, 2]. However, the aging process disrupts this delicate balance, leading to the dysregulation of bone metabolism and the development of bone diseases such as osteoporosis, OA, and bone tumors [3]. Conditions like osteosarcoma, malignant tumors with bone metastases, and other imbalances in bone metabolism can result in severe pain and a poor prognosis for patients [4]. Furthermore, orthopedic diseases, including OA, bone injuries, and degenerative disc disease, pose threats to health and reduce the quality of life for millions globally [5]. Current clinical treatments for orthopedic conditions include bed rest, non-steroidal anti-inflammatory drugs (NSAIDs), analgesics, lumbar discectomy, and interbody fusion [6], provide only temporary relief of symptoms and lack precision in targeting the pathogenesis of bone disease [7]. Hence, there is an urgent need to explore the mechanisms of bone diseases and develop safe and effective biomaterials/agents to improve the prognosis of patients with orthopedic diseases.
MSC are known for their pluripotent nature and the ability for self-renewal and multi-lineage differentiation [8], have been utilized in the past, either alone or in combination with biomaterials and growth factors, for treating musculoskeletal disorders [9]. However, the potential risks of immune rejection, tumorigenicity, and induction of tumor resistance associated with MSC transplantation have limited their use in orthopedic disease treatment [10]. Recent studies have revealed that the positive effects of MSC on bone disease may be mediated by paracrine mechanisms, particularly MSC-EVs [11, 12]. EVs represent heterogeneous membrane-bound vesicles of lipid bilayer, released into the microenvironment by all cells [13]. The researchers classified EVs into exosomes (30–150 nm), microvesicles (200–1000 nm) and apoptotic vesicles (800–5000 nm) based on the diameter of the EVs [14], the absence of a uniform nomenclature arises from overlapping characteristics in size, density, contents, and surface molecules among vesicles subtypes [15]. Consequently, for this study, the collective term “EVs” encompasses exosomes, microvesicles, and apoptotic vesicles. EVs serve as carriers for diverse cargo, including protein molecules, nucleic acids, pro-inflammatory factors, cytokines, and transcription factor receptors, enabling their participation in intercellular signaling through receptor-ligand interactions [16]. Furthermore, the genetic material within EVs, such as miRNAs, non-coding RNAs, and DNA, contributes to their involvement in immune response, signal transduction, and antigen presentation and influences cellular physiology and pathology [17, 18]. Functionally mirroring their parental cells, EVs play a crucial role in influencing disease progression by mediating angiogenesis through cell-to-cell communication and modulating immune responses [19].
Mounting evidence suggests the involvement of MSC-EVs in the regulation of bone-related diseases, including OA, fracture healing, degenerative bone diseases, and bone tumors, mediated by paracrine mediators [9, 20, 21]. Leveraging their commendable biocompatibility, tissue penetration, and pro-regenerative abilities akin to parental cells, MSC-EVs are increasingly harnessed as nano drug delivery carriers, finding applications in the treatment of diverse diseases such as oncology, neurodegenerative disorders, and immune disorders [22, 23]. Furthermore, the synergy of MSC-EVs with engineering techniques enhances the precision targeting of drugs for specific diseases [24]. This review provides a comprehensive summary of the regulatory role of MSC-EVs in the progression of orthopedic diseases. It explores their promising clinical application as nanocarriers for the treatment of orthopedic diseases, with the optimistic anticipation that cell-free therapies will pave the way for innovative perspectives in orthopedic disease treatment.
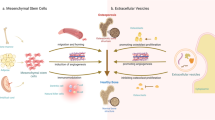
Regulation of osteoblast biological behavior by MSC-EVs
Operating at the nanoscale, these EVs are ubiquitously secreted by nearly all cell’s physiological or pathological conditions, utilizing endocytosis and biosynthetic pathways [25]. Activation of the endocytosis pathway, triggered by external or internal signals from the local environment, initiates membrane invagination, forming early endosomes [26, 27]. Guided by various cellular signaling pathways, these early endosomes transform into late endosomes. The subsequent fusion of multicellular bodies derived from late endosomes with the cell membrane culminates in the secretion of EVs [25, 28]. EVs, acting as messengers, transport nucleic acids, proteins, and lipids in a paracrine or endocrine manner, thereby regulating the biological functions of recipient cells [29]. Within the bone metabolism microenvironment, MSC-EVs emerge as pivotal players in the regulation of bone-related diseases, because MSC-EVs affect the progression of bone diseases by regulating signaling pathways, bone metabolism, inflammatory responses, angiogenesis, promoting osteoblast proliferation, and inhibiting ECM degradation (Fig. 1).
Regulating signaling pathway
MSC release EVs that exert a significant influence on the biological behavior of bone cells, thereby impacting the progression of bone diseases. MSC-EVs, enriched with various substances, including miRNAs and proteins, predominantly govern their regulatory effects on osteoblasts through the genetic material that they carry [30, 31]. Notably, specific miRNAs such as miRNA-935, miRNA-361-5p, miR-126, and lncRNA-H19 have demonstrated the ability to mitigate osteoporosis, OA, and bone damage by fostering osteoblast proliferation [32,33,34,35]. These signaling molecules (e.g., miRNAs), when introduced into recipient cells, profoundly affect bone formation and the advancement of skeletal diseases. Importantly, variations in genetic information lead to differential expression levels of signaling molecules, such as miRNAs, in osteoblasts [36]. Differences in the expression of miRNAs are evident in MSC-EVs promoting osteogenic differentiation. For instance, miR-135b, miR-203, miR-219, miR-299-5p, and miR-302b are significantly upregulated, while miR-155, miR-885-5p, miR-181a, and miR-320c are downregulated during osteogenic differentiation [37]. Moreover, the same miRNA may exhibit varying expression levels in different bone diseases. Therefore, when investigating bone diseases, it is crucial to consider the different effects of altered expression of signaling molecules from various aspects.
Cell growth regulation involves multiple growth factor receptors, where intracellular kinases activate or phosphorylate these receptors’ structural domains. This activation triggers downstream pro-growth signals through pathways like AKT (protein kinase B), protein kinase C (PKC), and MAP kinase, influencing osteoblast proliferation and disease progression [38, 39]. For instance, MSC-EVs have been shown to alleviate osteoporosis by promoting osteoblast proliferation through the mitogen-activated protein kinase (MAPK) pathway [38]. Additionally, MSC-EVs contribute to spinal cord injury alleviation by inhibiting TLR4/MyD88/NF-κB signaling pathways [40]. The presence of lncRNA XIST in MSC-EVs promotes osteosarcoma growth and metastasis through the miR-655/ACLY signaling pathway [41]. These findings underscore the pivotal role of MSC-EVs in regulating bone diseases [42], and future studies investigating the effects of remaining miRNAs/lncRNAs and other signaling pathways on bone diseases hold promise for advancing the utilization of MSC-EVs in clinical application.
Promoting osteoblast proliferation
As previously mentioned, MSC-EVs harbor a diverse array of signaling molecules, including proteins, lipids, and miRNAs, capable of influencing osteoblast activity and impacting bone formation. Notably, other signaling molecules may also contribute to the regulation of osteoblast proliferation and differentiation. Li et al. [43] observed that hypoxia inducible factor (HIF)-1α in MSC-EVs stimulated the expression of relevant osteogenic genes, rescuing bone ischemic necrosis by mitigating early steroid induction. Additionally, tsRNA-10277-containing MSC-EVs were found to regulate the lipogenic and osteogenic potential of bone marrow MSC (BM-MSC) [44].
Numerous cell biology studies have elucidated the mechanisms through which BM-MSC or MSC-EVs regulate osteoblast proliferation and differentiation, encompassing the following aspects: (1) EVs can induce the differentiation of BM-MSC into osteoblasts by regulating growth factors, osteogenic proteins, and transcription growth factor-β1 (TGF-β1) [45]. Furthermore, EVs can regulate protein expression through miRNA to facilitate the differentiation of BM-MSC into osteoblasts [46]. (2) MSC-EVs can directly enhance the proliferative capacity of osteoblasts through miRNAs or signaling pathways, thereby alleviating bone disease progression. For example, miR-935 carried by MSC-EVs supports the proliferation and differentiation of osteoblasts by targeting STAT1 [35]. Moreover, miR-1260b derived from MSC-EVs inhibits osteoclast activity, regulating the dynamic balance of osteoblasts through the Wnt5a-mediated RANKL pathway [47]. (3) Osteoblast proliferation and bone formation may be associated with enhanced vascularization, as miR-29a enriched in EVs secreted by BM-MSC promotes angiogenesis by being taken up by human umbilical vein endothelial cells [48]. (4) As mediators of cellular communication, MSC-EVs regulate immune responses and anti-apoptosis through cellular interactions, ultimately favoring osteoblast proliferation and bone formation [49]. For instance, MSC-EVs can alter macrophage polarization phenotypes by activating NF-κB signaling, promoting osteoblast differentiation [50]. Additionally, osteoblast-derived EVs inhibit osteoclast differentiation via the miR-503-3p/Hpse axis, thereby promoting bone formation [51].
The proliferation and differentiation of osteoblasts are thus recognized as dynamic processes involving multiple biological behaviors, where angiogenesis, immune cell phenotype, and bone metabolism play crucial roles.
Regulating angiogenesis
Angiogenesis plays a critical role in the evolution of musculoskeletal structures and the maintenance of normal physiological function. This process accelerates the delivery of nutrients, oxygen, and cells, thereby upholding the structural and functional integrity of joints and soft tissues. Moreover, angiogenesis facilitates the differentiation and mineralization of cartilage, promoting the establishment of bone homeostasis and bone healing [52]. Recent research suggests that MSC play a role in the in vivo generation of new blood vessels, attributed to their ability to secrete angiogenic factors and proteases, thereby promoting angiogenesis in disease-related signaling [53]. Critical players in this process include matrix metalloproteinase 2 (MMP-2), transforming growth factor-β (TGF-β), interleukin-6 (IL-6), and vascular endothelial growth factor (VEGF) are crucial [54, 55].
Interestingly, mounting evidence suggests that MSC-EVs contribute to the modulation of bone diseases through the remodeling of angiogenesis. Similar to their parental cells, MSC-EVs regulate bone formation via angiogenesis, involving multiple soluble mediators [56]. For instance, MSC-EVs promote angiogenesis through the VEGF and Hippo signaling pathways, enhancing tendon-bone healing [57]. Additionally, lncRNA-H19 in MSC-EVs supports bone formation by activating the ngpt1/Tie2-NO signaling pathway to promote angiogenesis [32]. Notably, VEGF emerges as a key mediator of angiogenesis, with its activity potentially linked to YAP/TAZ signaling and Hippo pathway activation [58]. However, the detailed mechanisms through which MSC-EVs ameliorate bone pathogenesis by promoting angiogenesis warrant further investigation. It would be interesting to explore whether MSC-EVs mediate their effects through specific cytokines (e.g., TGF-β, IL-6). Studies have shown that MSC-EVs promote angiogenesis through MMP-2, and MSC-EVs-derived TGF-β attenuates OA by regulating vascular activity [56, 59]. These findings confirm the potential clinical application of MSC-EVs in alleviating osteoarthropathy by promoting angiogenesis.
Inhibiting ECM degradation
Collagen, an essential component of tendon and bone, plays an indispensable role in maintaining the normal physiological function of the bone and joint [60]. Inflammatory cytokines, especially interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), stimulate the production of MMPs, leading to the degradation of all components of the extracellular matrix (ECM). This process results in permanent damage to cartilage, tendons, and bones, contributing to the development of diseases such as OA and RA [61]. Previous studies have established that collagenases, especially MMP-1 and MMP-13, play a pivotal role in inducing OA and RA by accelerating collagen degradation [62]. Interestingly, researchers have identified a unique ability of MSC-EVs to inhibit matrix degradation during arthritis progression. MSC-EVs induce the expression of type II collagen and aggregated glycans, expediting cartilage remodeling while simultaneously limiting matrix and collagen catabolism by inhibiting MMP-13 expression [63].
Furthermore, MSC-EVs may suppress osteoarticular inflammation by downregulating the expression of IL-1β, MMP-1, and MMP-13 [64]. Given that the regulation of ECM degradation by MSC-EVs is a multifactorial and correlated process, the activation of specific signaling pathways is equally noteworthy. By inhibiting ECM degradation along with the production of inflammatory factors, MSC-EVs present the possibility of inflammatory factors counteracting their matrix degradation inhibition by promoting ECM degradation [65]. For instance, MSC-EVs inhibit matrix degradation and the production of inflammatory factors through the miRNA-130b-3p-mediated LRP12/AKT/β-catenin axis, thereby alleviating OA progression [65]. Similarly, MSC-EVs rescue disc degeneration by promoting the proliferation of degenerating nucleus pulposus cells and synthesizing the ECM synthesis through the miR-129-5p/SOX4/Wnt/β-catenin axis [66]. Consequently, MSC-EVs hold significant promise in modulating ECM synthesis to reverse osteoarthropathy.
Regulating bone metabolism
Bone metabolism is intricately regulated by the balance between osteoblast-mediated bone formation and osteoclast-mediated bone resorption [67]. Imbalances, such as increased osteoclast activity and decreased osteoblast activity, can lead to delayed fracture healing or increased bone degeneration. The complex biological process of bone formation involves BM-MSC differentiating directly into osteoblasts through various biological factors. Notably, osteoblast-specific transcription factors, including runt-related transcription factor 2 (RUNX2) and osterix, play essential roles in osteoblast differentiation [68, 69]. Maintaining the balance of bone metabolism (osteoclast resorption and osteoblast remodeling) is vital for preventing the development of bone diseases. Increased osteolysis and bone resorption significantly impede bone growth into grafted tendons, affecting ECM synthesis and bone remodeling, ultimately leading to delayed early fracture healing [70]. Osteoclasts are the primary cell type responsible for the destruction and resorption of bone tissue in vivo, and inhibiting osteolysis while promoting osteogenesis and maintaining bone metabolism homeostasis are two central factors in promoting fracture healing and delaying joint degeneration [71].
Numerous preclinical and clinical studies have validated the potential of MSC-EVs in supporting bone healing by promoting osteogenic differentiation and inhibiting osteolysis. Feng et al. [72] proposed that miR-6924-5p, enriched in MSC-EVs, effectively inhibits tunnel osteolysis and enhances the biomechanical strength of tendon-bone healing by targeting two osteoclastic regulators, OCSTAMP and C-X-C motif chemokine ligand 12 (CXCL12). Simultaneously, MSC-EVs accelerated tendon-bone healing by delivering bone morphogenetic protein-2 (BMP-2), promoting the formation of bone and fibrocartilage tissues, as well as enhancing the stiffness and ultimate load strength of the tendon interface via the Smad/RUNX2 pathway [69]. Another intriguing study demonstrated that MSC-EVs loaded with recombinant C-Type lectin domain family 11, member A (CLEC11A) facilitate the transition of BM-MSC from lipogenic to osteogenic differentiation and inhibit osteoclast activity, ultimately alleviating osteoporosis [73]. Collectively, this evidence confirms that MSC-EVs can regulate bone metabolism to support fracture healing and alleviate osteoporosis. However, whether bone metabolism synergizes with biological behaviors such as angiogenesis and ECM degradation to improve the progression of related bone diseases is essential, as it may further explore the potential of MSC-EVs for clinical applications in orthopedic diseases [74].
Regulating inflammatory response
Inflammatory responses are widely involved in the regulation of physiological and pathological processes in musculoskeletal disorders [75]. In the physiological state, inflammation is essential for tissue repair and regeneration, such as in fracture repair, as well as an indicator of bone and joint infection [75]. However, numerous studies have affirmed that inhibiting inflammation can alleviate chronic inflammation in degenerative musculoskeletal diseases such as OA and disc degeneration [76]. Notably, damage-associated molecular patterns (DAMP) activated by degenerating or stressed cells mediate persistent noninfectious inflammatory responses. The activation of the inflammatory response triggers the release of pro-inflammatory cytokines/chemokines from innate immune cells [77]. Mechanistically, elevated DAMP levels activate inflammatory vesicles, inducing caspase-1 activation and ultimately promoting the release of IL-1β and IL-18 [78]. Furthermore, active inflammatory diseases, including RA, may result from differences in the distribution of functional pro-inflammatory helper T cells (Th17) and anti-inflammatory regulatory T cells (Tregs) [79]. Therefore, strategies involving the elimination of DAMP-induced inflammatory responses and the modulation of immune responses hold potential for the clinical management of inflammatory diseases.
Studies have demonstrated that MSC can inhibit the inflammatory response by promoting anti-inflammatory processes during tissue repair, thereby creating an appropriate microenvironment for cartilage and musculoskeletal regeneration [80]. For instance, MSC induce the polarization of anti-inflammatory macrophages (M2- macrophages) and reduce levels of IL-1, IL-6, IL-8, IL-17, TNF-α, and IFN-γ in inflamed tissues [80,81,82]. Similar to their parental cells, MSC-EVs also exhibit promising potential in modulating inflammatory and immune responses. MSC-EVs ameliorate osteoporosis by inhibiting NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome activation, as the negative regulation of NLRP3 inflammasome activation inhibits IL-1β and IL-18 secretion in osteoclasts, promoting recovery from bone loss [83, 84]. Additionally, macrophages have garnered attention for their potent immunomodulatory functions. MSC-EVs have been shown to reduce the inflammatory response of local tissues by polarizing macrophages from a pro-inflammatory M1 phenotype to an anti-inflammatory M2 phenotype, thereby promoting tendon-bone healing [57]. This may attributed to the M2 macrophage polarization, which decreases the expression levels of pro-inflammatory cytokines (IL-1β and IL-6) while stimulating the expression of anti-inflammatory cytokines (IL-10 and TGF-β) [85]. Given the immunomodulatory function of macrophage polarization, MSC-EVs may also suppress inflammation by inhibiting M1 macrophage polarization and promoting M2 macrophage infiltration [86]. Notably, specific signaling pathways may be associated with immune regulation in MSC-EVs. For instance, miR-23a-3p in BM-MSC-EVs supports tendon-bone healing by targeting and inhibiting IRF1 and NF-κB pathways in macrophages, promoting M2 macrophage polarization and suppressing inflammatory responses at the tendon-bone interface [87]. Furthermore, NF-κB is thought to be associated with GSDMD-mediated macrophage pyroptosis and inflammatory mediator release; however, the means by which MSC-EVs are involved in macrophage pyroptosis-based immune regulation are not yet known [88, 89]. Nevertheless, NF-κB may be a potential target for tendon-bone healing, and the detailed mechanisms by which MSC-EVs and NF-κB are involved in immune and inflammatory regulation must be further explored.
Potential application of MSC-EVs as drug carriers
Advantages of MSC-EVs as drug carriers
In comparison to synthetic biomaterials, EVs secreted by cells offer enhanced biocompatibility, lower immunogenicity, and greater target specificity [90]. The membranes of EVs are enriched with sphingolipids, signaling factors, and adhesion factors, facilitating their recognition and binding to target cells [91]. With a lipid molecular layer structure similar to cell membranes, EVs possess a robust ability to penetrate biological barriers (e.g., blood-brain barrier) and reach deeper tissues [92]. Additionally, the excellent tolerance of EVs allows them to evade immune phagocytosis, ensuring prolonged circulation [93]. These characteristics position EVs as effective drug carriers for delivering therapeutic agents to deep tissues.
In contrast to generic EVs, MSC-secreted EVs offer distinct advantages, rendering them exceptional drug carriers. MSC exhibit a heightened capacity to secrete EVs compared to other cells [94]. Concurrently, MSC-EVs demonstrate strong targeting abilities, and their low immunogenicity enables them to evade activating immune responses, resulting in a stronger circulating half-life [95]. Furthermore, MSC-EVs display effective lesion permeability and retention, allowing them to accumulate at the disease site [96, 97]. These unique attributes position MSC-EVs as highly valuable for clinical applications and ideal drug carriers for treating various diseases, including osteoarthropathies, neurodegeneration, and tumors.
The application of MSC-EVs as drug carriers
Currently, various techniques are available for loading exogenous drugs into EVs and delivering them to specific lesions, including incubation, electroporation, ultrasonication, extrusion, and freeze-thaw cycles [98]. Among these methods, incubation is commonly used due to its operational simplicity, but its drug-loading efficiency limits further development [99]. Although electroporation exhibits higher drug loading efficiency than incubation, the electric charge may induce the denaturation of EV surface proteins and structural disruption [100]. Both sonication and extrusion methods result in higher drug loading efficiency than incubation. However, sonication affects EVs’ physicochemical properties and triggers protein aggregation, while inappropriate mechanical compression (extrusion) disrupts EVs’ membrane structural integrity [101, 102]. Freeze-thaw cycling has the potential for large-scale applications, but rapid and repeated cycles alter the physicochemical properties of surface proteins and remain less efficient than sonication for drug encapsulation [103, 104]. Overall, each drug loading method has its advantages and disadvantages, with electroporation being the most commonly used method for EVs, considering factors like convenience and cost [105].
Several reports confirm the therapeutic potential of different drug-loading methods for MSC-EVs in skeletal diseases. For instance, BM-MSC-EVs can be loaded with miRNA-542-3p by electroporation [106], and Li et al. [107] used incubation to load exogenous lncRNA CAHM into MSC-EVs, modulating macrophage polarization to ameliorate disc degeneration. Additionally, exogenous drugs were loaded onto MSC-EVs by extrusion and sonication to alleviate OA progression [108, 109]. These cases demonstrate the promising clinical applications of MSC-EVs as drug carriers.
MSC-EVs targeted therapeutic potential
Notably, while the drug-loading efficiency of EVs can be improved in multiple ways, considering the prolonged retention of EVs in the blood circulation or tissues, improving the targeting of EVs would be beneficial to increase the efficiency of drug therapy. Current research focuses on improving EV targeting through engineering modifications.
Natural bone targeting capacity of MSC-EVs
Natural EVs released from cells inherently possess the ability to deliver their contents to host cells. Membrane surface proteins of EVs, such as tetraspanning proteins (CD9, CD63, and CD81), latex adhesion proteins (LA), Lamp-2b, and heat shock proteins (HSP), contribute to the targeting of EVs to host cells and mediate their entry into the recipient cells [110, 111]. Theoretically, EVs can be specifically internalized in a cell type-specific manner, depending on the identification of ligand proteins on the EV surface by the cell or tissue [112]. For instance, the interaction of CXC chemokine receptors (CXCR4) with stromal cell-derived factor 1 (SDF-1) can facilitate the targeted metastasis of MSC-EVs to osteosarcoma sites [113]. Furthermore, EVs secreted by different cells may contain signaling molecules that act as ligands for other cells, and these receptor-ligand interactions facilitate the enrichment of EVs in specific cells or tissues [114]. Zhang et al. [115] demonstrated that EVs derived from BM-MSC could target miR-206 for translocation into osteosarcoma tissues and regulate tumor growth. Induced by chemokines, MSC-EVs may accumulate in specific tissues through vascular leakage, revealing the homing effect of MSC [116]. The promising tissue-targeting properties of MSC-EVs hold much promise for the study and treatment of bone diseases, although more research is still worth exploring.
Engineering modifications of MSC-EVs to enhance bone targeting capability
Considering the inefficiency of the natural targeting of MSC-EVs, enhancing the bone targeting of MSC-EVs through engineering modifications is a potential option to improve the efficiency of bone disease treatment.
Direct modification of MSC-EVs
Click chemistry, membrane postinsertion, hydrophobic insertion, and receptor-ligand binding are typical approaches for direct modification of bone-targeted MSC-EVs.
Click chemistry involves coupling ligands to the outer surface of EVs, which is achieved by direct modification of EVs [117]. For example, EVs binding to alendronate can generate Ale-EVs through “click chemistry,” facilitating EV targeting to the bone via alendronate/hydroxyapatite binding and ultimately alleviating osteoporosis [118]. Copper-catalyzed azide-alkyne cycloaddition reactions (click chemistry) were utilized by Smyth et al. for bioconjugation of specific molecules to the surface of EVs, confirming its potential for improving bone-targeting ability [119].
Membrane postinsertion operation, due to its simplicity and flexibility, is considered an attractive EV modification strategy. This approach achieves covalent attachment of the target ligand to polyethylene glycol-lipid micelles, which are then co-incubated with selected liposomes before transferring to the liposome bilayer [120]. Yan et al. [121] demonstrated that this modification, involving folic acid (FA)-polyethylene glycol-cholesterol compound, enhances bone-targeting of EVs encapsulating dexamethasone sodium phosphate, contributing to RA amelioration. Incorporating folic acid onto EVs via membrane postinsertion strategy improves bone targeting and reduces synovial inflammation, showing good biocompatibility and extended circulating half-life [122,123,124].
Hydrophobic interactions have been employed to modify the surface of EVs through the insertion of hydrophobic diacyl lipids, facilitating the modification of EVs with bone-targeted peptides [125]. In this method, bone-targeting peptides are incubated with EVs, and hydrophobic interactions are harnessed to craft EVs capable of bone-targeting delivery. This approach involves modifying EVs through hydrophobic interactions and loading Shn3-expressing siRNAs into MSC-EVs by demonstrating the inherent bone-targeting properties of MSC-EVs, thereby further enhancing the therapeutic efficacy of osteoporosis treatment, as well as promoting osteoclast differentiation and facilitating angiogenesis [125, 126].
Currently, researchers employ receptor-ligand interactions to affix natural receptors onto the surface of EVs, creating targeted ligands [127]. Leveraging the selectivity of aptamers, various techniques are employed to modify the EV surface, enhancing bone targeting. Luo et al. [128] demonstrated that coupling of bone marrow stromal cell-derived EVs with BM-MSC-specific aptamers enables the targeted delivery of EVs to BM-MSC within the bone marrow. This approach avoids immune clearance and metabolism, thus offering potential options for treating osteoporosis and fractures. The glycoproteins present on EV membranes make glycosylphosphatidylinositol (GPI) an anchoring structure for functional ligands on the surface of EVs. The aptamer modification using GPI protects EV surface proteins from proteolytic degradation by proteases, thereby improving the targeting ability of EVs [129]. Furthermore, co-incubation of aldehyde-modified aptamers with BM-MSC-EVs facilitates the synthesis of aptamer-functionalized EVs, contributing to bone targeting and promoting bone repair and regeneration [128, 130].
Indirect modification of MSC-EVs
Recognizing the analogous properties of EVs to parental cells, a viable strategy for obtaining targeted EVs involves the genetic engineering modification of parental cells [131]. The modification of parental cells that produce EVs, such as directing signaling peptides to the surface of EVs, is achieved using plasmid vectors encoding target ligands fused to transmembrane proteins [110]. Lamp-2b, serving as a surface protein with a signal peptide for EVs, is often fused to targeting proteins to present the protein as a targeting site, ligand, or receptor on the EV surface. For instance, Liang et al. stably transfected dendritic cells with the CAP-Green fluorescent protein (GFP)-Lamp2b plasmid, obtaining EVs specifically targeted to chondrocytes. These EVs delivered miRNA-140 to the deep cartilaginous region by effectively targeting chondrocytes [132]. Similarly, chondrocyte-targeted EVs were engineered by genetically fusing a chondrocyte affinity peptide to the N-terminal end of the EV surface protein Lamp2b, demonstrating favorable osteoarticular targeting [133]. EVs obtained through genetic engineering of parental cells not only retained their bone-targeting properties but also exhibited increased stability. However, the degradation of peptides by endoplasmic proteases within cells poses a significant challenge to the yield of targeted peptide-functional EVs.
Indeed, specific metabolic reprogramming can enhance the acquisition of targeted EVs. For instance, metabolic glycoengineering (MGE) facilitates biorthogonal copper-free click chemical modification on the parental cell surface [134]. Dong et al. [135] utilized MGE by culturing parental cells with tetraacetylated N-azidoacetyl-D-mannosamine to generate unnatural azide moieties onto their membrane surfaces. Subsequently, dibenzocyclooctyne-coupled dextran sulfate (DBCO-DS) was attached via click chemistry to azide-containing parental cells, resulting in the creation of macrophage-targeted EVs. This innovative targeting modification holds promise for advancing orthopedic disease treatment by allowing the incorporation of targeting molecules into EVs, thereby achieving bon-specific targeting while maintaining structural stability.
MSC-EVs combined with biomaterials to improve bone targeting capabilities
MSC-EVs can be directly employed in bone disease treatment, typically through the injection of an aqueous solution containing MSC-EVs into the circulation or tissue to enhance tissue repair. However, the direct injection of free MSC-EVs in aqueous solutions poses challenges, as they are difficult to retain in the target region for extended periods and may be promptly removed, hindering the full utilization of their biological functions [136]. Consequently, direct injection of MSC-EVs in an aqueous solution may not be an optimal strategy. Currently, numerous studies have reported cases where MSC-EVs are combined with biomaterials to enhance clinical therapeutic efficacy. This approach aims to confer MSC-EVs with the capability of controlled drug release at the disease site in a dose- and time-dependent manner [136, 137]. The ideal biomaterials for combination with MSC-EVs should possess the following properties: I. The in vivo degradation rate of the material should not be excessively fast, ensuring the delivery of MSC-EVs to the target tissues and allowing control over the release rate of the loaded drug [138]. II. After the release of the MSC-EVs loaded drug, the biomaterial should degrade within the tissue, and the degradation rate should align with the regeneration rate. III. The biomaterials should be targeted and can be co-targeted with MSC-EVs for efficient delivery to the disease site [139].
Hydrogels, owing to their excellent biocompatibility, biodegradability, and advantageous properties for cell infiltration and adhesion, have been increasingly utilized in scientific research and clinical application [140]. Studies indicate that biomolecules bound to hydrogels maintain their structure and function for an extended period, attributed to the hydrogel’s ability to remodel the microenvironment, facilitating the storage and uptake of MSC-EVs while enabling targeted delivery to bone tissue [141]. For example, hydrogels loaded with MSC-EVs exhibit the capacity to target bone tissue, promoting the repair of bone tissue defects and mitigating disc degeneration [142, 143]. Mechanistically, the synergistic combination of hydrogels and MSC-EVs may offer enhanced efficacy for alleviating bone diseases by modulating angiogenesis, ameliorating inflammation, and remodeling the immune environment [140, 144]. Additionally, other biomaterials, including degradable implants, tissue-derived materials, and growth factors, may offer potential options to improve the targeting and therapeutic efficiency of MSC-EVs.
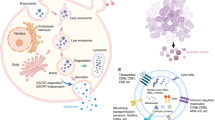
Therapeutic applications of MSC-EVs in bone-related diseases
Considering MSC-EVs’ ability to target specific cells or tissues and minimize the risk of toxicity to non-target tissues, they have been designed as a novel drug carrier for direct drug delivery to bone tissues, addressing various bone-related diseases, including OA, osteoporosis, RA, osteosarcoma (Fig. 2) (Table 1).
Osteoarthritis
Osteoarthritis (OA), a prevalent joint disease, is characterized by articular cartilage damage involving degeneration, fibrosis, fractures, defects, and overall joint surface impairment [145]. MSC-EVs emerge as promising drug carriers due to their capacity to deliver contents precisely to chondrocytes. For instance, MSC-EVs ameliorate OA by targeting histone deacetylase 3 and STAT1/NF-κB p65, delivering miR-326 to chondrocytes and cartilage, and inhibiting cell death within cartilage [146]. However, traversing the dense ECM of cartilage for molecules like miRNA remains challenging. Thus, the use of engineered EVs for delivery becomes a potential option. Liang et al. [133] engineered EVs by attaching chondrocyte affinity peptides to the N-terminal end of the EVs surface protein Lamp2b. Subsequently, they employed liposome membrane fusion to form hybridized chondrocyte-targeted EVs, encapsulating the CRISPR/Cas9 plasmid. The results demonstrated that such engineered EVs effectively targeted chondrocytes within cartilage injury, mitigating the degradation of ECM proteins and ultimately alleviating OA.
Osteoporosis
Osteoporosis, a systemic metabolic bone disease, primarily results from bone mass loss, destruction of bone tissue microstructure, and increased bone fragility, leading to fractures in patients [147]. The root cause of osteoporosis lies in the imbalance between osteoblasts and osteoclasts, disrupting bone metabolism and a shift in bone marrow cell lineage from osteoblasts to adipocytes. Current osteoporosis drugs, such as bisphosphonates, estrogens, and calcitonin, lack bone tissue targeting, resulting in poor therapeutic efficacy and associated side effects like nephrotoxicity and jaw necrosis [148, 149]. Given the excellent bone tissue targeting properties of MSC-EVs, they have been applied as drug carriers for osteoporosis treatment. For example, MSC-EVs-loaded LncRNA MALAT1 can target bone tissue by mediating the miRNA-34c/SATB2 axis, effectively alleviating osteoporosis [30]. Engineering modifications can further enhance the bone-targeting ability of MSC-EVs, thereby improving the therapeutic efficiency of osteoporosis drugs. The coupling of BM-MSC-EVs with BM-MSC-specific aptamers enhances the bone targeting of EVs, facilitating targeted drug delivery to bone tissue, promoting bone regeneration, and ameliorating osteoporosis [128]. Similarly, utilizing CXCR4-based EVs in combination with nanoparticles, expressing CXCR4 in the nature of EVs enables bone-targeted drug delivery, which could accumulate and release drugs in the bone marrow to reverse aging-related bone loss [150]. In summary, drug carriers based on MSC-EVs present a promising strategy against osteoporosis.
Rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic autoimmune disease with systemic involvement, characterized by joint inflammation, destruction of bone and cartilage, and loss of function [151]. Despite the use of NSAIDs, disease-modifying anti-rheumatic drugs (DMARDs), and biologics (e.g., TNF-α antagonists, IL-1 receptors and IL-6 receptors) in RA treatment, factors such as lack of specificity and low bioavailability continue to emphasize the need for innovating therapeutic approaches [152, 153]. Fortunately, MSC-EVs play a role in the amelioration of RA and can suppress inflammation through factors such as IL-1. This mechanism might be related to MSC-EVs’ ability to regulate the immune microenvironment by influencing the numbers of T cell and B cell subsets [154, 155]. Additionally, effective modulation of pro-inflammatory M1 and anti-inflammatory M2 macrophages, crucial in the inflammatory and autoimmune responses of RA, can mitigate the disease progression [156]. In a study, surface modification of macrophage-derived EVs with the anti-inflammatory cytokine interleukin-10, coupled with the encapsulation of the chemotherapeutic agent betamethasone sodium phosphate in EVs, facilitated targeted delivery to bone tissue, promoting M2 macrophage polarization and improving rheumatoid joint progression [157]. However, few studies have been reported on the use of engineered modified MSC-EVs for RA therapy. Considering the immunomodulatory capabilities of both MSC-EVs and macrophages, drug delivery to bone tissue through engineering modifications to MSC-EVs may present a potential therapeutic modality for RA [73, 158]. While more experiments are necessary to validate this speculation, natural MSC-EVs, as drug carriers, exhibited promising therapeutic effects in RA treatment [159].
Osteosarcoma
Osteosarcoma is a malignant tumor arising from mesenchymal cells, typically occurring in adolescents. Paradoxically, studies have revealed that MSC-EVs are involved in the dual regulation of osteosarcoma progression. On the one hand, they promote the proliferation and metastasis of osteosarcoma while simultaneously inhibiting its progression. For instance, lncRNA XIST in MSC-EVs can promote osteosarcoma growth and metastasis through the miR-655/ACLY signaling [41]. Conversely, miRNA-150 carried by MSC-EVs inhibits the proliferation and migration of osteosarcoma cells by regulating IGF2BP1 [160]. The contradiction arises from the diverse contents carried out by MSC-EVs. Nevertheless, it is undeniable that MSC-EVs can serve as effective drug carriers for targeted osteosarcoma treatment. For example, loading doxorubicin into MSC-EVs facilitates osteosarcoma therapy, with doxorubicin-loaded MSC-EVs targeting osteosarcoma therapy via the SDF1-CXCR4 axis [90, 113]. While there is a shortage of studies on the use of engineered MSC-EVs specifically for osteosarcoma treatment, insights from related cases can offer valuable perspectives. Huang et al. [161] demonstrated the preparation of c(RGDyK)-modified and MEG3-loaded EVs to enhance their targeting of osteosarcoma, effectively inhibiting osteosarcoma progression. This suggests the potential application of engineered MSC-EVs in advancing targeted therapies for osteosarcoma.
Importantly, the efficacy of MSC-EVs for the treatment of bone disease needs to be confirmed by more clinical studies. Currently, OA-based clinical studies have focused on MSC, and clinical studies have demonstrated that MSC can alleviate the symptoms of OA and promote cartilage regeneration [162, 163]. In addition, clinical studies have been used to confirm the efficacy of MSC-EVs in the treatment of OA, and unfortunately these two clinical studies have not yet been completed (NCT05520125, NCT04998058) [164]. Similar to OA, research on degenerative disc disease has focused on MSC and clinical studies have demonstrated that MSC can relieve back pain and slow the progression of degenerative disc disease (NCT04499105, NCT04759105) [165]. In addition, a Phase I clinical study of MSCEVs for the treatment of degenerative disc disease is ongoing and has not yet reached definitive conclusions (NCT04849429). For osteosarcoma, MSC-EVs have mainly been used as biomarkers for tumor diagnosis, and a clinical study at Ruijin Hospital in China confirmed the promising diagnostic potential of MSC-EVs in osteosarcoma (NCT05101655, NCT03108677) [166]. Considering that MSC-EVs have similar properties to MSC, MSC-EVs have a promising future for the treatment of bone diseases, but more research is needed to promote the clinical study and application of MSC-EVs for the treatment of bone diseases.
Conclusion and future perspectives
MSC-EVs are considered an effective tool for promoting tissue repair and regeneration. In recent years, extensive research has delved into the therapeutic potential of MSC-EVs in treating bone-related disorders. Their mechanisms and clinical applications have been widely explored. Functioning as bio messengers loaded with genetic material, MSC-EVs play a crucial role in regulating the progression of bone-related diseases. This involves actions such as promoting angiogenesis and maintaining bone metabolic homeostasis through miRNAs and lncRNAs, thereby alleviating conditions like OA, RA, and osteoporosis. Capitalizing on their exceptional properties, including high biocompatibility, low immunogenicity, easy penetration of biological barriers, and prolonged circulating half-life, MSC-EVs are acknowledged as promising vehicles for treating bone-related diseases. They can alleviate the disease symptoms by carrying various loads, such as NSAIDs, chemotherapeutic agents, and miRNAs. However, recognizing the inherent limitation of natural MSC-EVs in low bone targeting, their efficacy can be significantly enhanced through diverse engineering modification strategies. These modifications aim to improve the precision of drug delivery, increase loading efficiency, enable controlled release, and improve bone targeting. MSC-EVs exhibit the potential to revolutionize the treatment of bone-related diseases, thus a novel and promising therapeutic strategy.
However, the clinical application of MSC-EVs faces several challenges. First, there is a pressing need for effective procedures to purify clinical-grade MSC-EVs from samples, necessitating the development of robust purification guidelines. Second, the evaluation and analysis of heterogeneity within subpopulations of MSC-EVs pose challenges, given that individual MSC-EVs loaded with different biomolecules can significantly impact tissue biological functions. Thirdly, the administration route, optimal dosage, efficiency, and cost-effectiveness of MSC-EVs as drug carriers require thorough evaluation. Additionally, feasible strategies must be identified for engineering modifications on a large scale to enhance the therapeutic applications of MSC-EVs.
Furthermore, more extensive clinical research is essential to confirm the clinical utility and safety of bone-targeted MSC-EVs, especially when combined with biomaterials and promising AI strategies. The paradoxical role of MSC-EVs in regulating osteosarcoma progression, simultaneously promoting tumor proliferation and metastasis while inhibiting tumor progression, adds complexity. Consequently, careful consideration is necessary to avoid potential tumorigenic effects when utilizing MSC-EVs as drug carriers for osteosarcoma treatment. Despite these challenges, the potential of bone-targeted MSC-EVs as a next-generation therapeutic platform for bone-related diseases remains promising.
References
Shi C, Wu T, He Y, Zhang Y, Fu D. Recent advances in bone-targeted therapy. Pharmacol Ther. 2020;207:107473. https://doi.org/10.1016/j.pharmthera.2020.107473.
Cheng H, Chawla A, Yang Y, Li Y, Zhang J, Jang HL, et al. Development of nanomaterials for bone-targeted drug delivery. Drug Discov Today. 2017;22:1336–50. https://doi.org/10.1016/j.drudis.2017.04.021.
Bernardes de Jesus B, Schneeberger K, Vera E, Tejera A, Harley CB, Blasco MA. The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell. 2011;10:604–21. https://doi.org/10.1111/j.1474-9726.2011.00700.x.
Yang C, Tian Y, Zhao F, Chen Z, Su P, Li Y, et al. Bone microenvironment and osteosarcoma metastasis. Int J Mol Sci. 2020;21. https://doi.org/10.3390/ijms21196985.
Li J, Yin Z, Huang B, Xu K, Su J. Stat3 signaling pathway: a future therapeutic target for bone-related diseases. Front Pharmacol. 2022;13:897539. https://doi.org/10.3389/fphar.2022.897539.
Lu L, Xu A, Gao F, Tian C, Wang H, Zhang J, et al. Mesenchymal stem cell-derived exosomes as a novel strategy for the treatment of intervertebral disc degeneration. Front. Cell Dev Biol. 2021;9:770510. https://doi.org/10.3389/fcell.2021.770510.
Hu ZL, Li HY, Chang X, Li YY, Liu CH, Gao XX, et al. Exosomes derived from stem cells as an emerging therapeutic strategy for intervertebral disc degeneration. World J Stem Cells. 2020;12:803–13. https://doi.org/10.4252/wjsc.v12.i8.803.
Zhang F, Guo J, Zhang Z, Qian Y, Wang G, Duan M, et al. Mesenchymal stem cell-derived exosome: a tumor regulator and carrier for targeted tumor therapy. Cancer Lett. 2022;526:29–40. https://doi.org/10.1016/j.canlet.2021.11.015.
Gerami MH, Khorram R, Rasoolzadegan S, Mardpour S, Nakhaei P, Hashemi S, et al. Emerging role of mesenchymal stem/stromal cells (MSCs) and MSCs-derived exosomes in bone- and joint-associated musculoskeletal disorders: a new frontier. Eur J Med Res. 2023;28:86. https://doi.org/10.1186/s40001-023-01034-5.
Lu V, Tennyson M, Zhang J, Khan W. Mesenchymal stem cell-derived extracellular vesicles in tendon and ligament repair-a systematic review of in vivo studies. Cells. 2021;10. https://doi.org/10.3390/cells10102553.
Lui PPY. Mesenchymal stem cell-derived extracellular vesicles for the promotion of tendon repair - an update of literature. Stem Cell Rev Rep. 2021;17:379–89. https://doi.org/10.1007/s12015-020-10023-8.
Sun H, Pratt RE, Hodgkinson CP, Dzau VJ. Sequential paracrine mechanisms are necessary for the therapeutic benefits of stem cell therapy. Am J Physiol Cell Physiol. 2020;319:C1141–C1150. https://doi.org/10.1152/ajpcell.00516.2019.
Shah R, Patel T, Freedman JE. Circulating extracellular vesicles in human disease. N Engl J Med. 2018;379:958–66. https://doi.org/10.1056/NEJMra1704286.
Nooshabadi VT, Mardpour S, Yousefi-Ahmadipour A, Allahverdi A, Izadpanah M, Daneshimehr F, et al. The extracellular vesicles-derived from mesenchymal stromal cells: a new therapeutic option in regenerative medicine. J Cell Biochem. 2018;119:8048–73. https://doi.org/10.1002/jcb.26726.
Timmers L, Pasterkamp G, de Hoog VC, Arslan F, Appelman Y, de Kleijn DP. The innate immune response in reperfused myocardium. Cardiovasc Res. 2012;94:276–83. https://doi.org/10.1093/cvr/cvs018.
Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM. Exosome mediated communication within the tumor microenvironment. J Control Release: Off J Control Release Soc. 2015;219:278–94. https://doi.org/10.1016/j.jconrel.2015.06.029.
Marar C, Starich B, Wirtz D. Extracellular vesicles in immunomodulation and tumor progression. Nat. Immunol. 2021;22:560–70. https://doi.org/10.1038/s41590-021-00899-0.
Zhou E, Li Y, Wu F, Guo M, Xu J, Wang S, et al. Circulating extracellular vesicles are effective biomarkers for predicting response to cancer therapy. EBioMedicine. 2021;67:103365. https://doi.org/10.1016/j.ebiom.2021.103365.
Ohayon L, Zhang X, Dutta P. The role of extracellular vesicles in regulating local and systemic inflammation in cardiovascular disease. Pharmacol Res. 2021;170:105692. https://doi.org/10.1016/j.phrs.2021.105692.
Zou J, Yang W, Cui W, Li C, Ma C, Ji X, et al. Therapeutic potential and mechanisms of mesenchymal stem cell-derived exosomes as bioactive materials in tendon-bone healing. J Nanobiotechnol. 2023;21:14. https://doi.org/10.1186/s12951-023-01778-6.
Liu N, Dong J, Li L, Liu F. Osteoimmune interactions and therapeutic potential of macrophage-derived small extracellular vesicles in bone-related diseases. Int J Nanomed. 2023;18:2163–80. https://doi.org/10.2147/ijn.S403192.
Wei W, Ao Q, Wang X, Cao Y, Liu Y, Zheng SG, et al. Mesenchymal stem cell-derived exosomes: a promising biological tool in nanomedicine. Front Pharmacol. 2020;11:590470. https://doi.org/10.3389/fphar.2020.590470.
Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–90. https://doi.org/10.1016/j.biomaterials.2013.11.083.
Wang J, Li X, Wang S, Cui J, Ren X, Su J. Bone-targeted exosomes: strategies and applications. Adv Healthc Mater. 2023;12:e2203361. https://doi.org/10.1002/adhm.202203361.
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–28. https://doi.org/10.1038/nrm.2017.125.
Yin B, Ni J, Witherel CE, Yang M, Burdick JA, Wen C, et al. Harnessing tissue-derived extracellular vesicles for osteoarthritis theranostics. Theranostics. 2022;12:207–31. https://doi.org/10.7150/thno.62708.
Tamura T, Yoshioka Y, Sakamoto S, Ichikawa T, Ochiya T. Extracellular vesicles in bone homeostasis: key roles of physiological and pathological conditions. J Bone Miner Metab. 2023;41:345–57. https://doi.org/10.1007/s00774-022-01362-2.
Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89. https://doi.org/10.1146/annurev-cellbio-101512-122326.
Lin Z, Rodriguez NE, Zhao J, Ramey AN, Hyzy SL, Boyan BD, et al. Selective enrichment of microRNAs in extracellular matrix vesicles produced by growth plate chondrocytes. Bone. 2016;88:47–55. https://doi.org/10.1016/j.bone.2016.03.018.
Yang X, Yang J, Lei P, Wen T. LncRNA MALAT1 shuttled by bone marrow-derived mesenchymal stem cells-secreted exosomes alleviates osteoporosis through mediating microRNA-34c/SATB2 axis. Aging. 2019;11:8777–91. https://doi.org/10.18632/aging.102264.
Wu D, Chang X, Tian J, Kang L, Wu Y, Liu J, et al. Bone mesenchymal stem cells stimulation by magnetic nanoparticles and a static magnetic field: release of exosomal miR-1260a improves osteogenesis and angiogenesis. J Nanobiotechnol. 2021;19:209. https://doi.org/10.1186/s12951-021-00958-6.
Behera J, Kumar A, Voor MJ, Tyagi N. Exosomal lncRNA-H19 promotes osteogenesis and angiogenesis through mediating Angpt1/Tie2-NO signaling in CBS-heterozygous mice. Theranostics. 2021;11:7715–34. https://doi.org/10.7150/thno.58410.
Liu W, Li L, Rong Y, Qian D, Chen J, Zhou Z, et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020;103:196–212. https://doi.org/10.1016/j.actbio.2019.12.020.
Tao Y, Zhou J, Wang Z, Tao H, Bai J, Ge G, et al. Human bone mesenchymal stem cells-derived exosomal miRNA-361-5p alleviates osteoarthritis by downregulating DDX20 and inactivating the NF-κB signaling pathway. Bioorg Chem. 2021;113:104978. https://doi.org/10.1016/j.bioorg.2021.104978.
Zhang Y, Cao X, Li P, Fan Y, Zhang L, Ma X, et al. microRNA-935-modified bone marrow mesenchymal stem cells-derived exosomes enhance osteoblast proliferation and differentiation in osteoporotic rats. Life Sci. 2021;272:119204. https://doi.org/10.1016/j.lfs.2021.119204.
Wang X, Thomsen P. Mesenchymal stem cell-derived small extracellular vesicles and bone regeneration. Basic Clin Pharmacol Toxicol. 2021;128:18–36. https://doi.org/10.1111/bcpt.13478.
Xu JF, Yang GH, Pan XH, Zhang SJ, Zhao C, Qiu BS, et al. Altered microRNA expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PloS One. 2014;9:e114627. https://doi.org/10.1371/journal.pone.0114627.
Zhao P, Xiao L, Peng J, Qian YQ, Huang CC. Exosomes derived from bone marrow mesenchymal stem cells improve osteoporosis through promoting osteoblast proliferation via MAPK pathway. Eur Rev Med Pharmacol Sci. 2018;22:3962–70. https://doi.org/10.26355/eurrev_201806_15280.
Shen K, Duan A, Cheng J, Yuan T, Zhou J, Song H, et al. Exosomes derived from hypoxia preconditioned mesenchymal stem cells laden in a silk hydrogel promote cartilage regeneration via the miR-205-5p/PTEN/AKT pathway. Acta Biomater. 2022;143:173–88. https://doi.org/10.1016/j.actbio.2022.02.026.
Fan L, Dong J, He X, Zhang C, Zhang T. Bone marrow mesenchymal stem cells-derived exosomes reduce apoptosis and inflammatory response during spinal cord injury by inhibiting the TLR4/MyD88/NF-κB signaling pathway. Hum Exp Toxicol. 2021;40:1612–23. https://doi.org/10.1177/09603271211003311.
Zhu G, Xia Y, Zhao Z, Li A, Li H, Xiao T. LncRNA XIST from the bone marrow mesenchymal stem cell derived exosome promotes osteosarcoma growth and metastasis through miR-655/ACLY signal. Cancer Cell Int. 2022;22:330. https://doi.org/10.1186/s12935-022-02746-0.
Qi H, Liu DP, Xiao DW, Tian DC, Su YW, Jin SF. Exosomes derived from mesenchymal stem cells inhibit mitochondrial dysfunction-induced apoptosis of chondrocytes via p38, ERK, and Akt pathways. Vitr Cell Dev Biol Anim. 2019;55:203–10. https://doi.org/10.1007/s11626-019-00330-x.
Li H, Liu D, Li C, Zhou S, Tian D, Xiao D, et al. Exosomes secreted from mutant-HIF-1α-modified bone-marrow-derived mesenchymal stem cells attenuate early steroid-induced avascular necrosis of femoral head in rabbit. Cell Biol Int. 2017;41:1379–90. https://doi.org/10.1002/cbin.10869.
Fang S, He T, Jiang J, Li Y, Chen P. Osteogenic effect of tsRNA-10277-loaded exosome derived from bone mesenchymal stem cells on steroid-induced osteonecrosis of the femoral head. Drug Des Dev Ther. 2020;14:4579–91. https://doi.org/10.2147/dddt.S258024.
Xie Y, Chen Y, Zhang L, Ge W, Tang P. The roles of bone-derived exosomes and exosomal microRNAs in regulating bone remodelling. J Cell Mol Med. 2017;21:1033–41. https://doi.org/10.1111/jcmm.13039.
Cui K, Chen Y, Zhong H, Wang N, Zhou L, Jiang F. Transplantation of IL-10-overexpressing bone marrow-derived mesenchymal stem cells ameliorates diabetic-induced impaired fracture healing in mice. Cell Mol Bioeng. 2020;13:155–63. https://doi.org/10.1007/s12195-019-00608-w.
Nakao Y, Fukuda T, Zhang Q, Sanui T, Shinjo T, Kou X, et al. Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021;122:306–24. https://doi.org/10.1016/j.actbio.2020.12.046.
Lu GD, Cheng P, Liu T, Wang Z. BMSC-derived exosomal miR-29a promotes angiogenesis and osteogenesis. Front. Cell Dev Biol. 2020;8:608521. https://doi.org/10.3389/fcell.2020.608521.
Zhang L, Jiao G, Ren S, Zhang X, Li C, Wu W, et al. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res Ther. 2020;11:38. https://doi.org/10.1186/s13287-020-1562-9.
Qiu B, Xu X, Yi P, Hao Y. Curcumin reinforces MSC-derived exosomes in attenuating osteoarthritis via modulating the miR-124/NF-kB and miR-143/ROCK1/TLR9 signalling pathways. J Cell Mol Med. 2020;24:10855–65. https://doi.org/10.1111/jcmm.15714.
Wang Q, Shen X, Chen Y, Chen J, Li Y. Osteoblasts-derived exosomes regulate osteoclast differentiation through miR-503-3p/Hpse axis. Acta Histochem. 2021;123:151790. https://doi.org/10.1016/j.acthis.2021.151790.
Chen J, Hendriks M, Chatzis A, Ramasamy SK, Kusumbe AP. Bone vasculature and bone marrow vascular niches in health and disease. J Bone Miner Res Off J Am Soc Bone Miner Res. 2020;35:2103–20. https://doi.org/10.1002/jbmr.4171.
Meeson R, Sanghani-Keri A, Coathup M, Blunn G. VEGF with AMD3100 endogenously mobilizes mesenchymal stem cells and improves fracture healing. J Orthop Res Off Publ Orthop Res Soc. 2019;37:1294–302. https://doi.org/10.1002/jor.24164.
Wang CY, Yang HB, Hsu HS, Chen LL, Tsai CC, Tsai KS, et al. Mesenchymal stem cell-conditioned medium facilitates angiogenesis and fracture healing in diabetic rats. J Tissue Eng Regenerat Med. 2012;6:559–69. https://doi.org/10.1002/term.461.
Kasper G, Dankert N, Tuischer J, Hoeft M, Gaber T, Glaeser JD, et al. Mesenchymal stem cells regulate angiogenesis according to their mechanical environment. Stem Cells (Dayt, Ohio). 2007;25:903–10. https://doi.org/10.1634/stemcells.2006-0432.
Wang R, Xu B. TGFβ1-modified MSC-derived exosome attenuates osteoarthritis by inhibiting PDGF-BB secretion and H-type vessel activity in the subchondral bone. Acta Histochem. 2022;124:151933. https://doi.org/10.1016/j.acthis.2022.151933.
Huang Y, He B, Wang L, Yuan B, Shu H, Zhang F, et al. Bone marrow mesenchymal stem cell-derived exosomes promote rotator cuff tendon-bone healing by promoting angiogenesis and regulating M1 macrophages in rats. Stem Cell Res Ther. 2020;11:496. https://doi.org/10.1186/s13287-020-02005-x.
Wang X, Freire Valls A, Schermann G, Shen Y, Moya IM, Castro L, et al. YAP/TAZ orchestrate VEGF signaling during developmental angiogenesis. Dev Cell. 2017;42:462–478.e467. https://doi.org/10.1016/j.devcel.2017.08.002.
Tutuianu R, Rosca AM, Iacomi DM, Simionescu M, Titorencu I. Human mesenchymal stromal cell-derived exosomes promote in vitro wound healing by modulating the biological properties of skin keratinocytes and fibroblasts and stimulating angiogenesis. Int J Mol Sci. 2021; 22. https://doi.org/10.3390/ijms22126239.
Becerra J, Andrades JA, Guerado E, Zamora-Navas P, López-Puertas JM, Reddi AH. Articular cartilage: structure and regeneration. Tissue Eng. Part B, Rev. 2010;16:617–27. https://doi.org/10.1089/ten.TEB.2010.0191.
Nee LE, McMorrow T, Campbell E, Slattery C, Ryan MP. TNF-alpha and IL-1beta-mediated regulation of MMP-9 and TIMP-1 in renal proximal tubular cells. Kidney Int. 2004;66:1376–86. https://doi.org/10.1111/j.1523-1755.2004.00900.x.
Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4:157–64. https://doi.org/10.1186/ar401.
Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noël D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017;7:16214. https://doi.org/10.1038/s41598-017-15376-8.
van Buul GM, Villafuertes E, Bos PK, Waarsing JH, Kops N, Narcisi R, et al. Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthr Cartil. 2012;20:1186–96. https://doi.org/10.1016/j.joca.2012.06.003.
Zeng Z, Dai Y, Deng S, Zou S, Dou T, Wei F. Synovial mesenchymal stem cell-derived extracellular vesicles alleviate chondrocyte damage during osteoarthritis through microRNA-130b-3p-mediated inhibition of the LRP12/AKT/β-catenin axis. Immunopharmacol Immunotoxicol. 2022;44:247–60. https://doi.org/10.1080/08923973.2022.2038192.
Wang H, Li F, Ban W, Zhang J, Zhang G. Human bone marrow mesenchymal stromal cell-derived extracellular vesicles promote proliferation of degenerated nucleus pulposus cells and the synthesis of extracellular matrix through the SOX4/Wnt/β-Catenin axis. Front. Physiol. 2021;12:723220. https://doi.org/10.3389/fphys.2021.723220.
Zhou X, Cao H, Guo J, Yuan Y, Ni G. Effects of BMSC-derived EVs on bone metabolism. Pharmaceutics. 2022; 14. https://doi.org/10.3390/pharmaceutics14051012.
Lian JB, Stein GS, Javed A, van Wijnen AJ, Stein JL, Montecino M, et al. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr. Metab Disord. 2006;7:1–16. https://doi.org/10.1007/s11154-006-9001-5.
Han L, Liu H, Fu H, Hu Y, Fang W, Liu J. Exosome-delivered BMP-2 and polyaspartic acid promotes tendon bone healing in rotator cuff tear via Smad/RUNX2 signaling pathway. Bioengineered. 2022;13:1459–75. https://doi.org/10.1080/21655979.2021.2019871.
Hjorthaug GA, Søreide E, Nordsletten L, Madsen JE, Reinholt FP, Niratisairak S, et al. Negative effect of zoledronic acid on tendon-to-bone healing. Acta Orthopaedica. 2018;89:360–3. https://doi.org/10.1080/17453674.2018.1440189.
Zhao F, Ma X, Qiu W, Wang P, Zhang R, Chen Z, et al. Mesenchymal MACF1 Facilitates SMAD7 Nuclear Translocation to Drive Bone Formation. Cells. 2020; 9. https://doi.org/10.3390/cells9030616.
Feng W, Jin Q, Ming-Yu Y, Yang H, Xu T, You-Xing S, et al. MiR-6924-5p-rich exosomes derived from genetically modified Scleraxis-overexpressing PDGFRα(+) BMMSCs as novel nanotherapeutics for treating osteolysis during tendon-bone healing and improving healing strength. Biomaterials. 2021;279:121242. https://doi.org/10.1016/j.biomaterials.2021.121242.
Hu Y, Zhang Y, Ni CY, Chen CY, Rao SS, Yin H, et al. Human umbilical cord mesenchymal stromal cells-derived extracellular vesicles exert potent bone protective effects by CLEC11A-mediated regulation of bone metabolism. Theranostics. 2020;10:2293–308. https://doi.org/10.7150/thno.39238.
Matsuzaka Y, Yashiro R, Therapeutic Strategy of Mesenchymal-Stem-Cell-Derived Extracellular Vesicles as Regenerative Medicine. Int J Mol Sci. 2022; 23. https://doi.org/10.3390/ijms23126480.
Ogbechi J, Clanchy FI, Huang YS, Topping LM, Stone TW, Williams RO. IDO activation, inflammation and musculoskeletal disease. Exp Gerontol. 2020;131:110820. https://doi.org/10.1016/j.exger.2019.110820.
Demoruelle MK, Deane KD, Holers VM. When and where does inflammation begin in rheumatoid arthritis? Curr Opin Rheumatol. 2014;26:64–71. https://doi.org/10.1097/bor.0000000000000017.
Sebastião AI, Ferreira I, Brites G, Silva A, Neves BM, Teresa Cruz M, NLRP3 Inflammasome and Allergic Contact Dermatitis: A Connection to Demystify, Pharmaceutics. 12. https://doi.org/10.3390/pharmaceutics12090867 (2020)
Jia M, Lv Y, Xu Y, Gong Z. A comparative analysis of NLRP3-related inflammatory mediators in synovial fluid in temporomandibular joint osteoarthritis and internal derangement. BMC Musculoskelet. Disord. 2021;22:229. https://doi.org/10.1186/s12891-021-04092-0.
Weyand CM, Goronzy JJ. The immunology of rheumatoid arthritis. Nat. Immunol. 2021;22:10–18. https://doi.org/10.1038/s41590-020-00816-x.
Gao F, Chiu SM, Motan DA, Zhang Z, Chen L, Ji HL, et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7:e2062. https://doi.org/10.1038/cddis.2015.327.
Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong MH, Yoon SH, et al. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. 2014;46:e70. https://doi.org/10.1038/emm.2013.135.
Cheung TS, Galleu A, von Bonin M, Bornhäuser M, Dazzi F. Apoptotic mesenchymal stromal cells induce prostaglandin E2 in monocytes: implications for the monitoring of mesenchymal stromal cell activity. Haematologica. 2019;104:e438–e441. https://doi.org/10.3324/haematol.2018.214767.
Chen J, Liu R, Huang T, Sun H, Jiang H. Adipose stem cells-released extracellular vesicles as a next-generation cargo delivery vehicles: a survey of minimal information implementation, mass production and functional modification. Stem Cell Res Ther. 2022;13:182. https://doi.org/10.1186/s13287-022-02849-5.
Bouffi C, Bony C, Courties G, Jorgensen C, Noël D. IL-6-dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PloS One. 2010;5:e14247. https://doi.org/10.1371/journal.pone.0014247.
Shi Y, Kang X, Wang Y, Bian X, He G, Zhou M, et al. Exosomes derived from bone marrow stromal cells (BMSCs) enhance tendon-bone healing by regulating macrophage polarization. Med Sci Monit: Int Med J Exp Clin Res. 2020;26:e923328. https://doi.org/10.12659/msm.923328.
Xu J, Ye Z, Han K, Zheng T, Zhang T, Dong S, et al. Infrapatellar fat pad mesenchymal stromal cell-derived exosomes accelerate tendon-bone healing and intra-articular graft remodeling after anterior cruciate ligament reconstruction. Am J Sports Med. 2022;50:662–73. https://doi.org/10.1177/03635465211072227.
Li Z, Li Q, Tong K, Zhu J, Wang H, Chen B, et al. BMSC-derived exosomes promote tendon-bone healing after anterior cruciate ligament reconstruction by regulating M1/M2 macrophage polarization in rats. Stem Cell Res Ther. 2022;13:295. https://doi.org/10.1186/s13287-022-02975-0.
Heilig R, Dick MS, Sborgi L, Meunier E, Hiller S, Broz P. The Gasdermin-D pore acts as a conduit for IL-1β secretion in mice. Eur J Immunol. 2018;48:584–92. https://doi.org/10.1002/eji.201747404.
Zhou Q, Wang W, Yang F, Wang H, Zhao X, Zhou Y, et al. Disulfiram suppressed peritendinous fibrosis through inhibiting macrophage accumulation and its pro-inflammatory properties in tendon bone healing. Front Bioeng Biotechnol. 2022;10:823933. https://doi.org/10.3389/fbioe.2022.823933.
Wei H, Chen J, Wang S, Fu F, Zhu X, Wu C, Lin J. et al. A nanodrug consisting of doxorubicin and exosome derived from mesenchymal stem cells for osteosarcoma treatment in vitro. Int. J. Nanomed.2019;14:8603–10. https://doi.org/10.2147/ijn.S218988.
Kalluri R, LeBleu VS, The biology, function, and biomedical applications of exosomes. Science (New York, N.Y.). 2020; 367. https://doi.org/10.1126/science.aau6977.
Saint-Pol J, Gosselet F, Duban-Deweer S, Pottiez G, Karamanos Y. Targeting and crossing the blood-brain barrier with extracellular vesicles. Cells. 2020; 9. https://doi.org/10.3390/cells9040851.
Sharma S, Masud MK, Kaneti YV, Rewatkar P, Koradia A, Hossain MSA, et al. Extracellular vesicle nanoarchitectonics for novel drug delivery applications. Small (Weinh Der Bergstr, Ger). 2021;17:e2102220. https://doi.org/10.1002/smll.202102220.
Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, et al. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65:336–41. https://doi.org/10.1016/j.addr.2012.07.001.
Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32:252–60. https://doi.org/10.1038/nbt.2816.
Ortega-Pineda L, Sunyecz A, Salazar-Puerta AI, Rincon-Benavides MA, Alzate-Correa D, Anaparthi AL, et al. Designer extracellular vesicles modulate pro-neuronal cell responses and improve intracranial retention. Adv Healthc Mater. 2022;11:e2100805. https://doi.org/10.1002/adhm.202100805.
Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7:653–64. https://doi.org/10.1038/nrclinonc.2010.139.
Zhang F, Guo J, Zhang Z, Duan M, Wang G, Qian Y, et al. Application of engineered extracellular vesicles for targeted tumor therapy. J Biomed Sci. 2022;29:14. https://doi.org/10.1186/s12929-022-00798-y.
Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Controlled Release: Off J Controlled Release Soc. 2015;207:18–30. https://doi.org/10.1016/j.jconrel.2015.03.033.
Kooijmans SAA, Stremersch S, Braeckmans K, de Smedt SC, Hendrix A, Wood MJA, et al. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J Controlled Release: Off J Controlled Release Soc. 2013;172:229–38. https://doi.org/10.1016/j.jconrel.2013.08.014.
Jia Y, Lu T, Chen Q, Pu X, Ji L, Yang J, et al. Exosomes secreted from sonic hedgehog-modified bone mesenchymal stem cells facilitate the repair of rat spinal cord injuries. Acta Neurochirurgica. 2021;163:2297–306. https://doi.org/10.1007/s00701-021-04829-9.
Liu X, Wang J, Wang P, Zhong L, Wang S, Feng Q, et al. Hypoxia-pretreated mesenchymal stem cell-derived exosomes-loaded low-temperature extrusion 3D-printed implants for neural regeneration after traumatic brain injury in canines. Front Bioeng Biotechnol. 2022;10:1025138. https://doi.org/10.3389/fbioe.2022.1025138.
Bosch S, de Beaurepaire L, Allard M, Mosser M, Heichette C, Chrétien D, et al. Trehalose prevents aggregation of exosomes and cryodamage. Sci Rep. 2016;6:36162. https://doi.org/10.1038/srep36162.
Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed: Nanotechnol Biol Med. 2016;12:655–64. https://doi.org/10.1016/j.nano.2015.10.012.
Huang L, Rong Y, Tang X, Yi K, Qi P, Hou J, et al. Engineered exosomes as an in situ DC-primed vaccine to boost antitumor immunity in breast cancer. Mol Cancer. 2022;21:45. https://doi.org/10.1186/s12943-022-01515-x.
Xiong QH, Zhao L, Wan GQ, Hu YG, Li XL. Engineered BMSCs-derived exosomal miR-542-3p promotes cutaneous wound healing. Endocr Metab Immune Disord Drug Targets. 2023;23:336–46. https://doi.org/10.2174/1871530322666220523151713.
Li W, Xu Y, Chen W. Bone mesenchymal stem cells deliver exogenous lncRNA CAHM via exosomes to regulate macrophage polarization and ameliorate intervertebral disc degeneration. Exp Cell Res. 2022;421:113408. https://doi.org/10.1016/j.yexcr.2022.113408.
Pang L, Jin H, Lu Z, Xie F, Shen H, Li X, et al. Treatment with mesenchymal stem cell-derived nanovesicle-containing gelatin methacryloyl hydrogels alleviates osteoarthritis by modulating chondrogenesis and macrophage polarization. Adv Healthc Mater. 2023;12:e2300315. https://doi.org/10.1002/adhm.202300315.
Xia P, Wang Q, Song J, Wang X, Wang X, Lin Q, et al. Low-intensity pulsed ultrasound enhances the efficacy of bone marrow-derived mscs in osteoarthritis cartilage repair by regulating autophagy-mediated exosome release. Cartilage. 2022;13:19476035221093060. https://doi.org/10.1177/19476035221093060.
Salunkhe S, Basak DM, Chitkara D, Mittal A. Surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking: strategies and significance. J Controlled Release: Off J Controlled Release Soc. 2020;326:599–614. https://doi.org/10.1016/j.jconrel.2020.07.042.
Rana S, Zöller M. Exosome target cell selection and the importance of exosomal tetraspanins: a hypothesis. Biochem Soc Trans. 2011;39:559–62. https://doi.org/10.1042/bst0390559.
Matsuzaka Y, Tanihata J, Komaki H, Ishiyama A, Oya Y, Rüegg U, et al. Characterization and functional analysis of extracellular vesicles and muscle-abundant mirnas (mir-1, mir-133a, and mir-206) in c2c12 myocytes and mdx mice. PloS One. 2016;11:e0167811. https://doi.org/10.1371/journal.pone.0167811.
Wei H, Chen F, Chen J, Lin H, Wang S, Wang Y, et al. Mesenchymal stem cell derived exosomes as nanodrug carrier of doxorubicin for targeted osteosarcoma therapy via SDF1-CXCR4 axis. Int J Nanomed. 2022;17:3483–95. https://doi.org/10.2147/ijn.S372851.
Shefler I, Salamon P, Y. Hershko A, A. Mekori Y. Mast cells as sources and targets of membrane vesicles. Curr Pharm Des. 2011;17:3797–804. https://doi.org/10.2174/138161211798357836.
Zhang H, Wang J, Ren T, Huang Y, Liang X, Yu Y, et al. Bone marrow mesenchymal stem cell-derived exosomal miR-206 inhibits osteosarcoma progression by targeting TRA2B. Cancer Lett. 2020;490:54–65. https://doi.org/10.1016/j.canlet.2020.07.008.
Wu Q, Zhou L, Lv D, Zhu X, Tang H. Exosome-mediated communication in the tumor microenvironment contributes to hepatocellular carcinoma development and progression. J Hematol Oncol. 2019;12:53. https://doi.org/10.1186/s13045-019-0739-0.
Ailuno G, Baldassari S, Lai F, Florio T, Caviglioli G. Exosomes and extracellular vesicles as emerging theranostic platforms in cancer research. Cells. 2020; 9. https://doi.org/10.3390/cells9122569.
Wang Y, Yao J, Cai L, Liu T, Wang X, Zhang Y, et al. Bone-targeted extracellular vesicles from mesenchymal stem cells for osteoporosis therapy. Int J Nanomed. 2020;15:7967–77. https://doi.org/10.2147/ijn.S263756.
Smyth T, Petrova K, Payton NM, Persaud I, Redzic JS, Graner MW, et al. Surface functionalization of exosomes using click chemistry. Bioconjugate Chem. 2014;25:1777–84. https://doi.org/10.1021/bc500291r.
Jiang L, Luirink J, Kooijmans SAA, van Kessel KPM, Jong W, van Essen M, et al. A post-insertion strategy for surface functionalization of bacterial and mammalian cell-derived extracellular vesicles. Biochimica et. Biophysica acta Gen Subj. 2021;1865:129763. https://doi.org/10.1016/j.bbagen.2020.129763.
Yan F, Zhong Z, Wang Y, Feng Y, Mei Z, Li H, et al. Exosome-based biomimetic nanoparticles targeted to inflamed joints for enhanced treatment of rheumatoid arthritis. J Nanobiotechnol. 2020;18:115. https://doi.org/10.1186/s12951-020-00675-6.
Paulos CM, Turk MJ, Breur GJ, Low PS. Folate receptor-mediated targeting of therapeutic and imaging agents to activated macrophages in rheumatoid arthritis. Adv Drug Deliv Rev. 2004;56:1205–17. https://doi.org/10.1016/j.addr.2004.01.012.
Zhang N, Xu C, Li N, Zhang S, Fu L, Chu X, et al. Folate receptor-targeted mixed polysialic acid micelles for combating rheumatoid arthritis: in vitro and in vivo evaluation. Drug Deliv. 2018;25:1182–91. https://doi.org/10.1080/10717544.2018.1472677.
Kooijmans SAA, Fliervoet LAL, van der Meel R, Fens M, Heijnen HFG, van Bergen En Henegouwen PMP, et al. PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J. Controlled Release : Off. J. Controlled Release Soc. 2016;224:77–85. https://doi.org/10.1016/j.jconrel.2016.01.009.
Cui Y, Guo Y, Kong L, Shi J, Liu P, Li R, et al. A bone-targeted engineered exosome platform delivering siRNA to treat osteoporosis. Bioact Mater. 2022;10:207–21. https://doi.org/10.1016/j.bioactmat.2021.09.015.
Yallowitz AR, Shim JH, Xu R, Greenblatt MB. An angiogenic approach to osteoanabolic therapy targeting the SHN3-SLIT3 pathway. Bone. 2023;172:116761. https://doi.org/10.1016/j.bone.2023.116761.
Henning RJ. Cardiovascular exosomes and MicroRNAs in cardiovascular physiology and pathophysiology. J Cardiovasc Transl Res. 2021;14:195–212. https://doi.org/10.1007/s12265-020-10040-5.
Luo ZW, Li FX, Liu YW, Rao SS, Yin H, Huang J, et al. Aptamer-functionalized exosomes from bone marrow stromal cells target bone to promote bone regeneration. Nanoscale. 2019;11:20884–92. https://doi.org/10.1039/c9nr02791b.
Vidal M. Exosomes and GPI-anchored proteins: judicious pairs for investigating biomarkers from body fluids. Adv Drug Deliv Rev. 2020;161-162:110–23. https://doi.org/10.1016/j.addr.2020.08.006.
Sun S, Liu H, Hu Y, Wang Y, Zhao M, Yuan Y, et al. Selection and identification of a novel ssDNA aptamer targeting human skeletal muscle. Bioact Mater. 2023;20:166–78. https://doi.org/10.1016/j.bioactmat.2022.05.016.
Choi H, Choi Y, Yim HY, Mirzaaghasi A, Yoo JK, Choi C. Biodistribution of exosomes and engineering strategies for targeted delivery of therapeutic exosomes. Tissue Eng Regen Med. 2021;18:499–511. https://doi.org/10.1007/s13770-021-00361-0.
Liang Y, Xu X, Li X, Xiong J, Li B, Duan L, et al. Chondrocyte-targeted MicroRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl Mater Interfaces. 2020;12:36938–47. https://doi.org/10.1021/acsami.0c10458.
Liang Y, Xu X, Xu L, Iqbal Z, Ouyang K, Zhang H, et al. Chondrocyte-specific genomic editing enabled by hybrid exosomes for osteoarthritis treatment. Theranostics. 2022;12:4866–78. https://doi.org/10.7150/thno.69368.
Song S, Shim MK, Lim S, Moon Y, Yang S, Kim J, et al. In Situ One-Step fluorescence labeling strategy of exosomes via bioorthogonal click chemistry for real-time exosome tracking in vitro and in vivo. Bioconjugate Chem. 2020;31:1562–74. https://doi.org/10.1021/acs.bioconjchem.0c00216.
You DG, Lim GT, Kwon S, Um W, Oh BH, Song SH, et al., Metabolically engineered stem cell-derived exosomes to regulate macrophage heterogeneity in rheumatoid arthritis. Sci Adv. 2021; 7. https://doi.org/10.1126/sciadv.abe0083.
Xue Y, Riva N, Zhao L, Shieh JS, Chin YT, Gatt A, et al. Recent advances of exosomes in soft tissue injuries in sports medicine: A critical review on biological and biomaterial applications. J Controlled Release: Off J Controlled Release Soc. 2023;364:90–108. https://doi.org/10.1016/j.jconrel.2023.10.031.
Zhang Y, Xie Y, Hao Z, Zhou P, Wang P, Fang S, et al. Umbilical mesenchymal stem cell-derived exosome-encapsulated hydrogels accelerate bone repair by enhancing angiogenesis. ACS Appl Mater Interfaces. 2021;13:18472–87. https://doi.org/10.1021/acsami.0c22671.
Han C, Zhou J, Liang C, Liu B, Pan X, Zhang Y, et al. Human umbilical cord mesenchymal stem cell derived exosomes encapsulated in functional peptide hydrogels promote cardiac repair. Biomater Sci. 2019;7:2920–33. https://doi.org/10.1039/c9bm00101h.
Khayambashi P, Iyer J, Pillai S, Upadhyay A, Zhang Y, Tran SD. Hydrogel encapsulation of mesenchymal stem cells and their derived exosomes for tissue engineering. Int J Mol Sci. 2021; 22. https://doi.org/10.3390/ijms22020684.
Zhang H, Huang J, Alahdal M. Exosomes loaded with chondrogenic stimuli agents combined with 3D bioprinting hydrogel in the treatment of osteoarthritis and cartilage degeneration. Biomed Pharmacother = Biomedecine Pharmacotherapie. 2023;168:115715. https://doi.org/10.1016/j.biopha.2023.115715.
Pishavar E, Luo H, Naserifar M, Hashemi M, Toosi S, Atala A, et al. Advanced hydrogels as exosome delivery systems for osteogenic differentiation of MSCs: application in bone regeneration. Int J Mol Sci. 2021; 22. https://doi.org/10.3390/ijms22126203.
Xu T, Hua Y, Mei P, Zeng D, Jiang S, Liao C. Black phosphorus thermosensitive hydrogels loaded with bone marrow mesenchymal stem cell-derived exosomes synergistically promote bone tissue defect repair. J. Mater. Chem. B. 2023;11:4396–407. https://doi.org/10.1039/d3tb00341h.
Guan M, Liu C, Zheng Q, Chu G, Wang H, Jin J, et al. Exosome-laden injectable self-healing hydrogel based on quaternized chitosan and oxidized starch attenuates disc degeneration by suppressing nucleus pulposus senescence. Int J Biol Macromol. 2023;232:123479. https://doi.org/10.1016/j.ijbiomac.2023.123479.
Hu H, Zhang H, Bu Z, Liu Z, Lv F, Pan M, et al. Small extracellular vesicles released from bioglass/hydrogel scaffold promote vascularized bone regeneration by transferring miR-23a-3p. Int J Nanomed. 2022;17:6201–20. https://doi.org/10.2147/ijn.S389471.
Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H, et al. Osteoarthritis. Lancet (Lond, Engl). 2015;386:376–87. https://doi.org/10.1016/s0140-6736(14)60802-3.
Xu H, Xu B. BMSC-derived exosomes ameliorate osteoarthritis by inhibiting pyroptosis of cartilage via delivering miR-326 targeting HDAC3 and STAT1//NF-κB p65 to chondrocytes. Mediators Inflamm. 2021;2021:9972805. https://doi.org/10.1155/2021/9972805.
Song J, Zhao J, Liu T, Li Y, Dang X, Wang W. Prevalence and risk factors of osteoporosis in a chinese population: a cross-sectional study in Xi’an, Shaanxi Province, China. Med Sci Monit: Int Med J Exp Clin Res. 2023;29:e942346. https://doi.org/10.12659/msm.942346.
Khosla S, Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017;5:898–907. https://doi.org/10.1016/s2213-8587(17)30188-2.
Roux C, Briot K. Osteoporosis in 2017: addressing the crisis in the treatment of osteoporosis. Nat Rev Rheumatol. 2018;14:67–68. https://doi.org/10.1038/nrrheum.2017.218.
Hu Y, Li X, Zhang Q, Gu Z, Luo Y, Guo J, et al. Exosome-guided bone targeted delivery of Antagomir-188 as an anabolic therapy for bone loss. Bioact Mater. 2021;6:2905–13. https://doi.org/10.1016/j.bioactmat.2021.02.014.
Smith MH, Berman JR. What is rheumatoid arthritis? JAMA. 2022;327:1194. https://doi.org/10.1001/jama.2022.0786.
George MD, Baker JF, Winthrop K, Alemao E, Chen L, Connolly S, et al. Risk of biologics and glucocorticoids in patients with rheumatoid arthritis undergoing arthroplasty: a Cohort study. Ann Intern Med. 2019;170:825–36. https://doi.org/10.7326/m18-2217.
Burmester GR, Pope JE. Novel treatment strategies in rheumatoid arthritis. Lancet (Lond, Engl). 2017;389:2338–48. https://doi.org/10.1016/s0140-6736(17)31491-5.
Chang TH, Wu CS, Chiou SH, Chang CH, Liao HJ. Adipose-derived stem cell exosomes as a novel anti-inflammatory agent and the current therapeutic targets for rheumatoid arthritis. Biomedicines. 2022; 10. https://doi.org/10.3390/biomedicines10071725.
Tsujimaru K, Takanashi M, Sudo K, Ishikawa A, Mineo S, Ueda S, et al. Extracellular microvesicles that originated adipose tissue derived mesenchymal stem cells have the potential ability to improve rheumatoid arthritis on mice. Regenerative Ther. 2020;15:305–11. https://doi.org/10.1016/j.reth.2020.08.004.
Siouti E, Andreakos E. The many facets of macrophages in rheumatoid arthritis. Biochem Pharmacol. 2019;165:152–69. https://doi.org/10.1016/j.bcp.2019.03.029.
Li H, Feng Y, Zheng X, Jia M, Mei Z, Wang Y, et al. M2-type exosomes nanoparticles for rheumatoid arthritis therapy via macrophage re-polarization. J Controlled Release: Off J Controlled Release Soc. 2022;341:16–30. https://doi.org/10.1016/j.jconrel.2021.11.019.
Fan L, Guan P, Xiao C, Wen H, Wang Q, Liu C, et al. Exosome-functionalized polyetheretherketone-based implant with immunomodulatory property for enhancing osseointegration. Bioact Mater. 2021;6:2754–66. https://doi.org/10.1016/j.bioactmat.2021.02.005.
Chen Z, Wang H, Xia Y, Yan F, Lu Y. Therapeutic potential of mesenchymal cell-derived miRNA-150-5p-expressing exosomes in rheumatoid arthritis mediated by the modulation of MMP14 and VEGF. J Immunol (Baltim, Md: 1950). 2018;201:2472–82. https://doi.org/10.4049/jimmunol.1800304.
Xu Z, Zhou X, Wu J, Cui X, Wang M, Wang X, et al. Mesenchymal stem cell-derived exosomes carrying microRNA-150 suppresses the proliferation and migration of osteosarcoma cells via targeting IGF2BP1. Transl Cancer Res. 2020;9:5323–35. https://doi.org/10.21037/tcr-20-83.
Huang X, Wu W, Jing D, Yang L, Guo H, Wang L, et al. Engineered exosome as targeted lncRNA MEG3 delivery vehicles for osteosarcoma therapy. J Controlled Release : Off J Controlled Release Soc. 2022;343:107–17. https://doi.org/10.1016/j.jconrel.2022.01.026.
Khalifeh Soltani S, Forogh B, Ahmadbeigi N, Hadizadeh Kharazi H, Fallahzadeh K, Kashani L, et al. Safety and efficacy of allogenic placental mesenchymal stem cells for treating knee osteoarthritis: a pilot study. Cytotherapy. 2019;21:54–63. https://doi.org/10.1016/j.jcyt.2018.11.003.
Lamo-Espinosa JM, Blanco JF, Sánchez M, Moreno V, Granero-Moltó F, Sánchez-Guijo F, et al. Phase II multicenter randomized controlled clinical trial on the efficacy of intra-articular injection of autologous bone marrow mesenchymal stem cells with platelet rich plasma for the treatment of knee osteoarthritis. J Transl Med. 2020;18:356. https://doi.org/10.1186/s12967-020-02530-6.
Torrecillas-Baena B, Pulido-Escribano V, Dorado G, Gálvez-Moreno M, Camacho-Cardenosa M, Casado-Díaz A. Clinical potential of mesenchymal stem cell-derived exosomes in bone regeneration. J Clin Med. 2023; 12. https://doi.org/10.3390/jcm12134385.
Lu CH, Chen YA, Ke CC, Liu RS. Mesenchymal stem cell-derived extracellular vesicle: a promising alternative therapy for osteoporosis. Int J Mol Sci. 2021; 22. https://doi.org/10.3390/ijms222312750.
Zeng ZL, Xie H. Mesenchymal stem cell-derived extracellular vesicles: a possible therapeutic strategy for orthopaedic diseases: a narrative review. Biomater. Transl. 2022;3:175–87. https://doi.org/10.12336/biomatertransl.2022.03.002.
Huang CC, Kang M, Lu Y, Shirazi S, Diaz JI, Cooper LF, et al. Functionally engineered extracellular vesicles improve bone regeneration. Acta Biomaterialia. 2020;109:182–94. https://doi.org/10.1016/j.actbio.2020.04.017.
Wu XD, Kang L, Tian J, Wu Y, Huang Y, Liu J, et al. Exosomes derived from magnetically actuated bone mesenchymal stem cells promote tendon-bone healing through the miR-21-5p/SMAD7 pathway. Mater Today Bio. 2022;15:100319. https://doi.org/10.1016/j.mtbio.2022.100319.
Zhang J, Zhang J, Zhang Y, Liu W, Ni W, Huang X, et al. Mesenchymal stem cells-derived exosomes ameliorate intervertebral disc degeneration through inhibiting pyroptosis. J Cell Mol Med. 2020;24:11742–54. https://doi.org/10.1111/jcmm.15784.
Yuan Q, Wang X, Liu L, Cai Y, Zhao X, Ma H, et al. Exosomes derived from human placental mesenchymal stromal cells carrying AntagomiR-4450 alleviate intervertebral disc degeneration through upregulation of ZNF121. Stem Cells Dev. 2020;29:1038–58. https://doi.org/10.1089/scd.2020.0083.
Chen C, Fu L, Luo Y, Zeng W, Qi X, Wei Y, et al. Engineered exosome-functionalized extracellular matrix-mimicking hydrogel for promoting bone repair in glucocorticoid-induced osteonecrosis of the femoral head. ACS Appl Mater Interfaces. 2023;15:28891–906. https://doi.org/10.1021/acsami.3c01539.
Li F, Wu J, Li D, Hao L, Li Y, Yi D, et al. Engineering stem cells to produce exosomes with enhanced bone regeneration effects: an alternative strategy for gene therapy. J Nanobiotechnol. 2022;20:135. https://doi.org/10.1186/s12951-022-01347-3.
Lu X, Xu G, Lin Z, Zou F, Liu S, Zhang Y, et al. Engineered exosomes enriched in netrin-1 modRNA promote axonal growth in spinal cord injury by attenuating inflammation and pyroptosis. Biomater Res. 2023;27:3. https://doi.org/10.1186/s40824-023-00339-0.
Wang J, Li M, Jin L, Guo P, Zhang Z, Zhanghuang C, et al. Exosome mimetics derived from bone marrow mesenchymal stem cells deliver doxorubicin to osteosarcoma in vitro and in vivo. Drug Deliv. 2022;29:3291–303. https://doi.org/10.1080/10717544.2022.2141921.
Cheng J, Chen Z, Liu C, Zhong M, Wang S, Sun Y, et al. Bone mesenchymal stem cell-derived exosome-loaded injectable hydrogel for minimally invasive treatment of spinal cord injury. Nanomed (Lond, Engl). 2021;16:1567–79. https://doi.org/10.2217/nnm-2021-0025.
Qiu M, Liu D, Fu Q. MiR-129-5p shuttled by human synovial mesenchymal stem cell-derived exosomes relieves IL-1β induced osteoarthritis via targeting HMGB1. Life Sci. 2021;269:118987. https://doi.org/10.1016/j.lfs.2020.118987.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: this work is funded by own funding research.
Author information
Authors and Affiliations
Contributions
Jiandong Tang drafted this review and designed the figures; Xiangyu Wang completed the data collection; Xu Lin gave some valuable suggestions; Chao Wu provided the design and revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
The authors have consented to publish this article.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tang, J., Wang, X., Lin, X. et al. Mesenchymal stem cell-derived extracellular vesicles: a regulator and carrier for targeting bone-related diseases. Cell Death Discov. 10, 212 (2024). https://doi.org/10.1038/s41420-024-01973-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-024-01973-w