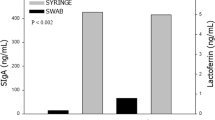
Abstract
Objective:
To determine whether the administration of mother’s colostrum into the buccal pouch in the first days of life alters the oral microbiota compared with control infants.
Study Design:
In this pilot study, 12 very low birth weight (VLBW) infants were randomly assigned to receive either colostrum from their mothers directly into the buccal pouch every 2 h for 46 h or standard care. We analyzed the oral microbiota at initiation and 48 and 96 h later using next-generation sequencing.
Result:
The oral microbiota changed markedly over the 96 h period in all babies. Patterns of colonization differed between groups with Planococcaceae, the dominant family at 48 and 96 h in the colostrum group, and Moraxellaceae and Staphylococcaceae, the dominant families at 48 and 96 h, respectively, in the control group.
Conclusion:
Buccal administration of mother’s colostrum to VLBW infants influenced the colonization of the oral cavity with differences persisting 48 h after completion of the intervention.
Similar content being viewed by others
Introduction
Human colostrum contains cytokines, antimicrobial peptides and proteins, hormones, cellular immune components and other biological substances that have immunomodulatory effects upon lymphoid tissues.1, 2, 3 These benefits may be especially important for very low birth weight (VLBW) infants, who are at greatly increased risk for infection due to prematurity. Ingested colostrum shapes the gut microbiota, decreases the risk of necrotizing enterocolitis4, 5 and provides protective anti-inflammatory molecules with the potential to blunt the often exuberant inflammatory response of premature infants.6 However, many small premature infants are not fed for several days after birth. Administration of small volumes of colostrum directly into the buccal cavity of intubated premature infants has been shown to be feasible and safe.7, 8 One study suggested that buccal colostrum may be nutritionally beneficial leading to improved growth,8 while another demonstrated a decreased risk of clinical sepsis (though this study was not powered to examine sepsis as an outcome).9
Additional theoretical benefits of buccal colostrum include stimulation of the oropharyngeal-associated lymphatic tissues,10 decreased risk of ventilator-associated pneumonia11 and alteration of the oral microbiota. Several neonatal intensive care units have adopted this practice12 although evidence for benefit is limited. Oral swabbing with chlorhexidine has been shown to decrease the risk of ventilator-associated pneumonia in adult intensive care unit patients.13 A recent retrospective cohort study of mechanically ventilated VLBW infants found oral care with mother’s own milk (colostrum, transitional milk and mature milk) was feasible and safe, however there were no differences in health outcomes (rate of positive tracheal aspirates, positive blood cultures, the number of ventilator days and length of stay) between the 68 infants receiving the intervention and the 70 infants that did not.14 The largest retrospective cohort study to date, comparing 89 premature infants who received ‘oropharyngeal’ colostrum and 280 premature infants who did not, demonstrated no differences in the incidence of necrotizing enterocolitis or nosocomial infection.8 To date, there is no evidence that oral care with colostrum alters the oral microbiota or decreases the risk of ventilator-associated pneumonia in premature infants. We sought to add to the literature by conducting a pilot study of the impact of colostrum on the composition of the oral microbiota in premature infants.
Methods
Study design
We conducted a randomized controlled clinical trial from November 2013 to October 2014 in the neonatal intensive care unit of the University of California Davis Children’s Hospital in Sacramento, California. The University Institutional Review Board reviewed and approved the protocol. The trial was registered at clinicaltrials.gov (NCT02306980). For this pilot study, a sample size of 12 patients was chosen based on feasibility of completion with recognition that such a study is only powered to demonstrate large differences in the primary outcome but is useful for the generation of preliminary data to more accurately justify a larger study. For example, assuming alpha 0.05 a sample size of six in each group could identify a change in the percentage of a single bacterial taxon from 95 to 8% with power 0.80.
Participants
Neonates were screened upon admission to the neonatal intensive care unit to determine eligibility. Inclusion criteria included birth weight <1500 g, age <7 days, intubation within 48 h of birth and availability of maternal colostrum. Neonates with a lethal medical condition were excluded. One of two investigators met with parents of eligible infants in person to inform them of the purpose of the study, describe the intervention and explain possible benefits and risks. Their questions were answered and additional meetings were arranged as needed to answer additional questions. After informed parental consent was obtained, each neonate was randomly assigned to the colostrum or standard care group. Twins were randomized together. The study was not blinded for two reasons. First, a placebo of sterile water does not look or smell like colostrum (even with taping or opaque syringes it is possible to see the liquid in the buccal pouch) and could potentially alter or dilute the oral microbiota. Second, administration of a standard placebo such as maltodextrin or lactose powder in water could potentially alter the oral microbiota as both are potential prebiotic substrates for oral microbes. Initiation and advancement of feedings were based on an established protocol.
Intervention and sample collection
Six of the twelve neonates were randomly assigned to the colostrum group using sealed opaque envelopes. Bedside nurses administered 0.2 ml of the mother’s colostrum via sterile syringe into the baby’s oral cavity (0.1 ml into each buccal pouch) every 2 h for 46 h regardless of whether the infant was receiving trophic feeds. The other six neonates received routine care. Using sterile cotton-tipped applicators, oral samples were collected from both groups just before initiation. Applicators were held in one lower buccal pouch for 5 s then gently swabbed inside the same cheek for 5 s, then repeated on the opposite cheek with the same applicator. Additional oral swabs were collected 2 h after completion of intervention (at 48 h) and 50 h after completion of intervention (at 96 h). Samples were collected from the control group at the same intervals, with initiation timed to availability of maternal colostrum for consistency. For uniformity of swabbing technique, three investigators collected all samples. Applicator tips were placed in sterile labeled 2-ml Eppendorf tubes and stored at −40 °C immediately after collection.
Next-generation sequencing
DNA extraction and library construction was performed as described previously15 with the following changes. DNA was extracted as described and the V4 region of the16S rRNA gene was amplified with barcoded primers F515 and R806. PCR amplification was carried out with initial denaturation of 2 min at 94 °C, followed by 30 cycles of 95 °C for 45 s, 50 °C for 60 s and 72 °C for 90 s, and a final extension step at 72 °C for 10 min. Samples were submitted to the UC Davis Genome Center DNA Technologies Core for sequencing on an Illumina MiSeq instrument (Illumina, San Diego, CA, USA). QIIME software package (University of Colorado, Boulder, CO, USA, version 1.8.0) was used for quality filtering and demultiplexing the resulting sequencing data.16 Operational taxonomic units were assigned using UCLUST (drive5.com, Tiburon, CA, USA) based on 97% pairwise identity17 and taxonomic classification was based on the Ribosomal Database Project classifier (Michigan State University, East Lansing, MI, USA) against a representative subset of the Greengenes 16S rRNA database (Second Genome, South San Francisco, CA, USA, gg_13_8 release).18, 19 Unassigned taxa mapping to human mitochondrial DNA were filtered out of the results before the final statistical analysis.
Statistical analysis
Linear discriminate analysis effect size (commonly referred to as LEfSe) is a useful tool for comparing complex microbial communities with low false-positive discovery rates; it utilizes the Kruskal–Wallis test and then the Wilcoxon test on subclasses to determine a signed (positive or negative) log score to estimate a biological effect.20 Comparisons between taxa at 48 and 96 h were also performed with the t-test assuming unequal variance (Stata version 12.1, StataCorp, College Station, TX, USA). As this is a pilot study with the purpose of generating hypotheses, we reported P-values <0.1.
Results
Of the 56 VLBW infants born or transferred to UCDMC neonatal intensive care units from November 2013 to October 2014, 12 were included and randomly assigned to the colostrum or usual care group (Figure 1). Consent was obtained within 36 h after birth for all infants. Table 1 shows the baseline characteristics of the study population; there were no significant differences between groups. Median age of colostrum initiation was 39 h (range 32 to 87). All six babies randomized to the treatment group completed 46 h of buccal colostrum administration (total of 24 doses per infant). A total of 36 specimens were analyzed for bacterial composition (three swabs from each infant).
Figure 2 shows the relative abundance of bacterial taxa at the family level. At enrollment, hour 0, the two groups were very similar. At 48 h the colostrum group had a significantly lower percentage of Moraxellaceae (t-statistic −2.91, Satterthwaite’s degrees of freedom 5.36, P=0.03) and at 96 h the colostrum group had a significantly lower percentage of Staphylococcaceae (t-statistic −3.21, Satterthwaite’s degrees of freedom 9.36, P=0.01) and a trend toward a greater percentage of Planococcaceae (t-statistic 2.06, Satterthwaite’s degrees of freedom 5.08, P=0.09). Figure 3 presents the microbiota for each individual infant at each time point. Note that by 96 h, for five of the six infants in the control group Staphylococcaceae are the dominant organism, whereas this was the case for only one of the six infants in the colostrum group.
The LEfSe analyses for all babies over the time of the study are summarized in Figure 4; at the phylum level Proteobacteria and Actinobacteria are significantly greater at time zero and Firmicutes are greater at 96 h (primarily explained by increases in the family Staphylococcaceae). The changes over time were more highly significant than the changes between groups.
Table 2 summarizes the clinical outcomes. In the colostrum group, one patient (infant E) developed late-onset Candida parapsilosis sepsis and endocarditis, diagnosed on day of life 19. One patient (infant J) developed ventilator-associated pneumonia on day of life 27, as evidenced by a single organism in the culture from the endotracheal aspirate (Enterobacter cloacae), an acute respiratory deterioration and pneumonia on chest radiograph. One patient (infant A) developed pneumonia on day of life 22, while on nasal cannula; aspirate from a freshly inserted endotracheal tube grew Staphylococcus aureus. One patient developed stage 3 necrotizing enterocolitis requiring colectomy at day of life 28 (infant A) and another developed stage 2 necrotizing enterocolitis at day of life 15 (infant I). Three patients developed chronic lung disease, defined as oxygen requirement at 36 weeks corrected gestational age or discharge if sooner.
In the control group, one patient (infant B) developed stage 2 necrotizing enterocolitis on day of life 21 with blood culture positive for Streptococcus bovis. One patient (infant G) had late-onset group B streptococcus sepsis at 6 weeks of life. One patient (infant H) developed early-onset Escherichia coli sepsis and meningitis and died on day of life 16. Two patients developed chronic lung disease.
Discussion
To the best of our knowledge, this is the first randomized controlled clinical trial to evaluate changes that occur in the oral microbiota of VLBW infants after the administration of mother’s colostrum into the buccal pouch. Although there have been studies evaluating the oral microbiome in humans, from the neonatal period through adulthood,21, 22, 23 we could find only one study of the oral microbiota of premature babies (analysis at 1 month of age).24
As many as 19 000 distinct phylotypes of organisms colonize the adult oral cavity.20 The predominant species of the buccal epithelium in adults have been shown to be Streptococcus and Gemella.21 During pregnancy, the amniotic fluid can become colonized with maternal oral microorganisms. These organisms, often associated with maternal periodontal disease, reach the normally sterile environment via transient bacteremia and likely represent a clinically significant risk factor for preeclampsia, preterm labor and low-birth weight babies.24, 25 The mouths of VLBW infants can, therefore, be colonized with these organisms even before birth.
Shortly after birth, neonatal bacterial communities are similar across anatomic sites and heavily influenced by delivery type (vaginal versus cesarean). Term infants born via vaginal birth typically have similar oral flora to their mother’s vaginal microbiota, which are predominantly Lactobacillus, Prevotella or Sneathia species, while the mouths of babies born by cesarean are typically colonized by bacteria similar to their mother’s skin, such as Staphylococcus, Corynebacterium and Propionibacterium.23 The low numbers of vaginal births in this pilot study preclude any observations about influence of delivery mode on response to buccal colostrum.
In the first days of life, neonates are exposed to bacteria from a variety of environmental sources heavily influenced by feeding and time in the hospital. In term neonates, the pioneers of oral colonization are predominantly Staphylococcus and Streptococcus.22 In premature infants, colonizers of the mouth by 1 month of age are predominantly Staphylococcus, Streptococcus, Corynebacterium, Pseudomonas, Enterobacter, Neisseria, Acinetobacter, Stenotrophomonas, Gamella, Propionibacterium, Enterococcus and Cedecea.26 In the first few days of life, at the family level we found the predominant oral bacteria in VLBW neonates to be Moraxellaceae, Mycoplasmataceae, Caulobacteraceae, Planococcaceae and Pseudomonadaceae with significant changes over the first days of life in both groups of infants.
Planococcacea are non-pathogenic environmental Gram-positive bacteria (phylum Firmicutes, order Bacillales) that are not associated with disease and not typical of the oral microbiota of the adult. Pasteurellaceae are Gram-negative bacteria (phylum Proteobacteria, order Pasteurellales), most of which are commensal organisms of the upper respiratory tract; while the species Haemophilus influenzae is in this family, it is not a common pathogen in the premature neonate. Moraxellaceae are also Gram-negative Proteobacteria (order Pseudomonadales), most of which are environmental organisms; while the upper airway pathogenic species Moraxella catarrhalis is in this family, it is also not a common pathogen in the premature neonate. Staphylococcaceae (Gram-positive, phylum Firmicutes, order Bacillales) are common colonizers of the skin and oral cavity of adults and infants. This family includes several commensal genera that colonize the mucous membranes as well as species that are common pathogens in premature infants including S. aureus and S. epidermidis.
Administration of mother’s colostrum into the mouth of the intubated premature infant has been proposed as a method of influencing the oropharyngeal lymphatic tissue with both local and systemic effects and of decreasing the risk of ventilator-associated pneumonia. Studies to date of this increasingly common intervention have demonstrated safety and feasibility and suggest a possible benefit in decreased time to full enteral feeding and decreased risk of sepsis.8, 10 Our study adds to the literature the first description of the oral microbiota of the VLBW infant in the first days of life (control group) and the first demonstration of alterations in the oral microbiota with colostrum. Large studies would be required to demonstrate a benefit of buccal administration of colostrum to prevent ventilator-associated pneumonia, sepsis or necrotizing enterocolitis (for instance, a recent study of probiotics to prevent sepsis included 1100 premature infants).27 Perhaps the most clinically significant change in this study is the marked differences seen in relative abundance of Staphylococcaceae at 96 h. On the basis of these pilot data, to confirm a similar difference in percentage of Staphylococcaceae of this magnitude (73% of the oral bacteria in the control group versus 17% in the colostrum group at 96 h) would require a sample size of 15 per group (assuming alpha 0.05 and beta 0.20).
This study has several limitations. The timing of the intervention and sample collection was dependent on availability of maternal colostrum, which was variable in both groups. Given the marked changes over time in the oral microbiota in this population and the small sample size, this may have influenced the ability to detect true differences between groups. In addition, the duration of the intervention (48 h) is less than some previous interventions and may have limited effects on the oral microbiota. Given the compelling evidence that colonizing microbes influence the host innate immune system,28 it would have been valuable to measure markers of immune response and correlate these with the oral microbiota; such correlations may be of value in determining mechanisms of protection in future studies. Finally, the small sample size precludes any definitive conclusions about prevention of oral dysbiosis with colostrum administration, however the purpose of pilot studies is to generate hypotheses and explore feasibility of future larger trials.
Conclusion
The results of this pilot study support the hypothesis that the oral microbiota of preterm babies is altered by colostrum administration, though the differences between groups were not as significant as the changes in the entire group over time.
There were no significant differences between groups in clinical outcomes in this study, though this study was not powered to determine differences in these outcomes. Larger studies, powered to determine differences in sepsis, chronic lung disease and pneumonia, are indicated before widespread adoption of this intervention. Inclusion of analysis of the oral microbiota and changes in markers of the innate and adaptive immune systems in these studies may help shed light on possible mechanisms of protection.
References
Smith DJ, Taubman MA . Ontogeny of immunity to oral microbiota in humans. Crit Rev Oral Biol Med 1992; 3 (1–2): 109–133.
Bocci V, von Bremen K, Corradeschi F, Luzzi E, Paulesu L . What is the role of cytokines in human colostrum? J Biol Regul Homeost Agents 1991; 5 (4): 121–124.
Garofalo R . Cytokines in human milk. J Pediatr 2010; 156 (2 Suppl): S36–S40.
Dai D, Walker WA . Protective nutrients and bacterial colonization in the immature human gut. Adv Pediatr 1999; 46: 353–382.
Miner CA, Fullmer S, Eggett DL, Christensen RD . Factors affecting the severity of necrotizing enterocolitis. J Matern Fetal Neonatal Med 2013; 26 (17): 1715–1719.
Walker A . Breast milk as the gold standard for protective nutrients. J Pediatr 2010; 156 (2, Suppl): S3–S7.
Rodriguez NA, Meier PP, Groer MW, Zeller JM, Engstrom JL, Fogg L . A pilot study to determine the safety and feasibility of oropharyngeal administration of own mother’s colostrum to extremely low-birth-weight infants. Adv Neonatal Care 2010; 10 (4): 206–212.
Seigel JK, Smith PB, Ashley PL, Cotten CM, Herbert CC, King BA et al. Early administration of oropharyngeal colostrum to extremely low birth weight infants. Breastfeed Med 2013; 8 (6): 491–495.
Lee J, Kim HS, Jung YH, Choi KY, Shin SH, Kim EK et al. Oropharyngeal colostrum administration in extremely premature infants: an RCT. Pediatrics 2015; 135 (2): e357–e366.
Gephart SM, Weller M . Colostrum as oral immune therapy to promote neonatal health. Adv Neonatal Care 2014; 14 (1): 44–51.
Rodriguez NA, Meier PP, Groer MW, Zeller JM . Oropharyngeal administration of colostrum to extremely low birth weight infants: theoretical perspectives. J Perinatol 2009; 29 (1): 1–7.
Pletsch D, Ulrich C, Angelini M, Fernandes G, Lee DS . Mothers’ “liquid gold”: a quality improvement initiative to support early colostrum delivery via oral immune therapy (OIT) to premature and critically ill newborns. Nurs Leadersh 2013; 26: 34–42.
Ozcaka O, Basoglu OK, Buduneli N, Tasbakan MS, Bacakoglu F, Kinane DF . Chlorhexidine decreases the risk of ventilator-associated pneumonia in intensive care unit patients: a randomized clinical trial. J Periodontal Res 2012; 47 (5): 584–592.
Thibeau S, Boudreaux C . Exploring the use of mothers’ own milk as oral care for mechanically ventilated very low-birth-weight preterm infants. Adv Neonatal Care 2013; 13 (3): 190–197.
Frese SA, Parker K, Calvert CC, Mills DA . Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome 2015; 3: 28.
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci USA 2011; 108 (Suppl 1): 4516–4522.
Edgar RC . Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010; 26 (19): 2460–2461.
Wang Q, Garrity GM, Tiedje JM, Cole JR . Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 2007; 73 (16): 5261–5267.
DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 2006; 72 (7): 5069–5072.
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS et al. Metagenomic biomarker discovery and explanation. Genome Biol 2011; 12 (6): R60.
Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE . Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 2005; 43 (11): 5721–5732.
Sampaio-Maia B, Monteiro-Silva F . Acquisition and maturation of oral microbiome throughout childhood: an update. Dent Res J (Isfahan) 2014; 11 (3): 291–301.
Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA 2010; 107 (26): 11971–11975.
Offenbacher S, Katz V, Fertik G, Collins J, Boyd D, Maynor G et al. Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol 1996; 67 (10 Suppl): 1103–1113.
Nabet C, Lelong N, Colombier ML, Sixou M, Musset AM, Goffinet F et al. Maternal periodontitis and the causes of preterm birth: the case-control Epipap study. J Clin Periodontol 2010; 37 (1): 37–45.
Hendricks-Munoz KD, Xu J, Parikh HI, Xu P, Fettweis JM, Kim Y et al. Skin-to-skin care and the development of the preterm infant oral microbiome. Am J Perinatol. (e-pub ahead of print 22 May 2015; doi:10.1055/s-0035-1552941).
Jacobs SE, Tobin JM, Opie GF, Donath S, Tabrizi SN, Pirotta M et al. Probiotic effects on late-onset sepsis in very preterm infants: a randomized controlled trial. Pediatrics 2013; 132 (6): 1055–1062.
Min YW, Rhee PL . The role of microbiota on the gut immunology. Clin Ther 2015; 37 (5): 968–975.
Acknowledgements
DAM acknowledges the Peter J. Shields Endowed Chair in Dairy Food Science at UC Davis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Sohn, K., Kalanetra, K., Mills, D. et al. Buccal administration of human colostrum: impact on the oral microbiota of premature infants. J Perinatol 36, 106–111 (2016). https://doi.org/10.1038/jp.2015.157
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2015.157
This article is cited by
-
Oropharyngeal administration of colostrum targeting gut microbiota and metabolites in very preterm infants: protocol for a multicenter randomized controlled trial
BMC Pediatrics (2023)
-
Dilemmas in feeding infants with intestinal failure: a neonatologist’s perspective
Journal of Perinatology (2023)
-
Variable preterm oral microbiome stabilizes and reflects a full-term infant profile within three months
Pediatric Research (2023)
-
Increasing early exposure to mother’s own milk in premature newborns
Journal of Perinatology (2022)
-
Oral Application of Colostrum and Mother’s Own Milk in Preterm Infants—A Randomized, Controlled Trial
Indian Journal of Pediatrics (2022)