Featured
-
-
Article |
 RETRACTED ARTICLE: Intrinsically unidirectional chemically fuelled rotary molecular motors
RETRACTED ARTICLE: Intrinsically unidirectional chemically fuelled rotary molecular motors
A homochiral rotary molecular motor shows autonomous unidirectional rotation around a single bond driven by a chemical fuel.
- Ke Mo
- , Yu Zhang
- & Depeng Zhao
-
News & Views |
 Synthesis reveals unexpected biological targets of a traditional medicine
Synthesis reveals unexpected biological targets of a traditional medicine
A compound made by plants used in traditional medicine has been prepared by chemical synthesis, providing enough for biological testing. The unexpected finding that it acts at opioid receptors raises prospects for drug discovery.
- Nicholas P. R. Onuska
- & Joshua G. Pierce
-
Obituary |
 David A. Evans (1941–2022)
David A. Evans (1941–2022)
Chemist who developed ways to synthesize and depict bioactive products.
- Mark Lautens
-
Article |
 Site-selective, stereocontrolled glycosylation of minimally protected sugars
Site-selective, stereocontrolled glycosylation of minimally protected sugars
A simplified synthesis strategy for stereo- and site-selective glycosylations, using minimally protected mono- and disaccharides and thiourea small-molecule catalysts, enables highly selective functionalization of carbohydrates.
- Qiuhan Li
- , Samuel M. Levi
- & Eric N. Jacobsen
-
News Feature |
 Why chemists can’t quit palladium
Why chemists can’t quit palladium
A retracted paper highlights chemistry’s history of trying to avoid the expensive, toxic — but necessary — catalyst.
- Ariana Remmel
-
Research Briefing |
 A radical way to forge carbon–carbon bonds
A radical way to forge carbon–carbon bonds
Carbon–carbon single bonds are found in most organic molecules. A new electrocatalytic method can create such bonds by uniting different alkyl carboxylic acids, substantially shortening synthetic routes to useful molecules. The reaction uses inexpensive reagents in a simple and scalable set-up, and allows the inclusion of many other functional groups.
-
Article |
 Cobalt-electrocatalytic HAT for functionalization of unsaturated C–C bonds
Cobalt-electrocatalytic HAT for functionalization of unsaturated C–C bonds
A perspective is given on how an electrocatalytic approach, inspired by decades of energy storage studies, can be used in the context of efficient cobalt-hydride generation with a variety of applications in modern organic synthesis.
- Samer Gnaim
- , Adriano Bauer
- & Phil S. Baran
-
Article |
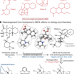 Synthesis and target annotation of the alkaloid GB18
Synthesis and target annotation of the alkaloid GB18
A synthetic route to GB18, an alkaloid from the bark of hallucinogenic Galbulimima sp., is developed, enabling its identification as an antagonist of κ- and μ-opioid receptors.
- Stone Woo
- & Ryan A. Shenvi
-
News & Views |
 A possible path towards encoded protein synthesis on ancient Earth
A possible path towards encoded protein synthesis on ancient Earth
How did the biological machinery for protein synthesis evolve from simple chemicals on ancient Earth? Experiments suggest an intriguing role for modified RNA nucleotides in directing stepwise peptide synthesis.
- Claudia Bonfio
-
Article
| Open Access A prebiotically plausible scenario of an RNA–peptide world
A prebiotically plausible scenario of an RNA–peptide world
Peptide synthesis can take place directly on RNA, which suggests how a nucleic acid–protein world might have originated on early Earth.
- Felix Müller
- , Luis Escobar
- & Thomas Carell
-
Article
| Open Access Catalytic synthesis of phenols with nitrous oxide
Catalytic synthesis of phenols with nitrous oxide
A study demonstrates that nitrous oxide can act as the source of O in a catalytic conversion of aryl halides to phenols, releasing N2 as by-product.
- Franck Le Vaillant
- , Ana Mateos Calbet
- & Josep Cornella
-
Research Highlight |
 Long-sought structure of Pepto-Bismol decoded
Long-sought structure of Pepto-Bismol decoded
Sugar-wafer-like layering could be key to how this antacid fights gastric discomforts.
-
Article |
 Multifunctional biocatalyst for conjugate reduction and reductive amination
Multifunctional biocatalyst for conjugate reduction and reductive amination
A biocatalytic enzyme originating from bacteria, EneIRED, facilitates amine-activated conjugate alkene reduction followed by reductive amination, efficiently preparing chiral amine diastereomers, which are commonly used in pharmaceuticals and agrochemicals.
- Thomas W. Thorpe
- , James R. Marshall
- & Nicholas J. Turner
-
Article |
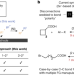 Ni-electrocatalytic Csp3–Csp3 doubly decarboxylative coupling
Ni-electrocatalytic Csp3–Csp3 doubly decarboxylative coupling
An Ni-electrocatalytic system can couple two different carboxylates using doubly decarboxylative cross-coupling, tolerating a range of functional groups, being scalable, used for the synthesis of 32 known compounds and reducing overall step counts by 73%.
- Benxiang Zhang
- , Yang Gao
- & Phil S. Baran
-
News & Views |
 Electrification promotes tricky synthetic chemical reactions
Electrification promotes tricky synthetic chemical reactions
Chemical synthesis often relies on reactions catalysed by transition metals. Electrochemical methods have now been developed that negate this need, opening up pathways to previously challenging reactions.
- Charlotte Willans
-
Article |
 [18F]Difluorocarbene for positron emission tomography
[18F]Difluorocarbene for positron emission tomography
Carbene chemistry is used to introduce difluoromethyl groups labelled with fluorine-18 into compounds for positron emission tomography imaging, using a reagent designed for high molar activity and versatility.
- Jeroen B. I. Sap
- , Claudio F. Meyer
- & Véronique Gouverneur
-
Article |
 Intermolecular [2π+2σ]-photocycloaddition enabled by triplet energy transfer
Intermolecular [2π+2σ]-photocycloaddition enabled by triplet energy transfer
A strain-release approach, realized by visible-light-mediated triplet energy transfer catalysis, enabled an intermolecular [2π+2σ]-photocycloaddition.
- Roman Kleinmans
- , Tobias Pinkert
- & Frank Glorius
-
Article |
 Electrochemically driven cross-electrophile coupling of alkyl halides
Electrochemically driven cross-electrophile coupling of alkyl halides
An electrochemical method is used to couple together two alkyl halides, enabling efficient cross-electrophile coupling of a variety of alkyl electrophiles with improved chemoselectivity compared with existing methods.
- Wen Zhang
- , Lingxiang Lu
- & Song Lin
-
Article |
 Automated iterative Csp3–C bond formation
Automated iterative Csp3–C bond formation
Identification of a hyperstable boronate enables automated lego-like synthesis to access a wider range of three-dimensionally complex small organic molecules rich in Csp3–C bonds.
- Daniel J. Blair
- , Sriyankari Chitti
- & Martin D. Burke
-
Article |
 Multicomponent alkene azidoarylation by anion-mediated dual catalysis
Multicomponent alkene azidoarylation by anion-mediated dual catalysis
A streamlined synthesis of β-arylethylamines using two distinct copper catalysts is reported, and an azide anion is proposed to both react to form the product and facilitate catalyst regeneration.
- Ala Bunescu
- , Yusra Abdelhamid
- & Matthew J. Gaunt
-
News |
 Busting benzene, lab-grown embryos — the week in infographics
Busting benzene, lab-grown embryos — the week in infographics
Nature highlights three key infographics from the week in science and research.
-
News & Views |
 Benzene rings broken for chemical synthesis
Benzene rings broken for chemical synthesis
Benzene rings are almost unbreakable in typical reaction conditions. Chemistry has now been developed that selectively breaks these rings open, highlighting their potential as building blocks for making open-chain molecules.
- Mark R. Crimmin
-
Article |
 Enantioselective synthesis of ammonium cations
Enantioselective synthesis of ammonium cations
Enantioselective supramolecular recognition allows for the asymmetric synthesis of nitrogen stereocentres, providing chiral ammonium cations in a dynamic crystallization process.
- Mark P. Walsh
- , Joseph M. Phelps
- & Matthew O. Kitching
-
Article |
 Metallaphotoredox-enabled deoxygenative arylation of alcohols
Metallaphotoredox-enabled deoxygenative arylation of alcohols
A metallaphotoredox-based cross-coupling platform is capable of activating a wide range of free alcohols using N-heterocyclic carbene salts, cleaving C–O bonds to form free carbon radicals that are then used to form new C–C bonds.
- Zhe Dong
- & David W. C. MacMillan
-
News & Views |
 Direct installation of boron groups offers boost to medicinal chemistry
Direct installation of boron groups offers boost to medicinal chemistry
Compounds called borylated azines have untapped potential for organic synthesis, but have faced problems associated with their preparation, stability and reactivity. A new class of these compounds provides a solution.
- Christine M. Le
-
Article |
 Cleaving arene rings for acyclic alkenylnitrile synthesis
Cleaving arene rings for acyclic alkenylnitrile synthesis
Common aromatic rings, such as anilines, arylboronic acids and aryl halides, can be opened up and converted to alkenyl nitriles through carbon–carbon bond cleavage using a copper catalyst.
- Xu Qiu
- , Yueqian Sang
- & Ning Jiao
-
Article |
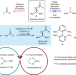 Photoinduced copper-catalysed asymmetric amidation via ligand cooperativity
Photoinduced copper-catalysed asymmetric amidation via ligand cooperativity
In the presence of three ligands and light, two distinct copper catalysts combine to produce enantioenriched secondary amides from racemic alkyl electrophiles and primary amide nucleophiles.
- Caiyou Chen
- , Jonas C. Peters
- & Gregory C. Fu
-
Article |
 Aziridine synthesis by coupling amines and alkenes via an electrogenerated dication
Aziridine synthesis by coupling amines and alkenes via an electrogenerated dication
The synthesis of aziridines—three-membered nitrogen-containing heterocycles—is achieved by a new method involving the electrochemical coupling of alkenes and amines, via a dicationic intermediate.
- Dylan E. Holst
- , Diana J. Wang
- & Zachary K. Wickens
-
Article |
 A radical approach for the selective C–H borylation of azines
A radical approach for the selective C–H borylation of azines
Selective borylation of azines—nitrogen-containing aromatic heterocycles used in the synthesis of many pharmaceuticals—is made possible by forming a radical from an aminoborane using a photocatalyst.
- Ji Hye Kim
- , Timothée Constantin
- & Daniele Leonori
-
Article |
 Skeletal editing through direct nitrogen deletion of secondary amines
Skeletal editing through direct nitrogen deletion of secondary amines
Nitrogen is ‘deleted’ from secondary amines using anomeric amide reagents, which react with the amine to form an isodiazene, after which nitrogen gas is released and the resulting carbon radicals combine to form a carbon–carbon bond.
- Sean H. Kennedy
- , Balu D. Dherange
- & Mark D. Levin
-
News & Views |
 Nitrogen deletion offers fresh strategy for organic synthesis
Nitrogen deletion offers fresh strategy for organic synthesis
Many scientific fields and industries rely on the synthesis of small organic molecules. A chemical reagent has been developed that allows such molecules to be made by ‘deleting’ nitrogen atoms from readily accessible precursors.
- William P. Unsworth
- & Alyssa-Jennifer Avestro
-
Article |
 Phosphorus-mediated sp2–sp3 couplings for C–H fluoroalkylation of azines
Phosphorus-mediated sp2–sp3 couplings for C–H fluoroalkylation of azines
Bench-stable fluoroalkylphosphine reagents are used to form C–CF2X bonds in a one-pot process, reliably fluoroalkylating pyridine building blocks and drug and agrochemical intermediates.
- Xuan Zhang
- , Kyle G. Nottingham
- & Andrew McNally
-
News & Views |
 Machine learning made easy for optimizing chemical reactions
Machine learning made easy for optimizing chemical reactions
An accessible machine-learning tool has been developed that can accelerate the optimization of a wide range of synthetic reactions — and reveals how cognitive bias might have undermined optimization by humans.
- Jason E. Hein
-
Article |
 Metallaphotoredox aryl and alkyl radiomethylation for PET ligand discovery
Metallaphotoredox aryl and alkyl radiomethylation for PET ligand discovery
A versatile and rapid metallaphotoredox catalytic method of making 3H- and 11C-labelled tracer compounds for use in positron emission tomography (PET) is reported.
- Robert W. Pipal
- , Kenneth T. Stout
- & David W. C. MacMillan
-
Article |
 Catalytic asymmetric addition of an amine N–H bond across internal alkenes
Catalytic asymmetric addition of an amine N–H bond across internal alkenes
Hydroamination with high enantio- and regioselectivity is achieved across a wide range of internal alkenes by using a cationic iridium complex that adds an ammonia surrogate containing a pyridine group.
- Yumeng Xi
- , Senjie Ma
- & John F. Hartwig
-
Article |
 Metal-free photoinduced C(sp3)–H borylation of alkanes
Metal-free photoinduced C(sp3)–H borylation of alkanes
Metal-free borylation of C(sp3)–H bonds by violet-light-induced hydrogen atom transfer is reported, demonstrating high selectivity for the substitution of methyl C–H bonds over other weaker C–H bonds.
- Chao Shu
- , Adam Noble
- & Varinder K. Aggarwal
-
Article |
 Computational planning of the synthesis of complex natural products
Computational planning of the synthesis of complex natural products
A synthetic route-planning algorithm, augmented with causal relationships that allow it to strategize over multiple steps, can design complex natural-product syntheses that are indistinguishable from those designed by human experts.
- Barbara Mikulak-Klucznik
- , Patrycja Gołębiowska
- & Bartosz A. Grzybowski
-
Article |
 Concentric liquid reactors for chemical synthesis and separation
Concentric liquid reactors for chemical synthesis and separation
In a rotating reactor, immiscible or pairwise-immiscible liquids organize into stable but internally agitated concentric layers, enabling multistep syntheses and separations of reaction mixtures.
- Olgierd Cybulski
- , Miroslaw Dygas
- & Bartosz A. Grzybowski
-
News & Views |
 Modular synthesis enables molecular ju-jitsu in the fight against antibiotic resistance
Modular synthesis enables molecular ju-jitsu in the fight against antibiotic resistance
An ancient resistance mechanism poses a problem when using streptogramin antibiotics. A modular approach to drug synthesis exploits this same mechanism to generate an antibiotic that avoids the emergence of resistance.
- Daniel J. Blair
- & Martin D. Burke
-
Article |
 Coupling dinitrogen and hydrocarbons through aryl migration
Coupling dinitrogen and hydrocarbons through aryl migration
An iron complex sequentially activates N2 and C–H bonds in benzene to form aniline, with coupling achieved through partial silylation of a reduced iron–nitrogen complex and phenyl migration.
- Sean F. McWilliams
- , Daniël L. J. Broere
- & Patrick L. Holland
-
News & Views |
 Reactions for making widely used aniline compounds break norms of synthesis
Reactions for making widely used aniline compounds break norms of synthesis
Chemists generally regard benzene rings as preformed units that are elaborated to build larger molecules. This idea has now been challenged in reactions for making anilines — precursors of many high-value chemical products.
- Valerie A. Schmidt
-
Article |
 A photochemical dehydrogenative strategy for aniline synthesis
A photochemical dehydrogenative strategy for aniline synthesis
A dual cobalt and photocatalysis system provides a way to assemble anilines from cyclohexanones and amines by progressively dehydrating the intermediate imine.
- Shashikant U. Dighe
- , Fabio Juliá
- & Daniele Leonori
-
News & Views |
 New class of molecule targets proteins outside cells for degradation
New class of molecule targets proteins outside cells for degradation
Molecules have previously been made that induce protein destruction inside cells. A new class of molecule now induces the degradation of membrane and extracellular proteins — opening up avenues for drug discovery.
- Claire Whitworth
- & Alessio Ciulli
-
Article |
 Lysosome-targeting chimaeras for degradation of extracellular proteins
Lysosome-targeting chimaeras for degradation of extracellular proteins
Lysosome-targeting chimaeras—in which a small molecule or antibody is connected to a glycopeptide ligand to form a conjugate that can bind a cell-surface lysosome-shuttling receptor and a protein target—are used to achieve the targeted degradation of extracellular and membrane proteins.
- Steven M. Banik
- , Kayvon Pedram
- & Carolyn R. Bertozzi
-
Nature Video |
 Your new lab partner: A mobile robot chemist
Your new lab partner: A mobile robot chemist
This autonomous mobile robot is designed to work alongside humans.
-
News & Views |
 How DNA and RNA subunits might have formed to make the first genetic alphabet
How DNA and RNA subunits might have formed to make the first genetic alphabet
Understanding the prebiotic origins of the nucleic acids is a long-standing challenge. The latest experiments support the idea that the first nucleic acid encoded information using a mixed ‘alphabet’ of RNA and DNA subunits.
- Kristian Le Vay
- & Hannes Mutschler
-
Nature Podcast |
 Podcast: Lab-made skin grows its own hair
Podcast: Lab-made skin grows its own hair
Listen to the latest science news, brought to you by Shamini Bundell and Nick Howe.
-
Article |
 Selective prebiotic formation of RNA pyrimidine and DNA purine nucleosides
Selective prebiotic formation of RNA pyrimidine and DNA purine nucleosides
A prebiotic synthesis of the purine DNA nucleosides (deoxyadenosine and deoxyinosine) in which the pyrimidine RNA nucleosides (cytidine and uridine) persist has implications for the coexistence of DNA and RNA at the dawn of life.
- Jianfeng Xu
- , Václav Chmela
- & John D. Sutherland
-
Article |
 Desymmetrization of difluoromethylene groups by C–F bond activation
Desymmetrization of difluoromethylene groups by C–F bond activation
Enantioselective activation of a single C–F bond in a difluoromethylene group is achieved using a chiral transition metal catalyst and a fluorophilic activator.
- Trevor W. Butcher
- , Jonathan L. Yang
- & John F. Hartwig

 Molecular editing of aza-arene C–H bonds by distance, geometry and chirality
Molecular editing of aza-arene C–H bonds by distance, geometry and chirality