Abstract
Study design:
This is a case report.
Objectives:
Spinal cord atrophy presenting with late progressive myelopathy after many years of clinical stability is a rare and unexplained phenomenon after cervical spine surgery. The authors report and discuss the etiologies and outcomes of late postoperative myelomalacic myelopathy.
Setting:
This study was conducted in the Department of Neurosurgery, Centro Hospitalar S. João, Porto, Portugal.
Methods:
We report on two patients with insidious chronic progressive myelopathy that developed more than 10 years after complete removal of a cervical intramedullary ependymoma and after transoral odontoidectomy and occipitocervical fusion for craniocervical junction malformation. Differential diagnoses were formulated and review of the literature was performed.
Results:
In both patients, after several years of clinical stability, insidious onset of debilitating myelopathy and dependency ensued. The clinical history, serology, imaging and neurophysiological investigation excluded several putative etiologies: arachnoid adhesions, tumor recurrence, late vertebral instability, trauma, syringomyelia, radiotherapy, and demyelinating or infectious causes.
Conclusion:
Late neurological deterioration after cervical spine surgery is usually related to disease progression or surgery-related complications. Nevertheless, in some patients late myelopathy can ensue even in the absence of identified causes.
Similar content being viewed by others
Introduction
Even though surgery remains the best treatment strategy for spinal ependymomas by allowing long-term disease-free survival, optimal neurological outcome mostly depends on complete lesion removal, tumor extending less than five levels and a McCormick score <3. Late neurological deterioration may occur owing to tumor recurrence, vertebral instability or radiation therapy.1
The outcome after surgery for cervicomedullary decompression and occipitocervical fusion varies according to the type of instrumentation and pathology. Nevertheless, myelopathy improves in most patients, and fusion rates are achieved in 95–100% of cases. Late complications are usually related to pseudarthrosis, adjacent-level degeneration and hardware complications.2
We report two cases of patients with spinal cord atrophy presenting with late progressive myelopathy more than 10 years after surgeries for intramedullary tumor and occipitocervical junction malformation. Long-term outcomes are presented and differentials are discussed. Literature review was performed focusing on delayed complications and long-term neurological outcome
Case report
Case 1
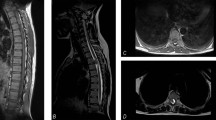
In 1993, a 39-year-old woman underwent complete surgical removal of a cervicodorsal intramedullary ependymoma (C3–D2). Her clinical presentation consisted of bimanual paresthesias, suspended dissociated sensory loss (C5–D2) and right-sided dominant tetraparesis. Postoperatively she maintained the sensory deficits and improved motor function. Gait was coordinated and there was sphincter function preservation. Clinical stability was maintained until 2008 when the patient presented with mild progressive tetraparesis, gait instability and urinary urgency. Neurological examination revealed nonspastic tetraparesis with dominant involvement of the lower extremities, D2–D3 sensory level, hypopalesthesia at the elbows and hyperreflexia. Magnetic resonance imaging (MRI) showed narrowing of the spinal cord between C4 and D2, whereas there were no medullary compressive lesions or neoplastic recurrence (Figure 1a).
T2-weighted magnetic resonance imaging showing spinal cord atrophy between C4 and D2 with no evidence of residual or recurrent medullary compressive lesions and anterior and posterior subarachnoid space showing patency of cerebral spinal fluid flow. (a) T2-weighted magnetic resonance imaging depicting focal area of myelomalacia at C1–C2 level with enlargement of the perimedullary subarachnoid space. (b) Subsequent imaging stability was documented despite ensuing debilitating myelopathy.
Additional investigation with brain MRI, vitamin B12, copper, ceruloplasmine, magnesium, iron kinetics, HIV, HBV, HCV, HTLV-1, syphilis serology and electromyography did not disclose additional causes for clinical deterioration. Genetic testing was not requested owing to the absence of other affected relatives.
At the last follow-up visit in June 2014, symptoms had slowly progressed with motor function deterioration, diminished left-hand grip, and increased dependence and difficulty in performing manual tasks. The patient was admitted to an intensive rehabilitation center.
Case 2
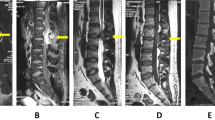
In 1995, a 38-year-old woman underwent a transoral odontoidectomy and posterior occipitocervical fusion for an occipitocervical junction malformation. She presented with motor deficit of the right lower limb and had a good postoperative recovery. Since 2005, the patient started complaining of decreased muscle strength and neuralgic pain, especially in the lower limbs, and urinary urgency. Neurological examination revealed spastic tetraparesis, predominantly in the right upper limb and both lower limbs, with overactive reflexes and bilateral Babinski sign. In addition, she had superficial and deep hypoesthesia in the four limbs and trunk, with a sensory level at C3–C4 and anesthesia from D6 to D10. Positive Romberg and paraparetic gait were evident. Cervical MRI showed signs of odontoidectomy and a myelomalacia area at C1–C2, as well as enlargement of the surrounding subarachnoid space (Figure 1b). Vertebral stability was demonstrated on dynamic radiographic studies. Laboratory investigation was normal, including vitamins, thyroid function, iron kinetics, syphilis serology, viral markers—HIV, HBV, HCV, HTLV-1—copper, ceruloplasmine and autoimmunity screen. Genetic testing was not requested owing to the absence of other affected relatives.
Peripheral nerve conduction studies and electromyography ruled out the possibility of peripheral lesions. Brain and full spinal cord MRI showed no further pathology.
At the last follow-up visit in late 2013, the patient showed worsened gait owing to severe spasticity, which interfered with function and daily activities. Intrathecal baclofen pump was considered, but screening test was negative.
Discussion
Causes of chronic progressive myelopathy other than traumatic or surgical sequelae are numerous and may be divided into five main groups (Table 1). Clinical history, examination, laboratory and imaging studies have allowed us to exclude these causes in the two reported cases. As many as 38% of patients with progressive myelopathy remain a diagnostic enigma, but, despite this important percentage, little attention has been given to this group.3
Published series on long-term outcome after surgery for spinal cord ependymomas focus on progression-free survival and its relation to complete lesion removal and preoperative functional status as predictors of postoperative neurological outcome.1, 4, 5 In fact, tumor recurrence can occur up to 18 years after surgery.6 Raco et al.7 describe functional outcome in a case series and reported that 66% of patients maintained preoperative deficits, 25% improved and 9% had further neurologic deterioration after 7.1 years.
Some authors propose treatment-related factors as predictors for functional outcome, namely the choice of laminoplasty versus laminectomy, claiming that it avoids delayed vertebral instability, which may cause myelopathy, while not compromising the desired decompressive effect.1, 8 Rates of deformity seem to vary from 30 to 60%, but its true frequency in the adult population with intradural tumor is unknown, as well as its long-term neurological consequences.
Some authors have also described poorer functional outcomes with tumors of the cervicothoracic region, presumably owing to the particularly tenuous blood supply to the thoracic cord.8
Only Hoshimaru et al.8 and Samii9 mention spinal cord atrophy in association with arachnoid scarring, presumably owing to mechanical irritation of the arachnoid caused by swelling of the spinal cord in long-standing intramedullary tumors.
According to the systematic review by Winegar et al.,2 a high fusion rate (93%) can be accomplished for occipitocervical fusion, and this has significant correlation with neurological improvement. Inflammatory diseases have the lowest rate of instrumentation failure and the highest rate of neurological improvement (91%) following the use of screw/rod techniques.
Deutsch et al.10 reported that 86% of the patients had symptom improvement after 36 months of follow-up, whereas one-third markedly improved the Nurick grade. In this series, complications occurred in 30% of patients, with late complications being pseudarthrosis, adjacent-level degeneration, infection and occipital neuralgia.
No progressive myelomalacic myelopathy following occipitocervical fusion is reported in the literature to the best of our knowledge, but, nonetheless, mean follow-up is limited.
The cases presented stand out for the presence of focal spinal cord atrophy with no evidence of recurrent/residual tumor, infection, vertebral instability, hardware dysfunction or nonfusion. In this setting, one could ask what causes myelopathy to ensue years after cervical surgery and how spinal cord atrophy evolves and contributes to the pathogenesis of this late neurologic deterioration.
Differential diagnosis in patients with progressive myelopathy following spinal surgery is difficult, owing to the similar clinical picture of various conditions. Avrahami et al.11 reviewed the MRI findings of 31 patients with insidious tetra/paraparesis 2–8 years after cervical surgery. All had spinal cord atrophic changes, and progression of myelopathy was considerably faster in the neoplasm and in vascular, rather than in degenerative, etiology.
The dominant feature of the presented patients was severe spinal cord atrophy, for which many causes are reported in the literature: malformations, central nervous system infection, trauma, radiotherapy, immune disorders, demyelination, axonal injury, Wallerian degeneration and vascular abnormalities.12 In addition, chronic compression, deformity and cord edema can result in ischemia with subsequent demyelination and gliosis.13 Finally, reduced neuronal reserve pool and age-related neuronal cell death can lead to accelerated reduction in function.
After careful investigation of our patients, we have not found a rationale for the late progressive myelopathy. Trauma, cyst formations and cord tethering were excluded in contrast to previous reports, with myelomalacia being the only finding.
Conclusion
Some patients are unable to maintain neurological condition in the long term following cervical spine surgery for intramedullary tumor or occipitocervical junction anomalies. Etiology is usually attributed to progression of disease, cystic cavities, trauma or postoperative deformity. Nevertheless, late myelopathy can ensue even in the absence of identified causes.
References
Eroes CA, Zausinger S, Kreth FW, Goldbrunner R, Tonn JC . Intramedullary low grade astrocytoma and ependymoma. Surgical results and predicting factors for clinical outcome. Acta Neurochir 2010; 152: 611–618.
Winegar CD, Lawrence JP, Friel BC, Fernandez C, Hong J, Maltenfort M et al. A systematic review of occipital cervical fusion: techniques and outcomes. J Neurosurg Spine 2010; 13: 5–16.
Jeffery DR . Chronic progressive myelopathy: diagnostic analysis of cases with and without sensory involvement. J Neurol Sci 1996; 142: 153–156.
Cooper PR . Outcome after operative treatment of intramedullary spinal cord tumors in adults: intermediate and long-term results in 51 patients. Neurosurgery 1989; 25: 855–859.
Garces-Ambrossi GL, McGirt MJ, Mehta VA, Sciubba DM, Witham TF, Bydon A et al. Factors associated with progression-free survival and long-term neurological outcome after resection of intramedullary spinal cord tumors: analysis of 101 consecutive cases. J Neurosurg Spine 2009; 11: 591–599.
Brotchi J, Fischer G . Spinal cord ependymomas. Neurosurg Focus 1998; 4: e2.
Raco A, Esposito V, Lenzi J, Piccirilli M, Delfini R, Cantore G . Long-term follow-up of intramedullary spinal cord tumors: a series of 202 cases. Neurosurgery 2005; 56: 972–981.
Hoshimaru M, Koyama T, Hashimoto N, Kikuchi H . Results of microsurgical treatment for intramedullary spinal cord ependymomas: analysis of 36 cases. Neurosurgery 1999; 44: 264–269.
Samii M, Klekamp J . Surgical results of 100 intramedullary tumors in relation to accompanying syringomyelia. Neurosurgery 1994; 35: 865–873.
Deutsch H, Haid RW, Rodts GE, Mummaneni PV . Occipitocervical fixation: long-term results. Spine 2005; 30: 530–535.
Avrahami E, Tadmor R, Cohn DF . Magnetic resonance imaging in patients with progressive myelopathy following spinal surgery. J Neurol Neurosurg Psychiatry 1989; 52: 176–181.
Finsterer J, Lubec D, Samec P . Generalised spinal cord atrophy, Chiari-I malformation, and syringomyelia. Spinal Cord 2001; 39: 184–188.
Shimizu K, Nakamura M, Nishikawa Y, Hijikata S, Chiba K, Toyama Y . Spinal kyphosis causes demyelination and neuronal loss in the spinal cord: a new model of kyphotic deformity using juvenile Japanese small game fowls. Spine 2005; 30: 2388–2392.
Acknowledgements
We thank Dr. Ricardo Cardoso for professional language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
de Carvalho, B., Barros, P., Pereira, P. et al. Late postoperative myelomalacic myelopathy. Spinal Cord 53 (Suppl 1), S27–S29 (2015). https://doi.org/10.1038/sc.2014.239
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2014.239