Abstract
Study design:
This is a cross-sectional validation study.
Objective:
The objective of this study was to adapt and validate a self-report version of the Spinal Cord Independence Measure (SCIM III) for the Spanish population.
Methods:
A cross-cultural adaptation of the self-report version of the SCIM III for the Spanish population was performed on the basis of international guidelines. A total of 100 patients with spinal cord injury (SCI) were recruited. A team of healthcare professionals administered the SCIM III by observation. In addition, the patients completed the Spanish self-report version (eSCIM-SR). Data from both questionnaires were analysed jointly.
Results:
A high correlation was observed between SCIM III and eSCIM-SR. Lin’s concordance correlation coefficient for the global score was 0.998 (95% confidence interval: 0.997, 0.998), and the subscale scores were 0.988 (0.982, 0.992) for self-care, 0.992 (0.988, 0.995) for respiration and sphincter management and 0.997 (0.995, 0.998) for Mobility. Bland–Altman plots showed a small bias of −0.32 (95% limits of agreement: −3.01, 2.37). The estimated bias was low in all three domains, with values of −0.22 (−2.12, 1.68), −0.1 (−2.02, 1.82) and −0.03 (−1.69, 1.63) for the self-care, respiration and sphincter management and mobility subscales, respectively.
Conclusion:
Our study validates the eSCIM-SR as a tool for the functional assessment of patients with SCI, principally in the outpatient setting.
Similar content being viewed by others
Introduction
Short- and long-term complications in patients with spinal cord injury (SCI) cause significant disability and diminish functional abilities that must be accurately assessed using scales that have been designed specifically for this purpose. It is particularly important to evaluate patient functional status and objectively assess treatment effectiveness in rehabilitation medicine.1
In this regard, the third version of the Spinal Cord Independence Measure (SCIM III) has demonstrated satisfactory psychometric properties, reliability, validity and sensitivity to functional changes in multicentre studies. It is the only scale that was specifically designed to evaluate the functional capacity of patients with SCI.2, 3, 4, 5, 6, 7
The SCIM III evaluates three specific and highly relevant functional areas in patients with SCI: self-care (six items, scores range from 0 to 20), respiration and sphincter management (four items, scores range from 0 to 40) and mobility (nine items, scores range from 0 to 40). Each subscore is scored according to its proportional weight in the patient’s overall activity. The total score of the scale is 100 points, with higher scores indicating higher levels of functional independence. A team of healthcare professionals evaluates the patients by observation.8 Administration of the scale by observation requires time and is primarily performed in the hospital setting.3
In light of the need for an instrument to assess SCI patients’ functional independence that consumes fewer resources with rapid data collection using little effort, and that can be administered regardless of setting, in Switzerland in 2013 Fekete et al.9 developed a self-report version of the SCIM III (SCIM-SR) in German. The new scale consists of 17 items (4 of which have multiple choices) and the same subscales as the SCIM III. Both scales and their scores are equivalent. With a sample of 100 patients with SCI, Fekete et al. achieved a high correlation with the professional-rated SCIM III scale. The SCIM-SR has proven to be an important instrument for evaluating SCI patients’ functional status in the long term and can even be performed in their own homes.
The SCIM-SR has been translated into English, French and Italian, but only the German version has been validated. Before its use in clinical care and research, it must be validated for different countries and cultures.10
The aim of this work is to perform a transcultural adaptation and validation of the SCIM-SR for the Spanish population as a tool to measure functional capacity in patients with SCI.
Materials and methods
This cross-sectional validation study was approved by the Clinical Research Ethics Board of the Hospital Universitario y Politécnico La Fe de Valencia, Spain. The use of the SCIM-SR was authorised by Fekete. Verbal informed consent was obtained from all study participants before their inclusion.
This study includes two well-defined parts: transcultural adaptation and validation.
Transcultural Adaptation
The translation and transcultural adaptation of the scale were conducted in accordance with the international guidelines and proposals put forth by Sperber,11 as detailed below.
Translation into Spanish
Two professional, native Spanish-speaking translators who were members of the Interuniversity Institute of Applied Modern Languages (IULMA) at the University of Valencia made two independent translations of the English version of the SCIM-SR.9 Each translator provided a written report that the study authors combined into a single Spanish version.
Back translation
The Spanish version of the SCIM-SR was translated back into English by two other professional translators from the Translation Service at the Hospital Universitario y Politécnico La Fe de Valencia. Both translators were native English speakers and were unaware of the original English version of the questionnaire. Each translator provided a different report that the study authors again combined into a single English version.
Linguistic validation
Thirty reviewers with a high level of English who were specialised translation students at the Faculty of Philology, Translation, and Communication at the University of Valencia, Spain, independently compared each item of the new English version with the original questionnaire. The purpose of this process was to identify potentially problematic items so that they could be re-translated until they were interpreted in the same way in both languages. Two scales were used: one to analyse the comparability of the language and another to analyse the similarity of its interpretability. Both were Likert-type scales and were scored from 1 (extremely comparable/extremely similar) to 7 (not comparable at all/not similar at all). A mean score was obtained for each item. Items with a mean score of three or less were directly incorporated into the Spanish version, whereas items with a mean score >3 were revised and translated again until a mean score of 3 or less was obtained.
This phase was considered complete only when all of the items reached a mean score of 3 or less.
Pilot study
The Spanish version of the SCIM-SR (eSCIM-SR) was tested in 20 subjects who had been diagnosed with SCI. Each subject provided feedback regarding his/her understanding of the questionnaire, which was used to make final corrections. The study authors discussed the information obtained. Once these changes were made, the final Spanish version was obtained.
Validation of the Spanish version of the SCIM-SR
Patients
A total of 100 patients with SCI who were aged over 18 years and cared for at the Spinal Cord Unit at the Hospital Universitario y Politécnico la Fe de Valencia between February and April 2014 were included in this study. The inclusion criteria were inpatients or outpatients with either traumatic or non-traumatic SCI, a time since the injury of ⩾1 month, a sufficient level of Spanish (tested by the study authors before study inclusion) and the ability to read and answer questions from a questionnaire without assistance. Individuals with severe health issues or cognitive impairment, or those who had undergone surgery within seven days before the study, were excluded.
Procedure
Researchers who were experienced with the use of the SCIM III and had a high level of English administered the English questionnaire by observation to each study participant. Patients were then given the eSCIM-SR to be completed independently or with writing assistance if the level of their injury impeded the use of a pen. No more than three days elapsed between completion of the SCIM III and eSCIM-SR.
Sociodemographic (age, gender and education level) and injury-related (date, aetiology and vertebral level) data were recorded, as was whether the patients were inpatients or outpatients.
We declare that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Statistical analysis
From the collected data, we calculated the mean, s.d., median and first and third quartiles in the case of numeric variables and relative frequencies in the case of categorical variables. Concordance between the original SCIM III and Spanish self-report version was assessed by Lin’s12 concordance correlation coefficient and by Bland–Altman plots.13 The mean bias and 95% limits of agreement between both tests were also estimated. In addition to overall agreement, partial concordance among the different domains of the questionnaires was assessed using the same approaches. All statistical analyses were performed using the R software (version 3.1.0) (Auckland, New Zealand).
Results
Characteristics of the study population are presented in Table 1.
The total and subscale scores of the SCIM III and the eSCIM-SR are presented in Table 2. The mean scores were similar between the questionnaires for both the total and each subscale.
The distribution of the total scores was similar between SCIM III and eSCIM-SR, as shown in Figure 1.
Table 3 shows Lin’s correlations and Bland–Altman differences between SCIM III and eSCIM-SR. Concordance between both tests was almost complete,14 with a Lin’s concordance correlation coefficient of 0.998 (95% confidence interval; 0.997, 0.998).
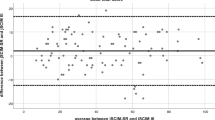
The Bland–Altman plot showed a small bias of −0.32 (95% limits of agreement; −3.01, 2.37). The concordance of the different domains was also very high, with a Lin’s concordance correlation coefficient of 0.988 (95% confidence interval; 0.982, 0.992) for the self-care subscale, 0.992 (95% confidence interval; 0.988, 0995) for the respiration and sphincter management subscale and 0.997 (95% confidence interval; 0.995, 0.998) for the mobility subscale. Estimated bias was low in all three domains, with values of −0.22, −0.1 and −0.03 for the Self-Care, respiration and sphincter management and mobility subscales, respectively. The 95% limits of agreement were also low, ranging from the lowest limit of −2.12 for the self-care subscale to the highest limit of 1.82 for the respiration and sphincter management subscales.
Figure 2 shows the Bland–Altman plots for the agreement of the total score between SCIM III and eSCIM-SR and for the partial agreement of the subscale scores between SCIM III and eSCIM-SR.
Discussion
The results of this study demonstrate the validity of the eSCIM-SR relative to the SCIM III, yielding a Lin’s correlation value of 0.998 for the total score and a Lin’s correlation value exceeding 0.98 for each of the three subscale scores.12
Only the study by Fekete et al.9 has validated a self-reported scale based on SCIM III (SCIM-SR) and was performed in German-speaking inpatients with SCI, resulting in low Pearson's correlation values (that is, above 0.7). Fekete et al. attributed the low concordance between the SCIM III and SCIM-SR to greater differences among the inpatients, as the questionnaire reflected the patients’ current situation instead of their situation at the time of admission. In our study, 84% of the subjects were outpatients with stable health conditions, which may have accounted for the high concordance between the SCIM III and eSCIM-SR.
Our findings also validate the eSCIM-SR as a useful assessment tool of functional independence in patients with SCI in an outpatient setting, and its use has the advantage of requiring less healthcare resources for its application.
In our study, the greatest correlation was observed for the Mobility subscale, which may have occurred because it is one of the easiest areas to substantiate by both external observation and the patients themselves.
As in the study by Fekete et al., the limitations of our study are related to item number 6 (Bladder Management) of the SCIM III, because it had to be modified for a self-report version as it is impossible for patients to measure their residual volume of urine. However, as with the study by Fekete et al., a high correlation was obtained between the item 6 scores of the SCIM III and their equivalents on the eSCIM-SR.
The eSCIM-SR is the second self-report version of the SCIM III to be validated. Our findings are considered valid only for the Spanish population. However, it is worth noting that both the German SCIM-SR and our Spanish eSCIM-SR were derived from the English version of the SCIM-SR. We believe that validation of the English SCIM-SR is warranted and eagerly await this research.
One of the strengths of our study is the process of transcultural adaptation through which the Spanish version was created, which conforms with the guidelines and international criteria proposed by Sperber.11 In addition, we believe that our sample size (n=100) was sufficiently large, with no missing values for any of the variables analysed.
Conclusion
Our study validates the eSCIM-SR as a tool for the functional assessment of patients with SCI, principally in the outpatient setting.
DATA ARCHIVING
There were no data to deposit.
References
Zarco-Perpiñán MJ, Barrera-Chacón MJ, García Obrero I, Méndez Ferrer JB, Alarcón LE, Echevarría-Ruiz de Vargas C . Development of the Spanish version of the Spinal Cord Independence Measure version III: cross-cultural adaptation and reliability and validity study. Disabil Rehabil 2014; 36: 1644–1651.
Catz A, Itzkovich MA . Spinal Cord Independence Measure: comprehensive ability rating scale for the spinal cord lesion patient. J Rehabil Res Dev 2007; 44: 65–68.
Itzkovich M, Tamir A, Philo O, Steinberg F, Ronen J, Spasser R et al. Reliability of the Catz-Itzkovich spinal cord independence measure assessment by interview and comparison with observation. Am J Phys Med Rehabil 2003; 82: 267–272.
Anderson K, Aito S, Atkins M, Biering-Sørensen F, Charlifue S, Curt A et al. Functional recovery measures for spinal cord injury: an evidence-based review for clinical practice and research report of the National Institute on Disability and Rehabilitation Research Spinal Cord Injury Measures Meeting. J Spinal Cord Med 2008; 31: 133–144.
Anderson KD, Acuff ME, Arp BG, Backus D, Chun S, Fisher K et al. United States (US) multi-center study to assess the validity and reliability of the Spinal Cord Independence Measure (SCIM III). Spinal Cord 2011; 49: 880–885.
Bluvshtein V, Front L, Itzkovich M, Aidinoff E, Gelernter I, Hart I . SCIM III J is reliable and valid in a separate analysis for traumatic spinal cord lesions. Spinal Cord 2011; 49: 292–296.
Scivoletto G, Tamburella F, Laurenza L, Molinari M . The spinal cord independence measure: how much change is clinically significant for spinal cord injury subjects. Disabil Rehabil 2013; 35: 1808–1813.
Catz A, Itzkovich M, Steinberg F, Philo O, Ring H, Ronen J et al. Disability assessment by a single rater or a team: a comparative study with the Catz-Itzkovich spinal cord independence measure. J Rehabil Med 2002; 34: 226–230.
Fekete C, Eriks-Hoogland I, Baumberger M, Catz M, Itzkovich M, Lüthi H et al. Development and validation of a self-report version of the Spinal Cord Independence Measure (SCIM III). Spinal Cord 2013; 51: 40–47.
Meneghini R, Packer AL . Is there science beyond English? Initiatives to increase the quality and visibility of non-English publications might help to break down language barriers in scientific communication. EMBO Rep 2007; 8: 112–116.
Sperber AD . Translation and validation of study instruments for cross-cultural research. Gastroenterology 2004; 126: S124–S128.
Lin L . A note on the concordance correlation coefficient. Biometrics 2000; 56: 324–325.
Bland J, Altman D . Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 327: 307–310.
McBride GB . A proposal for strength-of-agreement criteria for Lin's Concordance Correlation Coefficient. NIWA Client Report: HAM2005062, 2005.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Aguilar-Rodríguez, M., Peña-Pachés, L., Grao-Castellote, C. et al. Adaptation and validation of the Spanish self-report version of the Spinal Cord Independence Measure (SCIM III). Spinal Cord 53, 451–454 (2015). https://doi.org/10.1038/sc.2014.225
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2014.225
This article is cited by
-
Exoskeleton-based training improves walking independence in incomplete spinal cord injury patients: results from a randomized controlled trial
Journal of NeuroEngineering and Rehabilitation (2023)
-
Validation of the Thai version of the Spinal Cord Independence Measure Self-Report (SCIM-SR-Thai)
Spinal Cord (2022)
-
Functional independence in the Finnish spinal cord injury population
Spinal Cord (2022)
-
Translation and validation of the Chinese version of the Spinal Cord Independence Measure (SCIM III) Self-Report
Spinal Cord (2021)
-
Cross-cultural adaptation and psychometric testing of the Thai version of the Spinal Cord Independence Measure III—Self Report
Spinal Cord (2021)