Abstract
Human metabolism is complex, and is impacted by genetics, cohabitation, diet, health, and environmental inputs. As such, we applied untargeted LC-MS metabolomics to 1425 saliva samples from a diverse group of elementary school-aged children and their caregivers collected during the Family Life Project, of which 1344 were paired into caregiver/child dyads. We compared metabolomes within and between homes, performed population-wide “metabotype” analyses, and measured associations between metabolites and salivary biomeasures of inflammation, antioxidant potential, environmental tobacco smoke (ETS) exposure, metabolic regulation, and heavy metals. Children and caregivers had similar salivary metabolomes, and dyad explained most metabolomic variation. Our data clustered into two groups, indicating that “metabotypes” exist across large populations. Lastly, several metabolites—putative oxidative damage-associated or pathological markers—were correlated with the above-mentioned salivary biomeasures and heavy metals. Implications of the family environment’s effects on metabolomic variation at population, dyadic, and individual levels for human health are discussed.
Similar content being viewed by others
Introduction
Metabolomics allows for the assay and quantification of thousands of chemical compounds in a biological sample1,2,3,4,5. In humans, metabolomics correlates with the overall metabolism of biochemical pathways in not only the individual, but also microbes that inhabit specific areas of the body1,6. Previous studies have shown that metabolomics is a useful tool that has the potential to find biomarkers for diseases and stress, generally through analyzing bodily fluids from subjects7,8,9. One such sample specimen is saliva - a complex matrix of chemical compounds, proteins, and cells important in lubricating the oral cavity, digesting food, and protecting against infection3,10,11. As a minimally-invasive biofluid to collect, saliva offers a straightforward way to assay metabolites, proteins, and xenobiotic compounds to find biomarkers for disease or environmental stress exposure12,13,14. Aside from host metabolites, saliva also notably contains microbially-produced compounds and provides a medium to associate host and microbial relationships1,6,15. In this study, we concentrate on inter/intrafamily relationships of the salivary metabolome, and associations between this metabolome and biomeasures relating to environmental tobacco smoke (ETS) exposure (the nicotine breakdown product cotinine)16, antioxidant potential (uric acid; a compound considered to be a strong antioxidant and is abundant and active in saliva)12,17,18, inflammation (C-reactive protein; CRP)19, and the modulation of glucose and lipid metabolism (adiponectin; a compound thought to be anti-inflammatory in serum)13, along with a selection of metals (chromium, copper, lithium, manganese, and zinc)14.
Family members are known to share similar oral health attributes, living environments, and overall diets. Likely due to this close social ecology of the home, several studies have shown that the oral microbiome is more similar within cohabitating family members than those outside of the home20,21,22,23,24. Similarly, the overall metabolomes of parental caregivers and their children are more similar than nonfamily individuals, and several metabolites are strongly correlated between family members25,26,27,28,29,30. While the concept of interfamilial metabolic similarity is not novel, many studies concentrate on well-established health biomarkers such as cholesterol, amino acids, and hormones. However, due to the targeted nature of these analytical methods, they likely do not capture overall metabolic relationships within families, and may miss currently-unknown biomarkers of oral health8,31. Much like the salivary microbiome1,20, the salivary metabolome can be categorized into multiple “ecotypes” of metabolites that may correspond to oral dysbiosis, altered biochemical profiles, or baseline metabolism1,9,32, but exactly how widespread this clustering is across large populations remains largely unknown.
The human oral cavity contains a multitude of chemicals derived from host biofluids, and as mentioned above, allows for investigations into the environment in which people live through salivary analyses10,14. For example, kit-based spectrophotometric techniques and mass-spectrometry-based methods can detect the presence of analytes involved in nicotine metabolism (i.e., cotinine), drugs and medications, xenobiotics, and metals14,33,34. Similarly, through untargeted metabolomics, we may be able to discover novel biomarkers of oral dysbiosis (or, conversely, health) that provide new diagnostic tools and establish baseline measurements of chemicals within saliva1,3,8,15. Families living in rural or lower-socioeconomic areas are often exposed to high pollution burdens14,35,36, which likely contributes to chronic stress. Furthermore, individuals exposed to heavy metals are known to have higher metabolites associated with oxidative stress and damage (reviewed in Bonvallot 201837. As part of further understanding the effects that environmental stressors have on children38, we chose to analyze salivary metals content and associate them with our untargeted metabolomes. Many metals are known to be associated with physiological and neurological problems when observed at high levels39,40,41. These elements serve as reliable indicators of exposure, and we seek to uncover potential biomarkers for metals exposure, which may help us understand unexplored pathways underlying health outcomes, and add to the growing field of “exposomics”42,43,44.
Given the importance of understanding human metabolism and how it relates to families, we investigated the salivary metabolome in the context of children and their caregivers as part of the Family Life Project (FLP), a large-scale prospective longitudinal study38. For example, if caregivers are smoking - with negative impacts on oral and other health measures—are these markers shared with children who are not smoking, but share the same household? We asked several questions of our data: First, does the salivary metabolome differ between children and adults (caregivers, henceforth), and is there metabolic concordance within families? Second, does the salivary metabolome associated with biomeasures of ETS exposure, antioxidant potential, metabolic regulation, or inflammation, and are there chemicals in saliva that may serve as additional biomarkers for the above-mentioned biomeasures? Lastly, are there associations between salivary metals burden and metabolites, and might these associations indicate potential negative health outcomes?
Methods
Study participants
The Family Life Project (FLP) is a longitudinal study of families residing in Pennsylvania or North Carolina. FLP began in 2003–2004, when a representative sample of 1292 children whose families resided in the target communities at the time the mothers gave birth were recruited and enrolled. Detailed descriptions of the sampling and recruitment procedures are available in Vernon-Feagans, Cox, and the FLP Key Investigators, 201338. Briefly, families with a child born between September 2003 and August 2004 were recruited from hospitals, and multiple subsequent FLP study visits have been conducted with consented participants from children aged 2-months to age 20 years. The current analyses focused on a subset of data collected at the child’s 90-month at-home follow-up visit, where children and their primary caregivers provided unstimulated, resting/baseline saliva samples via passive drool collection. These saliva samples were assayed and archived in −80 ◦C freezers, and biospecimens with adequate saliva remaining for metabolome analysis were examined in this study. Out of the total FLP sample, we assessed 1425 saliva samples (Table 1) (child N for this subsample = 719; female = 353, male = 366; age = 79–100 months [average = 87 months], caregiver N = 706; female = 692, male = 27; age = 22–65 years [average = 34 years) of which 1344 were paired into 672 caregiver/child dyads. Procedures for this study were approved by the Institutional Review Boards of the University of North Carolina (IRB # 07–0646 and 16–2751) and New York University (IRB # IRB-FY2017-69) using deidentified data. For children, informed consent was obtained from the child’s parent or guardian. Informed consent was also obtained from all adult participants in the study. Sample IDs were further randomized prior to analysis and reporting.
Saliva sample handling for biomeasure analyses
All salivary biomarker analyses were conducted at the UC Irvine Institute for Interdisciplinary Salivary Bioscience Research (IISBR), where samples were stored at −80 °C as previously described20. Briefly, we thawed the samples, vortexed, then centrifuged samples for 15 min at 3500 RPM, then analyzed the supernatant for adiponectin, CRP, cotinine, and uric acid concentrations as follows below. We note that only children’s samples were assayed for all biomeasures, while caregiver samples were subject to cotinine analysis only. Samples numbers for each analyte in children were: adiponectin (N = 701), CRP (N = 616), cotinine (N = 714), and uric acid (N = 630), while caregivers’ cotinine was measured in (N = 672) samples (Table 1).
Adiponectin, C-reactive protein, cotinine, and uric acid assays
We analyzed salivary adiponectin with the Human Adiponectin MSD assay kit (Meso Scale Discovery, Rockville, MD, USA). We diluted samples fivefold, then assayed following the manufacturer’s supplied protocol using a four-log standard curve and read the sample concentrations on a Meso Quickplex SQ120 spectrophotometer (Meso Scale Discovery, Rockville, MD). Concentrations were derived using MSD Discovery Workbench software v4.0 using curve fit models, with an assay range of sensitivity of 0.06 to 1000 ng/mL.
We assayed CRP in duplicate with the Human CRP V-Plex MSD Multi-spot Assay kit (Meso Scale Discovery, Rockville, MD, USA). We diluted samples five- or ten-fold, then assayed following the manufacturer’s protocol using a modified four-log standard and read the sample concentrations on a Meso Quickplex SQ120 spectrophotometer. The assay range sensitivity was 1.33–46,600 pg/mL CRP.
We measured salivary cotinine concentrations in both children and caregivers using the Salimetrics Salivary Cotinine ELISA kit (Salimetrics, Carlsbad, CA, USA) following the manufacturer’s protocol. We analyzed 20 uL of sample in duplicate by incubating samples with kit reagents for 90 min with shaking at 37 °C, and diluted samples tenfold if subjects reported nicotine use. As above, we washed the plates and then added TMB followed by room temperature incubation for 30 min in the dark. We added 2 M sulfuric acid and read the results on a PowerWave HT spectrophotometer (BioTek/Agilent Technologies, Santa Clara, CA) spectrophotometer, and computed a standard curve using a four-parameter non-linear regression curve fit. The assay range of sensitivity was 0.15 to 200 ng/mL for neat saliva and 1.5 to 2000 ng/mL for tenfold diluted saliva, and we substituted values of ½ the lower limit of measurement for each sample under the lowest reliable measurement for 316 samples (N = 138 caregivers and N = 178 children).
We analyzed uric acid with the Salimetrics Salivary Uric Acid Assay kit (Salimetrics, Carlsbad, CA, USA). We mixed 10 uL of the sample with 190 uL of uric acid reagent in duplicate following the manufacturer’s protocol, then measured the results on a PowerWave HT spectrophotometer (BioTek/Agilent Technologies, Santa Clara, CA). The uric acid assay had a range of sensitivity from 0.07–20 mg/dL.
Salivary metals data
We obtained salivary metal concentrations in children from ref. 14 and report their methods here for clarity. Briefly, Gatzke-Kopp and colleagues used Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) to measure the concentration of metals in aliquots of saliva that we matched to our own. We obtained data from N = 237 samples for chromium, copper, manganese, and zinc, while only N = 204 samples yielded detectible lithium measurements (Table 1).
Metabolome sample preparation and mass-spectrometry
We sent frozen saliva samples on dry ice to the West Coast Metabolomics Center (WCMC) at the University of California, Davis, for sample preparation and data collection. Briefly, WCMC extracted 100 uL of saliva with 3:3:2 acetonitrile/isopropanol/water and then evaporated the samples. WCMC ran 5 uL of the sample through hydrophilic interaction chromatography (HILIC) and then used an Agilent 6530 Quadrupole Time of Flight MS/MS mass spectrometer in both positive and negative ion modes to capture ion intensities. WCMC used published protocols to collect data, and ran all samples, quality control pool samples, and method blanks45,46,47 in the same manner and identified mass-spectra using the MassBank of North America database. WCMC normalized the peak areas of samples with Systematic Error Removal Using Random Forest (SERRF)48, and we note that within each sample, WCMC added stable isotope “internal control standards” (iSTDs) to the samples before injection to calibrate retention times. We received the data as an Excel file with metabolite names (for identified metabolites only), adducts, ion mode, retention times, mass/charge ratios (m/z), and peak heights for each ion (Dryad dataset49). We then assigned “chemical taxonomy” to the named metabolites with the chemical classification software “ClassyFire”50.
Metabolome data analyses
We used R51 for data analyses and manipulation. Before any analyses, we removed iSTD ions from the dataset, as the WCMC added these for quality control. We also filtered out ions with an average relative peak height of less than twofold higher in samples or quality control samples as compared to blanks. We normalized the ions to their within-sample relative peak heights and used these values for downstream analyses. Throughout the analyses of the data, we used a “metabology” approach—where metabolomics data are analyzed through community ecology tools to understand the relationships between ions and adequately handle sparse, compositional, multidimensional data52,53,54. We used these data to tabulate Shannon diversity and Bray-Curtis indexes and visualized the data with nonmetric multidimensional scaling ordinations (NMDS) with “ggplot2”55 and “patchwork”56 with categorical variables self-reported on a questionnaire: “caregiver/child”, “female/male” for children, “smoking/nonsmoking” for caregivers, and “<1 ng/mL or >1 ng/mL” cotinine concentration for children as an estimate for ETS exposure16. We tested these variables for statistical significance with Adonis (PERMANOVA with 999 permutations) in the R package “vegan”57 for multivariate statistics and linear mixed-effects models on individual named metabolites with “lmerTest”58 in R. We obtained salivary metals data from Gatzke-Kopp et al 202314 on sample aliquots from the same original source, and refer to their publication for all relevant materials and methods to metals concentration analyses. Similarly, we assigned metals concentrations to tertile based on concentration within the entire subsample and ran Adonis tests on those data as above.
We used the R package “Hmisc”59 to generate Spearman correlations between the relative peak heights of ions and the concentrations of the biomeasures adiponectin, cotinine, CRP, and uric acid, along with each metal for children and cotinine for caregivers only, then corrected the resulting P values for multiple comparisons via the Benjamini–Hochberg method. We also used distance-based Redundancy Analysis (db-RDA) in “vegan” to assess the contributions of continuous variables to metabolome variability and plotted the resulting ordinations with “ggplot2.”
Lastly, we performed stomatotype analysis (as in refs. 20,60,61) on the metabolomes of paired caregiver/child dyads using Jensen–Shannon distances and partitioning around medoid (PAM) clustering with the R packages “ade4”62 and “cluster”63. We estimated optimal cluster number by Silhouette values, then used lmerTest for univariate analyses between PAM clusters, adjusted p values for multiple comparisons with the Benjamini–Hochberg method, and plotted the resulting PCoA ordination and boxplots of the data with “ggplot2” and “patchwork.”
Results
We obtained ion profiles from both caregivers (N = 706) and children (N = 719), along with extraction method blanks (N = 153), and quality control pool samples (N = 157) (Dryad dataset49). From these metabolomes, we detected 2881 unique ions, of which 281 were identified by name, and filtering left us 2046 total ions (both positive and negative ions), of which 210 were named. Overall, the 104 most proportionally represented ions comprised 50.02% of the relative peak heights in our samples (Fig. S1), of which 1409 were present at >0.01% relative peak height. Likewise, identified metabolites only comprised 15.56% of the relative peak heights of ions, while unknown ions accounted for 84.45% (Figs. S1–S4). We used the ClassyFire chemical classifier to assign “chemical taxonomy” to each identified ion and plotted the relative peak heights of the ten most proportionally represented chemical superclasses as stacked bar plots (Figs. S2–S4).
We paired 1426 samples into 672 caregiver/child dyads. We compared the diversity of all 2046 ions (as measured by Bray-Curtis dissimilarities) and found that children and caregivers significantly differed (F = 37.2, R2 = 0.02, P < 0.001), and dyad explained most of the variation between our subjects (F = 1.8, R2 = 0.62, P < 0.001, Fig. 1a), while alpha diversity was not different between caregivers and children (H(1) = 1.6, P = 0.20). Then, we used linear mixed-effects models with dyad as a random variable on ions at greater than 0.01% relative peak height and showed that 95 identified metabolites differed between caregivers and children (Padj < 0.05, supplemental dataset SF1). Of these, 20 metabolites were highly significantly different between caregivers and children (Padj < 2 × 10−15): Theophylline-, caffeine+, 4-imidazoleacrylic acid±, ribose-5-phosphate-, inosine-, agmatine+, 2-amino-1-phenylethanol+, and amino acids and their derivatives (Fig. 1 and Supplemental Dataset SF1).
Comparisons of caregivers and children by (A) NMDS ordination and (B) linear mixed-effects models of the relative peak heights of the 20 most significantly differentially abundant metabolites (Padj < 2 × 10–15). Dyad explained most of the variation between our subjects (F = 1.8, R2 = 0.62, P < 0.001 while categorical age explained ~2% of metabolome variation (F = 37.2, R2 = 0.02, P < 0.001). (+) or (−) denotes positive or negative metabolite ions.
We analyzed the metabolomes of paired caregiver/child dyads (N = 1344) through PAM clustering and observed that these metabolomes clustered into two overlapping groups of individuals based on Silhouette values (Silhouette = 0.12) and plotted the resulting PCoA ordination (Fig. 2a). Ion beta diversity differed between the two clusters (F = 189.6, R2 = 0.12, P < 0.001). We then ran linear mixed-effects models on ions at greater than 0.1% relative peak height with dyad as a random variable to univariately compare the clusters and showed that 25 identified metabolites significantly differed between the clusters (Padj < 0.05, Fig. 2b and Supplemental Dataset SF1). Of these, 20 metabolites had Padj values of <1 × 10−6: Hypoxanthine+, oxypuranol-, 2-amino-1-phenylethanol+, 2-(4-amino-1-piperidinyl)ethanol+, 4-imidazoleacrylic acid+, histamine+, nudifloramide+, phenylacetaldehyde B+, and amino acids and derivatives (including nonproteinogenic) (Fig. 2 and Supplemental Dataset SF1).
Analyses of the metabolomes of paired subjects by (A) PAM clustering and (B) linear mixed-effects models of the relative peak heights of the 25 most significantly differentially abundant metabolites (Padj < 0.001). Only metabolites at greater than 0.1% relative peak heights were analyzed by LMER, and (+) or (−) denotes positive or negative metabolite ions.
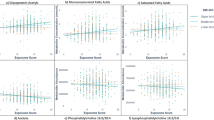
We were interested in the effects of biological sex on the metabolomes of children, but found no differences between the alpha or beta diversity of boys’ and girls’ overall metabolomes (alpha: [H(1) = 0.44, P = 0.51]; beta: F = 1.14, R2 = 0.002, P = 0.25), and did not observe any significant univariate associations between individual ion and sex. We assessed the associations between concentrations of adiponectin, CRP, cotinine, and uric acid with the overall metabolomes of children (N = 538) through distance-based redundancy analysis (db-RDA). We observed that the four biomeasures cumulatively explained 5.3% of metabolome variation (Overall F = 7.5, P < 0.001), with each biomeasure significantly associated with the metabolomes (P < 0.026 for each, Fig. 3 and Supplemental Dataset SF1).
A db-RDA analysis of children’s metabolomes with kit-assayed biomeasure concentrations overlayed as vectors. Overall, the four measured biomarkers were significantly associated with metabolome separation (F = 7.5, P < 0.001), with each ion significantly associating with the metabolomes (P < 0.026 for each). B, C Significant Spearman correlations (−0.15 < ρ > 0.15 shown only) of identified metabolites with kit-assayed adiponectin and uric acid. (+) or (−) denotes positive or negative metabolite ions.
We ran univariate Spearman’s correlations on ions at greater than 0.01% average relative peak height to find associations between specific ions and each of the salivary biomeasures examined. We report the sample sizes for each analysis as each biomeasure had a different number of measurements. Concentrations of adiponectin were significantly correlated with 41 metabolites (N = 701, −0.16 < ρ < 0.35, Padj < 0.05, Fig. 3 and Supplemental Dataset SF1). CRP levels were significantly correlated with 22 metabolites (N = 616, −0.13 < ρ < 0.15, Padj < 0.05, Fig. 3 and Supplemental Dataset SF1). Lastly, uric acid concentrations were significantly correlated with 94 metabolites (N = 630, −0.49 < ρ < 0.57, Padj < 0.05, Fig. 3 and Supplemental Dataset SF1). We also ran these correlation tests on kit-measured cotinine concentrations and reported those results with the smoking/nonsmoking analyses.
We compared the associations between ETS exposure and children’s (N = 714) and caregivers’ (N = 672) metabolomes separately because of the different routes of smoking exposure. We analyzed children’s metabolomes by categorically classifying children by their kit-measured cotinine concentration (children’s cotinine levels <1 ng/uL and >1 ng/uL) but did not see a significant relationship between ETS exposure and metabolic alpha (H(1) = 0.25, P = 0.61) or beta diversity (F = 1.1, R2 = 0.002, P = 0.29, Fig. 4a). We then ran generalized linear models on each metabolite and found that only nicotine+ was significantly different between groups (Padj < 0.001, Supplemental Dataset SF1).
A NMDS ordination of the metabolomes of children with salivary cotinine <1 ng/uL versus >1 ng/uL and B caregivers who reported smoking or not. ETS exposure did not significantly alter the metabolome of children (F = 1.1, R2 = 0.002, P = 0.29), but did affect caregivers’ (F = 8.6, R2 = 0.01, P < 0.001. C, D Significant Spearman correlations (Padj < 0.05, (−0.15 < ρ > 0.15 shown only) of salivary cotinine and kit-measured cotinine in (C) children or D caregivers. E Boxplots of the relative peak heights of significantly different metabolites between smoking and nonsmoking caregivers as tested by generalized linear models (Padj < 0.05, only Padj < 0.01 shown). (+) or (−) denotes positive or negative metabolite ions.
As the intensity of ETS exposure likely causes different effects on the metabolome we ran Spearman correlations between kit-assayed cotinine and individual metabolites in children (N = 714). Cotinine was significantly correlated with 14 metabolites (−0.11 < ρ < 0.26, Padj < 0.05, Fig. 4 and Supplemental Dataset SF1).
We found that self-reported smoking status slightly related to the metabolome beta diversity of caregivers (F = 8.6, R2 = 0.01, P < 0.001, Fig. 4), but not alpha diversity (H(1) = 0.44, P = 0.51). We used generalized linear models on individual ions at greater than 0.01% relative peak height to find associations with caregivers’ smoking status. Only 22 identified metabolites significantly differed between groups: Nicotine+, 4-imidazoleacrylic acid+, phenylethanolamine B+, 5-aminosalicylic acid+, N-omega-acetylhistamine+, histamine+, 2’-deoxyguanosine+, theophylline-, phenylacetaldehyde B+, 2’-deoxyadenosine+, inosine-, hypoxanthine+, choline+, nudifloramide+, and amino acids and derivatives (Fig. 4 and Supplemental Dataset SF1).
We ran Spearman correlations between individual ions and kit-measured cotinine in caregivers in the same fashion as above in children. Cotinine was significantly correlated with 39 identified metabolites (−0.32 < ρ < 0.67, Padj < 0.05, Fig. 4 and Supplemental Dataset SF1).
We measured the associations between the metals lithium (N = 204; average = 13.9 μg/L, range = 0.11–845.5 μg/L), chromium (N = 237; average = 7.3 μg/L, range = 0.07–22.5 μg/L), copper (N = 237; average = 29.0 μg/L, range = 0.23–844.2 μg/L), manganese (N = 237; average = 8.6 μg/L, range = 0.04–122.5 μg/L), and zinc (N = 237; average = 60.9 μg/L, range = 0.09–566.0 μg/L) measured in saliva and the metabolomes in children. As the metals were reported as concentrations (ug/L), we split the metals results into tertiles (i.e., 1st, 2nd, 3rd) to analyze the associations categorically. Salivary metal tertile for each element significantly associated with metabolome beta diversity ([Li: R2 = 0.01, F = 1.5, P = 0.044], Cr: [R2 = 0.01, F = 1.5, P = 0.039], Cu: [R2 = 0.02, F = 2.1, P = 0.004], Mn: [R2 = 0.02, F = 2.7, P < 0.001], Zn: [R2 = 0.01, F = 1.6, P = 0.038], Fig. 5), but not metabolome alpha diversity (P > 0.05 for each, Fig. 5). We then used db-RDA on the samples with measured concentrations of all metals (N = 204) and found that these metals explained 4.6% of metabolome variation (Overall F = 1.9, P < 0.001), but only chromium, manganese, and copper contributed meaningfully to this variation, while lithium and zinc did not (Cr, Mn, Cu: P < 0.05, Li and Zn: P > 0.05, Fig. 5 and Supplemental Dataset SF1).
NMDS ordinations of metabolomic diversity as a function of salivary metal tertiles for (A) chromium, B copper, C lithium, D manganese, and E zinc for each metal tested. Metals significantly associated with metabolome beta diversity ([Li: R2 = 0.01, F = 1.5, P = 0.044], Cr: [R2 = 0.01, F = 1.5, P = 0.039], Cu: [R2 = 0.02, F = 2.1, P = 0.004], Mn: [R2 = 0.02, F = 2.7, P < 0.001], Zn: [R2 = 0.01, F = 1.6, P = 0.038]). Panel (F) is a db-RDA of all metal concentrations. Metals explained 4.6% of metabolome variation (Overall F = 1.9, P < 0.001), and chromium, manganese, and copper contributed significantly to the variation, while lithium and zinc did not (Cr, Mn, Cu: P < 0.05, Li and Zn: P > 0.05).
We also measured univariate Spearman correlations between metals concentration and individual metabolites at greater than 0.01% relative peak height. Lithium was significantly correlated with six metabolites (ρ < −0.21, Padj < 0.05, Fig. 6, Supplemental Dataset SF1), chromium was significantly correlated with nine metabolites (ρ < −0.18, Padj < 0.05, Fig. 6, Supplemental Dataset SF1), copper was significantly correlated with 42 metabolites (−0.46 < ρ < 0.44, Padj < 0.05, Fig. 6, Supplemental Dataset SF1), manganese was significantly correlated with 52 metabolites (−0.47 < ρ < 0.38, Padj < 0.05, Fig. 6, Supplemental Dataset SF1), and zinc significantly correlated with 29 metabolites (−0.43 < ρ < 0.31, Padj < 0.05, Fig. 6, Supplemental Dataset SF1).
Discussion
Through our exploratory analyses of a large-scale sample from the 90-month assessment of the Family Life Project cohort38, we were able to assay the salivary metabolome and its associations with biomeasures relating to antioxidant potential, environmental tobacco smoke (ETS) exposure, and systemic inflammation. Most of the variance in salivary metabolomes were explained by family dyad, supporting the view that home environments are incredibly impactful on people, and the closeness of families often result in similar metabolisms and health measures. We also find that a small but significant proportion of the variance is explained by how children’s and adults’ metabolomes differed overall25,26,27,28,29. Extending this concept further, subjects generally clustered into two overlapping metabolomic groups, suggesting that people fall into sub-groups with shared features, although we are unable to discern exactly what causes this grouping1. We observed significant but minor shifts in smokers’ metabolomes, but not in their children, indicating that primary ETS exposure can affect salivary metabolites and may be associated with increased inflammation and polyamine turnover4,33,64,65. We also show that kit-based biomeasures correspond well with metabolites intermediate in their respective biochemical pathways, suggesting both kit-based spectrophotometry and mass-spectrometry-based approaches are useful to assaying metabolism. Lastly, salivary metals were associated with altered children’s metabolomes, and were often anticorrelated with putative proteolysis products, suggesting that environmental metals may affect metabolism66, and that saliva is a useful biofluid in which to assay metals14. Collectively, our study represents a large-scale investigation into the metabolism of families and how these chemicals may relate to cohabitating individuals.
Our study shows that the caregiver/child dyad explained most of the salivary metabolome variation, supporting others’ similar findings in specific metabolites25,26,27,28, but previously unknown in large-scale studies measuring the overall metabolome. Many factors are known to affect metabolism, such as age, sex, diet, and, increasingly, familial environment, and our work adds an important dimension to this metabolic variability1,28,67. By applying untargeted metabolomics, we can start to unravel the complex interactions that family and social structure has on the metabolism of individuals. Even though the child/caregiver dyad explained the vast majority of metabolic variation, several ions’ relative peak heights differed between children and caregivers - many of which are “lifestyle,” medicinal, or environmental molecules. Chemicals such as nicotine, caffeine, theophylline, phenylacetaldehyde, acetaminophen, and salicylic acid were all higher in adults, likely due to diet or medication4. Similarly, adults generally had higher relative peak areas of dipeptides (suggesting proteolysis or oxidative damage), while children had more ions basal or intermediate to chemical pathways, perhaps due to children’s higher basal metabolic rate and growth. As amino acid metabolism has been shown to change with age4 and differs across populations1, more research is needed to understand amino acid metabolism associations with medical conditions or normal development. We also note that large proportions of our data consist of unidentified ions and are likely important to a discerning child from a caregiver, but we are unable to classify these peaks and cannot rule out that the observed spectral peaks are not distinct ions. We appreciate that the magnitude of these differences, while significant, are slight, further stressing the impact and importance of family (relatedness and/or cohabitation) on metabolism, and that our sample population was targeted to specific locations and demographics, reducing the generalizability of our results. We also recognize that our metabolomics data are biased by only using one type of chromatography, which likely overrepresents biogenic amines at the cost of other ion species68, and that matrix effects and ionization efficiencies may impact the interpretation of our results69.
Unsupervised clustering of large-scale, multidimensional metabolomics data has been used to group diseased and healthy individuals by common metabolic patterns and provide insights into their underlying physiology1,9,32,70,71,72. In our study, subjects’ metabolomes tended to cluster into two overlapping groups which did not obviously correspond to any of our metadata categories (i.e., sex, age, smoking, state of residence, etc.). These clusters were somewhat separated by free dipeptides, a Lewis X trisaccharide (LeX) and intermediates of the urea cycle (i.e., citrulline, arginine, and ornithine), but mainly differed by the proportion of unidentified, ions and we note that the overall differences between clusters were relatively minor as seen before1. Previous cluster-based metabolomics studies have shown that differences in amino acid metabolism can cause cluster separation1,9,32, and oral microorganisms impact the concentrations of these metabolites1,15, so future studies should incorporate “multi-‘omic” approaches to understanding the chemical and microbial ecology of the mouth. Likewise, urea cycle metabolites were important in cluster separation. Intermediates of the urea cycle have been implicated as potential indicators for diverse maladies, such as hypertension73, breast cancer32, severe inflammation74, and urea cycle disorders75, suggesting that untargeted metabolomics and cluster analyses are useful to find compounds that may serve as biomarkers for disease. While the clusters largely separate in ordination space, we note that there is substantial overlap, indicating variability and noise in our data, which may be affecting our interpretations. Similarly, as we are unable to pinpoint exactly what is driving metabolome clustering, our results may be influenced by diet or other personal choices that are not reflected in our metadata1,2,4,15,76,77, or unknown interactions/ion detection phenomena69.
Adiponectin, CRP, and uric acid are significantly associated with altered metabolomes in children, with uric acid explaining the most variance. As the endpoint in purine degradation78, uric acid was (as expected) strongly anticorrelated with intermediates of purine metabolism. Conversely, acylcarnitines were correlated with uric acid, which may indicate consumption of a high-fat/high-protein diet and subsequent lipid accumulation79, and an increase in creatine with uric acid may also be due to protein intake and subsequent purine metabolism or antioxidant activity80,81. We found many positive correlations between free dipeptides and adiponectin—a molecule predicted to modulate inflammation and oxidative stress13,82. As dipeptides are thought to be biomarkers for proteolysis83 and may have antioxidant activity84 the correlations of adiponectin and dipeptides could indicate infections or inflammation in children’s mouths, and so may serve as a useful salivary biomeasure for immune activity. Lastly, CRP had very slight associations with overall salivary metabolomes and individual metabolites, suggesting that while CRP is a useful biomarker for systemic inflammation, it is also nonspecific85, and may not associate well with metabolic pathways in saliva15. Collectively, these results suggest that the spectrophotometry kit-based biomeasures capture useful physiological markers for their respective pathways or functions, and indicate that saliva is a worthwhile biospecimen for assaying these measures10.
Environmental tobacco smoke (ETS) exposure is a major, worldwide cause of morbidity and has been shown to alter human metabolism4,33,64. Smoking minorly affected caregivers’ overall metabolomes, but exposure to ETS did not alter children’s—likely because children were only exposed passively and did not receive the same nicotine dose as active smokers, although this is speculative because we do not have survey data reflecting total nicotine consumption. In both children and caregivers, several metabolites were correlated with kit-assayed cotinine (the major final metabolite of nicotine), including phenylethanolamine, phenylacetaldehyde, choline, creatine, N8-acetylspermidine, and unsurprisingly nicotine, along with a strong apparent histamine response in caregivers. As an agonist of acetylcholine receptors, nicotine has been shown to increase acetylcholine demand (and therefore choline catabolized from phospholipids)86, which may be driving our perceived proportional increase in salivary choline87, along with increasing phenylethanolamine N-methyltransferase activity (possibly observed in our study as increased phenylethanolamine)88. Likewise, smoking reduces creatine kinase activity (converts creatine to phosphocreatine), which may cause higher levels of creatine with increasing nicotine concentrations89,90. In regard to N8-acetylspermine—a molecule indicative of polyamine turnover and associated with vascular pathologies65,91—again, we observed a correlation with nicotine. Lastly, histamine was markedly correlated to cotinine concentration, suggesting that histamine production, mast cell activations, and inflammation are affected by nicotine use in caregivers92,93. While the overall metabolomic associations with self-reported smoking status and cotinine concentration were minor, the above individual correlations indicate that saliva metabolomics are useful to find potential biomarkers that may indicate altered physiology related to tobacco smoke exposure4,33,64.
Salivary metal concentrations were slightly associated with altered child metabolomes, suggesting that metal consumption/exposure potentially affects metabolism34,94,95,96,97. As the metals cooccurred in the samples, we analyzed them simultaneously, and show that there are likely synergistic associations with the metabolome, which shifts the individuals’ overall metabolism96. When specifically considering zinc, copper, and manganese, metals concentration were often anticorrelated with free dipeptides—potential markers for proteolysis and oxidative stress—suggesting that these metals may act as antioxidants reducing the body’s need to produce its own dipeptide antioxidants83,84. Conversely, there were positive associations between these metals and free amino acids, which may indicate a shift toward complete protein degradation or proteosome activation66,97. Taken together, these results suggest that metals are involved in protein metabolism or that metals may be acting as or along with antioxidants, but more research into oral metabolomics is needed, and we recognize that our results are correlative. Still, our work suggests that metals exposure and the metabolome interact, and more studies should incorporate these types of analyses to better understand human metabolism.
Conclusions
Metabolomics allows for the simultaneous detection of thousands of chemicals and can provide insights into the complex biochemical activity of human metabolism. Our study suggests that there are both large-scale patterns and subtle differences in the salivary metabolome between populations and within families, and that cohabitation likely affects metabolism. Taken together, metabolomics and kit-based biomeasure analyses indicate that tobacco smoke affects primary users’ metabolism, and that there are several putative biomeasures for antioxidant potential, tobacco smoke ETS exposure, and systemic inflammation along with metals concentrations, that can be studied for further use in understanding the biochemistry of environmental exposures and stress. As our study is mainly discovery-based, we suggest that future research investigate the interactions between the oral microbiome and metabolome in a more targeted way, and that “multi-omic” approaches be applied to family-based or large-populational studies to understand the complex microbes and molecules that underly human health.
Data availability
Tabulated mass-spectrometry metabolomics data are freely available on Dryad (https://doi.org/10.5061/dryad.66t1g1k88)49.
References
Zaura, E. et al. On the ecosystemic network of saliva in healthy young adults. ISME J 11, 1218–1231 (2017).
Bar, N. et al. A reference map of potential determinants for the human serum metabolome. Nature https://doi.org/10.1038/s41586-020-2896-2 (2020).
Dame, Z. T. et al. The human saliva metabolome. Metabolomics 11, 1864–1883 (2015).
Dunn, W. B. et al. Molecular phenotyping of a UK population: defining the human serum metabolome. Metabolomics 11, 9–26 (2015).
De Filippis, F. et al. The same microbiota and a potentially discriminant metabolome in the saliva of omnivore, ovo-lacto-vegetarian and vegan individuals. PLoS ONE 9, e112373 (2014).
Nguyen, T. et al. Host-microbe interactions: profiles in the transcriptome, the proteome, and the metabolome. Periodontol. 2000 82, 115–128 (2020).
Bertram, H. C., Eggers, N. & Eller, N. Potential of human saliva for nuclear magnetic resonance-based metabolomics and for health-related biomarker identification. Anal. Chem. 81, 9188–9193 (2009).
Gardner, A., Carpenter, G. & So, P.-W. Salivary metabolomics: from diagnostic biomarker discovery to investigating biological function. Metabolites 10, 2 (2020).
Su, M.-W. et al. Blood multiomics reveal insights into population clusters with low prevalence of diabetes, dyslipidemia and hypertension. PLoS ONE 15, e0229922 (2020).
Granger, D. A. & Taylor, M. K. Salivary Bioscience: Foundations of Interdisciplinary Saliva Research and Applications (Springer International Publishing, 2020).
Maughan, H. & Whiteson, K. in Salivary Bioscience: Foundations of Interdisciplinary Saliva Research and Applications (eds. Granger, D. A. & Taylor, M. K.) Ch. 7 (Springer International Publishing, 2020).
Riis, J. L. et al. The validity, stability, and utility of measuring uric acid in saliva. Biomark. Med. 12, 583–596 (2018).
Riis, J. L. et al. Adiponectin: Serum-saliva associations and relations with oral and systemic markers of inflammation. Peptides 91, 58–64 (2017).
Gatzke-Kopp, L. M. et al. Environmental tobacco smoke exposure is associated with increased levels of metals in children’s saliva. J. Expo. Sci. Environ. Epidemiol. https://doi.org/10.1038/s41370-023-00554-w. (2023)
Tang, Z.-Z. et al. Multi-omic analysis of the microbiome and metabolome in healthy subjects reveals microbiome-dependent relationships between diet and metabolites. Front. Genet. 10, 454 (2019).
Benowitz, N. L. Biomarkers of environmental tobacco smoke exposure. Environ. Health Perspect. 107, 349–355 (1999).
Becker, B. F. Towards the physiological function of uric acid. Free Radic. Biol. Med. 14, 615–631 (1993).
Battino, M., Ferreiro, M. S., Gallardo, I., Newman, H. N. & Bullon, P. The antioxidant capacity of saliva: The antioxidant capacity of saliva. J. Clin. Periodontol. 29, 189–194 (2002).
Paraskevas, S., Huizinga, J. D. & Loos, B. G. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J. Clin. Periodontol. 35, 277–290 (2008).
Rothman, J. A. et al. Oral microbial communities in children, caregivers, and associations with salivary biomeasures and environmental tobacco smoke exposure. mSystems 8, e0003623 (2023).
Lira-Junior, R., Åkerman, S., Klinge, B., Boström, E. A. & Gustafsson, A. Salivary microbial profiles in relation to age, periodontal, and systemic diseases. PLoS ONE 13, e0189374 (2018).
Cephas, K. D. et al. Comparative analysis of salivary bacterial microbiome diversity in edentulous infants and their mothers or primary care givers using pyrosequencing. PLoS ONE 6, e23503 (2011).
Burcham, Z. M. et al. Patterns of oral microbiota diversity in adults and children: a crowdsourced population study. Sci. Rep. 10, 2133 (2020).
Foxman, B. et al. The effects of family, dentition, and dental caries on the salivary microbiome. Ann. Epidemiol. 26, 348–354 (2016).
Andraos, S. et al. Population epidemiology and concordance for plasma amino acids and precursors in 11-12-year-old children and their parents. Sci. Rep. 11, 3619 (2021).
Andraos, S. et al. Plasma trimethylamine N-oxide (TMAO) and its precursors: population epidemiology, parent-child concordance, and associations with reported dietary intake in 11-12-year-old children and their parents. Curr. Dev. Nutr. https://doi.org/10.1093/cdn/nzaa103. (2020)
Andraos, S. et al. Plasma B vitamers: population epidemiology and parent-child concordance in children and adults. Nutrients 13, 821 (2021).
Voerman, E. et al. A population-based resource for intergenerational metabolomics analyses in pregnant women and their children: the Generation R Study. Metabolomics 16, 43 (2020).
Ellul, S. et al. Metabolomics: population epidemiology and concordance in Australian children aged 11-12 years and their parents. BMJ Open 9, 106–117 (2019).
Foxman, B. et al. Exploring the effect of dentition, dental decay and familiality on oral health using metabolomics. Infect. Genet. Evol. 22, 201–207 (2014).
Peisl, B. Y. L., Schymanski, E. L. & Wilmes, P. Dark matter in host-microbiome metabolomics: Tackling the unknowns-A review. Anal. Chim. Acta 1037, 13–27 (2018).
Gal, J. et al. Comparison of unsupervised machine-learning methods to identify metabolomic signatures in patients with localized breast cancer. Comput. Struct. Biotechnol. J. 18, 1509–1524 (2020).
Gu, F. et al. Cigarette smoking behaviour and blood metabolomics. Int. J. Epidemiol. 45, 1421–1432 (2016).
Papaioannou, N. et al. Multi-omics analysis reveals that co-exposure to phthalates and metals disturbs urea cycle and choline metabolism. Environ. Res. 192, 110041 (2021).
Jones, D. H., Yu, X., Guo, Q., Duan, X. & Jia, C. Racial disparities in the heavy metal contamination of urban soil in the southeastern United States. Int. J. Environ. Res. Public Health 19, 1105 (2022).
Masri, S. et al. Risk assessment of soil heavy metal contamination at the census tract level in the city of Santa Ana, CA: implications for health and environmental justice. Environ. Sci. Process. Impacts 23, 812–830 (2021).
Bonvallot, N. et al. Metabolomics as a powerful tool to decipher the biological effects of environmental contaminants in humans. Curr. Opin. Toxicol. 8, 48–56 (2018).
Vernon-Feagans, L., Cox, M. & Key, F. L. F. The family life project: an epidemiological and developmental study of young children living in poor rural communities. Monogr. Soc. Res. Child Dev. 78, 1–150 (2013).
Guilarte, T. R. Manganese neurotoxicity: new perspectives from behavioral, neuroimaging, and neuropathological studies in humans and non-human primates. Front. Aging Neurosci. 5, 23 (2013).
Rechtman, E. et al. Sex-specific associations between co-exposure to multiple metals and visuospatial learning in early adolescence. Transl. Psychiatry 10, 358 (2020).
Chasapis, C. T., Ntoupa, P.-S. A., Spiliopoulou, C. A. & Stefanidou, M. E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 94, 1443–1460 (2020).
Wei, S. et al. Metabolomics as a valid analytical technique in environmental exposure research: application and progress. Metabolomics 18, 35 (2022).
Sun, J. et al. A review of environmental metabolism disrupting chemicals and effect biomarkers associating disease risks: where exposomics meets metabolomics. Environ. Int. 158, 106941 (2022).
Bessonneau, V., Pawliszyn, J. & Rappaport, S. M. The saliva exposome for monitoring of individuals’ health trajectories. Environ. Health Perspect. 125, 077014 (2017).
Cajka, T. & Fiehn, O. Toward merging untargeted and targeted methods in mass spectrometry-based metabolomics and lipidomics. Anal. Chem. https://doi.org/10.1021/acs.analchem.5b04491 (2016).
Kind, T. et al. Identification of small molecules using accurate mass MS/MS search. Mass Spectrom. Rev. https://doi.org/10.1002/mas.21535 (2018).
Kind, T. et al. FiehnLib: mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. https://doi.org/10.1021/ac9019522 (2009).
Fan, S. et al. Systematic error removal using random forest for normalizing large-scale untargeted lipidomics data. Anal. Chem. 91, 3590–3596 (2019).
Rothman, J. et al. Data for: The salivary metabolome of children and parental caregivers in a large-scale family environment study. Dryad dataset https://doi.org/10.5061/dryad.66t1g1k88 (2024).
Djoumbou Feunang, Y. et al. ClassyFire: automated chemical classification with a comprehensive, computable taxonomy. J. Cheminform. 8, 61 (2016).
R Core Team. R: a language and environment for statistical computing. (R Foundation for Statistical Computing, 2016).
Dunham, S. J. B. et al. Sex-specific associations between AD genotype and the microbiome of human amyloid beta knock-in (hAβ-KI) mice. Alzheimers Dement. https://doi.org/10.1002/alz.13794 (2024).
Passos Mansoldo, F. R., Garrett, R., da Silva Cardoso, V., Alves, M. A. & Vermelho, A. B. Metabology: analysis of metabolomics data using community ecology tools. Anal. Chim. Acta 1232, 340469 (2022).
Dunham, S. J. B. et al. Longitudinal analysis of the microbiome and metabolome in the 5xfAD mouse model of Alzheimer’s Disease. MBio 13, e0179422 (2022).
Wickham, H. Ggplot2: Elegant Graphics for Data Analysis (Springer-Verlag, 2009).
Pedersen, T. L. Patchwork: the composer of plots. R package version 1, 182 (2020).
Oksanen, J. et al. Vegan: community ecology package. (2017).
Kuznetsova, A., Brockhoff, P. B. & Christensen, R. H. B. lmerTest package: tests in linear mixed effects models. J. Stat. Softw. 82, 1–26 (2017).
Harrell, F. E. Hmisc: Harrell Miscellaneous. (2019).
Willis, J. R. et al. Citizen science charts two major “stomatotypes” in the oral microbiome of adolescents and reveals links with habits and drinking water composition. Microbiome 6, 218 (2018).
Arumugam, M. et al. Enterotypes of the human gut microbiome. Nature 473, 174–180 (2011).
Dray, S. & Dufour, A. B. The ade4 package: implementing the duality diagram for ecologists. J. Stat. Softw 22, 1–20 (2007).
Maechler, M., Rousseeuw, P., Struyf, A., Hubert, M. & Hornik, K. Cluster: cluster analysis basics and extensions. (2019).
Hsu, P.-C. et al. Metabolomic profiles of current cigarette smokers. Mol. Carcinog. 56, 594–606 (2017).
Nayak, A. et al. N8-Acetylspermidine: a polyamine biomarker in ischemic cardiomyopathy with reduced ejection fraction. J. Am. Heart Assoc. 9, e016055 (2020).
Tamás, M. J., Sharma, S. K., Ibstedt, S., Jacobson, T. & Christen, P. Heavy metals and metalloids as a cause for protein misfolding and aggregation. Biomolecules 4, 252–267 (2014).
Saben, J. L., Sims, C. R., Piccolo, B. D. & Andres, A. Maternal adiposity alters the human milk metabolome: associations between nonglucose monosaccharides and infant adiposity. Am. J. Clin. Nutr. 112, 1228–1239 (2020).
Konieczna, L. et al. Analytical approach to determining human biogenic amines and their metabolites using eVol microextraction in packed syringe coupled to liquid chromatography mass spectrometry method with hydrophilic interaction chromatography column. Talanta 150, 331–339 (2016).
Chamberlain, C. A., Rubio, V. Y. & Garrett, T. J. Impact of matrix effects and ionization efficiency in non-quantitative untargeted metabolomics. Metabolomics 15, 135 (2019).
Haslam, D. E. et al. Associations of network-derived metabolite clusters with prevalent type 2 diabetes among adults of Puerto Rican descent. BMJ Open Diabetes Res. Care 9, e002298 (2021).
Goudo, M., Sugimoto, M., Hiwa, S. & Hiroyasu, T. The usefulness of sparse k-means in metabolomics data: an example from breast cancer data. Preprint at bioRxiv https://doi.org/10.1101/2022.02.05.479235 (2022)
Kim, J. O. et al. Data-driven identification of plasma metabolite clusters and metabolites of interest for potential detection of early-stage non-small cell lung cancer cases versus cancer-free controls. Cancer Metab. 10, 16 (2022).
Mehanna, M. et al. Influence of genetic West African ancestry on metabolomics among hypertensive patients. Metabolites 12, 783 (2022).
Li, T. et al. Longitudinal metabolomics reveals ornithine cycle dysregulation correlates with inflammation and coagulation in COVID-19 severe patients. Front. Microbiol. 12, 723818 (2021).
Burrage, L. C. et al. Untargeted metabolomic profiling reveals multiple pathway perturbations and new clinical biomarkers in urea cycle disorders. Genet. Med. 21, 1977–1986 (2019).
Kisuse, J. et al. Urban diets linked to gut microbiome and metabolome alterations in children: a comparative cross-sectional study in thailand. Front. Microbiol. 9, 1345 (2018).
Rasmussen, L. G. et al. Standardization of factors that influence human urine metabolomics. Metabolomics 7, 71–83 (2011).
Pedley, A. M. & Benkovic, S. J. A new view into the regulation of purine metabolism: the purinosome. Trends Biochem. Sci. 42, 141–154 (2017).
Khodorova, N. V. et al. Urinary medium-chained acyl-carnitines sign high caloric intake whereas short-chained acyl-carnitines sign high -protein diet within a high-fat, hypercaloric diet in a randomized crossover design dietary trial. Nutrients 13, 1191 (2021).
Nishida, Y. Relation between creatinine and uric acid excretion. Ann. Rheum. Dis. 51, 101–102 (1992).
Barros, M. P. et al. Effects of acute creatine supplementation on iron homeostasis and uric acid-based antioxidant capacity of plasma after wingate test. J. Int. Soc. Sports Nutr. 9, 25 (2012).
Zyśk, B., Ostrowska, L. & Smarkusz-Zarzecka, J. Salivary adipokine and cytokine levels as potential markers for the development of obesity and metabolic disorders. Int. J. Mol. Sci. 22, 11703 (2021).
Thirumalaikumar, V. P., Wagner, M., Balazadeh, S. & Skirycz, A. Autophagy is responsible for the accumulation of proteogenic dipeptides in response to heat stress in Arabidopsis thaliana. FEBS J 288, 281–292 (2021).
Elias, R. J., Kellerby, S. S. & Decker, E. A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 48, 430–441 (2008).
Luan, Y.-Y. & Yao, Y.-M. The clinical significance and potential role of c-reactive protein in chronic inflammatory and neurodegenerative diseases. Front. Immunol. 9, 1302 (2018).
Summers, K. L. & Giacobini, E. Effects of local and repeated systemic administration of (-)nicotine on extracellular levels of acetylcholine, norepinephrine, dopamine, and serotonin in rat cortex. Neurochem. Res. 20, 753–759 (1995).
Lockman, P. R. et al. Nicotine exposure does not alter plasma to brain choline transfer. Neurochem. Res. 31, 503–508 (2006).
Evinger, M. J., Ernsberger, P., Regunathan, S., Joh, T. H. & Reis, D. J. A single transmitter regulates gene expression through two separate mechanisms: cholinergic regulation of phenylethanolamine N-methyltransferase mRNA via nicotinic and muscarinic pathways. J. Neurosci. 14, 2106–2116 (1994).
Reznick, A. Z. et al. Modification of plasma proteins by cigarette smoke as measured by protein carbonyl formation. Biochem. J. 286, 607–611 (1992).
Anbarasi, K., Vani, G., Balakrishna, K. & Devi, C. S. S. Creatine kinase isoenzyme patterns upon chronic exposure to cigarette smoke: protective effect of Bacoside A. Vascul. Pharmacol. 42, 57–61 (2005).
Zhu, L. et al. Spermine on endothelial extracellular vesicles mediates smoking-induced pulmonary hypertension partially through calcium-sensing receptor. Arterioscler. Thromb. Vasc. Biol. 39, 482–495 (2019).
Wang, C. et al. Nicotine accelerates atherosclerosis in apolipoprotein e-deficient mice by activating α7 nicotinic acetylcholine receptor on mast cells. Arterioscler. Thromb. Vasc. Biol. 37, 53–65 (2017).
Pesci, A. et al. Mast cells in the airway lumen and bronchial mucosa of patients with chronic bronchitis. Am. J. Respir. Crit. Care Med. 149, 1311–1316 (1994).
Feng, P. et al. Human supplementation with Pediococcus acidilactici GR-1 decreases heavy metals levels through modifying the gut microbiota and metabolome. NPJ Biofilms Microbiomes 8, 63 (2022).
Booth, S. C., Workentine, M. L., Weljie, A. M. & Turner, R. J. Metabolomics and its application to studying metal toxicity. Metallomics 3, 1142–1152 (2011).
Deng, P. et al. Application of metabolomics to characterize environmental pollutant toxicity and disease risks. Rev. Environ. Health 34, 251–259 (2019).
Fu, Z. & Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 30, 167–176 (2020).
Acknowledgements
This study is part of the Family Life Project (FLP). We thank Kaitlin Smith and Tatum Stauffer for technical assistance with salivary biospecimen testing and the Family Life Project (FLP) Investigators. We would like to express our gratitude to the families, children, and teachers who participated in this research and to the FLP research assistants for their hard work and dedication.
Author information
Authors and Affiliations
Contributions
J.A.R.: Validation, formal analysis, investigation, resources, data curation, writing—original draft, writing— review and editing, visualization, funding acquisition; H.L.P.: Formal analysis, investigation, resources, data curation, writing—original draft, writing—review and editing; S.J.B.D.: Validation, methodology, writing— review and editing, funding acquisition; J.L.R.: Conceptualization, formal analysis, investigation, resources, data curation, writing—original draft, writing—review and editing, supervision, project administration, funding acquisition; D.A.G.: Conceptualization, resources, writing—original draft, writing—review and editing, supervision, project administration, funding acquisition; E.A.T.: Conceptualization, investigation, resources, data curation, writing—original draft, writing—review and editing, supervision, project administration, funding acquisition; K.L.W.: Conceptualization, resources, writing—original draft, writing— review and editing, supervision, project administration, funding acquisition. All authors have read and approved the publication of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
In the interest of full disclosure, DAG is the founder and Chief Scientific and Strategy Advisor at Salimetrics LLC and Salivabio LLC. These relationships are managed by the policies of the committees on conflict of interest at the Johns Hopkins University School of Medicine and the University of California, Irvine. No other authors have conflicts to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplemental_information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rothman, J.A., Piccerillo, H.L., Dunham, S.J.B. et al. The salivary metabolome of children and parental caregivers in a large-scale family environment study. npj Metab Health Dis 2, 22 (2024). https://doi.org/10.1038/s44324-024-00024-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44324-024-00024-3