Abstract
Aging exerts profound and paradoxical effects on the immune system, at once impairing proliferation, cytotoxicity and phagocytosis, and inducing chronic inflammation. Previous studies have focused on individual tissues or cell types, while a comprehensive multisystem study of tissue-resident and circulating immune populations during aging is lacking. Here we reveal an atlas of age-related changes in the abundance and phenotype of immune cell populations across 12 mouse tissues. Using cytometry-based high parametric analysis of 37 mass-cytometry and 55 spectral flow-cytometry parameters, mapping samples from young and aged animals revealed conserved and tissue-type-specific patterns of both immune atrophy and expansion. We uncovered clear phenotypic changes in both lymphoid and myeloid lineages in aged mice, and in particular a contraction in natural killer cells and plasmacytoid dendritic cells. These changes correlated with a skewing towards myelopoiesis at the expense of early lymphocyte genesis in aged mice. Taken together, this atlas represents a comprehensive, systematic and thorough resource of the age-dependent alterations of the mammalian immune system in lymphoid, barrier and solid tissues.
Similar content being viewed by others
Main
The continuously increasing life expectancy of the global population is associated with a growing number of morbid years in older adults. This aged population is more prone to infections and cancer, and alterations to the aging immune system are held to be the underlying mechanism. Age-related changes within the immune system can be broken down into two main processes: first, a gradual decline in the function of all major cellular leukocyte subsets, termed ‘immunosenescence’1; and second, the emergence of chronic low-level inflammation—so-called inflammaging2—across various tissues3,4. Together, these age-related processes are thought to be risk factors for infectious diseases, increased mortality from seasonal viruses5, cancer6, cardiovascular diseases7 and chronic inflammatory disorders8. In addition, due to impaired immunological memory in the older adults, low response to vaccination has become a substantial population-wide issue9. Consequently, it is important to decipher the changes to the immune system with age to understand the role of such alterations in increased morbidity, and how they might be ameliorated in the future.
Previous research has primarily focused on describing the age-related changes to adaptive immunity10 and the consequences of aging lymphocytes on mounting an immune response to bacterial11 or viral12 infections. Several studies have characterized the populations of lymphoid cells in the blood of aging humans13,14, but it is unknown how circulating levels of immune cell populations compare to those in tissues, which are profoundly important for overall health. Mouse studies have documented a decreased number of classic T cells during aging15,16, but again a comprehensive assessment of lymphoid populations and their relative abundance across various tissues is missing. Similarly for myeloid cells, reports of the phenotypic and functional changes across monocytes, macrophages and polymorphonucleated phagocytes have been contradictory17 or are focused on an exemplary tissue or organ4,18. There is thus the clear need to map systematically the age-related changes within different myeloid subsets, particularly under physiological conditions and across various tissues. Phagocytes are highly auto-fluorescent, a feature that greatly interferes with classic polychromatic-based cytometry analysis. Furthermore, because numerous canonical myeloid cell markers are shared across most lineages, to identify all myeloid lineages reliably within a range of organs, an exhaustive combination of cell surface markers is required.
Here we present a comprehensive single-cell immunophenotyping atlas of the immune cell populations in 12 different tissues from young and aged C57/BL6 inbred mice. To overcome the technical issues described above, we used mass cytometry, also termed cytometry by time-of-flight (CyTOF), to characterize the myeloid cell subpopulations using a single comprehensive panel and found both tissue-specific and conserved cross-tissue differences between young adult and aged mice. We subsequently went on to confirm our findings with two validation cohorts and a different, independent technique—namely spectral flow cytometry—to establish the robustness of our initial conclusions (two independent cohorts of n = 10 young and n = 10 aged mice) and further interrogate the lymphoid and hematopoietic stem cell (HSC) compartments with high resolution. By comparing tissues classified as internal organs, barrier and lymphoid tissues, we observed patterns of age-related alterations across tissue-resident innate immune cell populations, with implications for the capacity of tissues to regenerate and combat pathogenic threats. These data (https://zenodo.org/deposit/5593273) provide a valuable resource into the changes of leukocyte composition, phenotype and function in mammalian tissues during aging.
Results
Generating an immune atlas from young and aged mouse tissues
First, we sought to design a mass-cytometry panel and experimental protocol to identify all major innate myeloid lineages across 12 tissues: lung, liver, visceral adipose tissue (VAT), brown adipose tissue (BAT), small intestine, thymus, testes, mesenteric lymph nodes (MesLNs), skin-draining lymph nodes (sdLNs), spleen, blood and bone marrow (BM). For our initial analysis cohort, we devised four experimental groups (n = 4 young adult male, n = 4 young adult female; n = 4 aged female; and n = 3 aged male C57/BL6 mice) to allow immune comparisons between age as well as sex. We devised similar group sizes for our subsequent validation cohorts, as described in Extended Data Fig. 1 and the Methods section.
Male and female mice aged around 7–8 weeks (‘young’), 16 weeks (‘middle’) or 18–20 months (‘aged’) were acquired from the same vendor. Our mice showed no physical signs of illness or macroscopic signs of cancerous growths at the time of analysis. Both our initial and validation cohorts of mice have been housed under identical conditions from birth until purchase by the vendor. This was done to avoid facility-specific and induced differences in the readouts, such as alterations to the microbiome of the mice. Furthermore, we aimed to increase the standards of our study’s reproducibility by acquiring a mouse cohort which would be available to other researchers for purchase and confirmation experiments.
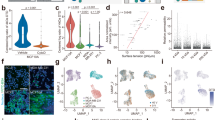
We first analyzed the cells of the immune compartment using mass cytometry (Fig. 1a). Using unsupervised representation learning techniques and meta-clustering based on self-organizing maps19,20, we identified all canonical immune lineages in tissues from both young and old mice (Fig. 1a).
a, Schematic diagram of experimental workflow. Briefly, two cohorts of young adult and aged mice of both sexes were culled using an overdose of pentobarbital. Organs were harvested, and the tissues were digested to extract tissue-resident leukocytes. Following antibody labeling, marker expression data were acquired using a mass cytometer or spectral flow cytometer. The data were further analyzed using high-dimensional analysis tools incorporating machine-learning algorithms. b, Modified scaffold map of the total CD45pos compartment of exemplary mass-cytometry data (cells pooled from n = 1 liver, n = 1 lung and n = 1 spleen). Corresponding heatmap depicts median marker expression within each FlowSOM-derived cluster. c, Modified scaffold map of the total myeloid compartment of exemplary mass-cytometry data, after gating out the lymphoid cells presented in b (cells pooled from n = 1 liver, n = 1 lung and n = 1 spleen). Corresponding heatmap depicts median marker expression of FlowSOM-derived clusters. Also see details in Extended Data Fig. 1.
To gather an overview of the immune populations present in our samples, mass-cytometry data were visualized using uniform manifold approximation and projection (UMAP)-dimensionality reduction21 on the total concatenated leukocyte fraction from all mice and within each tissue group. The total number of cells depicted per UMAP visualization was normalized per experimental group. Cells were then further grouped using FlowSOM, a self-organizing map that clusters cells (here depicted in different colors) depending on their overall similarities in marker expression19. All live/CD45+ or myeloid cells per sample were included for the FlowSOM analysis in order better to train the algorithm and improve cluster identification. We identified the major T cell lineages (CD3posCD90pos cells), further identified by CD4pos (T helper cells), CD8pos (cytotoxic T cells), or non-αβ T cells (CD3posCD4lowCD8low cells such as γδ T cells among other unconventional T cell subsets), B cells (B220highMHCIIhighSiglec-Hneg), natural killer (NK) cells (NK1.1high) and Innate Lymphoid Cells (ILCs) (CD90posCD3negNK1.1low) (Fig. 1b).
As lymphoid cells are many times more abundant in most tissues compared to myeloid cells, we then gated out the lymphocytes subsets to enable the identification of all myeloid lineages, including smaller populations such as tissue-resident dendritic cell (DC) subsets. We further quantified the phagocytic populations within all the analyzed organs as a percentage of the total myeloid compartment, as opposed to the total CD45pos fraction. As visualized in the representative heatmap (Fig. 1c), using additional markers22,23,24, we were able to discern the commonly present myeloid populations across most tissues. In the majority of tissues we defined classic monocytes (Ly6ChighCCR2high); non-classic monocytes (expressing Ly6Cint/lowCX3CR1highPD-L1high in combination with CD88highF4/80high); monocyte-derived cells (Ly6ChighMHCIIhigh); DC subsets (CD11cposMHCIIpos cells, with XCR1highCD24high representing conventional dendritic cells 1 (cDC1s), and CD11bhighCD172ahigh cDC2s, B220posSiglec-Hpos plasmacytoid dendritic cells (pDCs)); various tissue macrophage subsets (expressing CD11b with a combination of markers such as F4/80high, MerTKhigh, CD64high, among other tissue-specific markers); as well as granulocytes such as neutrophils (Ly6Gpos); eosinophils (Siglec-Fpos); and mast cells (FcεR1apos) (Fig. 1c). While it has recently been shown that the majority of pDCs arise from lymphoid progeny25, we have here analyzed these cells alongside their myeloid counterparts due to their main tissue functions (namely antigen expression) matching their myeloid kin; their effector role in bridging the two arms of the immune response; and to increase the resolution on this small population within tissues. By identifying all major myeloid and lymphoid subsets using a single comprehensive panel applicable to all tissues, we demonstrated the robustness and specificity of the mass-cytometry panel designed in this study.
To create a comprehensive immune-phenotyping map as a resource to compare cellular changes between young and aged mice, we aimed to measure age-related alterations in the relative abundance of tissue leukocytes across representative lymphoid and non-lymphoid murine tissues. In addition, we assessed the frequency changes and their relative abundance between sexes in aging. A summary of those features is available for download (https://zenodo.org/deposit/5593273). Furthermore, while the immunophenotyping has been carried out for the full list of 12 organs listed previously, to depict the scope of our aging immune atlas, we here go on to present exemplary murine tissues with noteworthy age-related changes in cellular composition and phenotypic variances, while introducing our analysis pipeline as well as the different classes of murine tissues we have analyzed. To highlight the general agreement between male and female age-related leukocyte frequency changes, we applied multidimensional scaling to the total leukocytes, in which the distances, or relative similarities, between experimental groups were calculated by pooling the frequencies of all leukocyte populations as per age and sex group26. We then visualized the mean antigen expressions across leukocytes using a heatmap with unsupervised hierarchical clustering27 (Extended Data Fig. 2) and found that peripheral tissues have very few sex dimorphisms. However, in the BM we observed a neutrophil subsets-driven sex dimorphism particularly in young mice (Extended Data Fig. 2c)28. In this case, we present the lung, sdLN and VAT as representative barrier, lymphoid and specialized connective tissues.
Aged lung exhibits pan-reduction of tissue-resident cells
The lung represents one of the best studied murine tissues with regards to the myeloid compartment29, both in development30 and aging31. Thus, the lung represents a canonical barrier tissue affected by age-related functional decline.
Our initial characterization of the lung lymphoid populations (Fig. 2a,b) showed a significant (P = 0.006) mean reduction in T helper cell frequency in lungs from aged mice (approximately 50% decline relative to lungs from young mice). This age-driven phenotypic change has been reported previously32. Similarly, we noted a significant drop in NK cell frequency with age (more than halving from an average of 16.5% in the young adult group to 7.1% in the aged mice, P = 0.006) (Fig. 2b). In turn, unconventional T cells (for example γδT and NKT cells) were twice as abundant in lungs from old versus young mice (P = 0.01). Taken together, we observed an overall reduction in canonical lymphocytes from the aging lung, compensated by an increase in quasi-innate lymphocytes during aging, that may then occupy the cellular niche of the departed αβ T cells.
a, UMAP with FlowSOM overlay showing total CD45 compartment of young adult and aged lungs. b, Violin plots (n = 8 young, n = 7 aged) depicting frequencies of FlowSOM-generated immune cell clusters shown in a. Statistical analysis was carried out using nonparametric Mann–Whitney–Wilcoxon tests. The P value was then adjusted for multiple comparisons using a BH test. P values of less than 0.05 were considered significant and are indicated by the numerical value on the respective graphs. c, UMAP with FlowSOM overlay showing total myeloid compartment (as shown in a, dark blue cluster) of young adult and aged lungs. d, Violin plots (n = 8 young, n = 7 aged) depicting frequencies of FlowSOM-generated immune cell clusters shown in c. All depicted P values are significant (<0.05) with a BH false discovery rate of less than 5%. e, Dot plots showing the gating strategy of alveolar macrophages (left panel). Histogram and subsequent heatmap depicting differential median marker expression in alveolar macrophages of young adult and aged lungs (two right-most panels). Statistical analysis was carried out using nonparametric Mann–Whitney–Wilcoxon tests. The P value was then adjusted for multiple comparisons using a BH test. P values of less than 0.05 were considered significant and are indicated by the numerical value on the respective graphs. f, Violin plots (n = 10 young, n = 10 aged) depicting frequencies of immune cell clusters acquired through the validation FACS cohort. Statistical analysis was carried out using nonparametric Mann–Whitney–Wilcoxon tests. The P value was then adjusted for multiple comparisons using a BH test. P values of less than 0.05 were considered significant and are indicated by the numerical value on the respective graphs. Also see details in Extended Data Fig. 2.
Alongside, we observed an overall increase in the frequency of total myeloid cells in the leukocyte fraction of lungs from aged mice (from 26.6% in young, to 41.6%, P = 0.01) (Fig. 2c,d). When we analyzed the composition of the myeloid compartment in the murine lung, we observed a significant decrease in frequency of cellular subsets associated with phagocytosis and debris clearance, as well as antigen processing and presentation, in aged compared to young mice: alveolar macrophages (termed AM, identified as CD11blow CD11chigh Siglec-Fhigh) dropped from 8.0% to 1.6% (P < 0.001) and eosinophils declined from 3.8% to 1.1% (P = 0.013); while cDC1 dropped by almost half in the aged lung (P = 0.004). Furthermore, we also observed a significant drop of the frequency of pDCs (P = 0.0001)—another common feature shared across a plethora of tissues (Fig. 2d).
Furthermore, our analysis also highlighted interesting phenotypic changes of these subpopulations: in particular, we saw a shift of AM within the dimensionality reduction representation (red cluster in Fig. 2c), that is mainly driven by an increased MHC-II expression on AMs in the aged lung (Fig. 2e). This phenotype suggests an increased MHC-II-mediated communication between T cells and tissue-resident macrophages, which occurs especially in inflammatory disease settings33,34. This may be also a result of interferon gamma (IFN-γ), a main driver of MHC-II expression35, usually presented by adaptive T cells in the lung microenvironment36. Our validation cohort acquired using spectral flow cytometry reflects the same age-related frequency changes within the myeloid fraction of the lung (Fig. 2f).
In summary, the aging lung reveals a decline in several immune compartments. Most notably, we identified a reduction in T helper cell as well as NK cell frequencies, which was accompanied by an increase in the total myeloid cell fraction. A general trend in loss of specialized tissue-resident macrophages37 as well as DCs38 was previously reported.
Aging induces organ-specific immune adaptations
To highlight the robustness of our analysis, we present the immune compartments of a further representative secondary lymphoid tissue (SLT) (sdLN) and soft connective tissue (VAT). In the former, we found a relative reduction in the NK (P = 0.05) and T helper cell (P = 0.003) fractions in aged compared to young mice (Fig. 3a,b). Simultaneously, and likely as a result of an empty cellular niche, we observed a relative increase in B cells (P = 0.06) within the sdLNs (Fig. 3b), thus confirming previous findings39. In the sdLNs we observed relative stability in total myeloid abundance (Fig. 3c), despite the reduced CD4 T cell and innate lymphoid cell numbers (Fig. 3d). Within the myeloid compartment of the LNs, we once again observed a significant (P = 0.02) decline in the frequency of pDCs, as well as NK cells (P = 0.05). Taken together, whereas aging barrier tissues, such as the lung, showed a marked alteration within the tissue-resident and surveilling myeloid compartment, the overall abundance of total myeloid cells in the aging lymph nodes (LNs) remains relatively stable.
a, UMAP with FlowSOM overlay showing total CD45 compartment of young adult and aged sdLN. b, Violin plots (n = 8 young, n = 7 aged) depicting frequencies of FlowSOM-generated immune cell clusters shown in a. Statistical analysis was carried out using nonparametric Mann–Whitney–Wilcoxon tests. The P value was then adjusted for multiple comparisons using a BH test. P values of less than 0.05 were considered significant and are indicated by the numerical value on the respective graphs. c, UMAP with FlowSOM overlay showing total myeloid compartment (as shown in a, dark blue cluster) of young adult and aged SdLN. d, Violin plots (n = 8 young, n = 7 aged) depicting frequencies of FlowSOM-generated immune cell clusters shown in c. Statistical analysis was carried out using nonparametric Mann–Whitney–Wilcoxon tests. The P value was then adjusted for multiple comparisons using a BH test. P values of less than 0.05 were considered significant and are indicated by the numerical value on the respective graphs. e, UMAP with FlowSOM overlay showing total CD45 compartment of young adult and aged VAT. f, Violin plots (n = 8 young, n = 7 aged) depicting frequencies of FlowSOM-generated immune cell clusters shown in e. Statistical analysis was carried out using nonparametric Mann–Whitney–Wilcoxon tests. The P value was then adjusted for multiple comparisons using a BH test. P values of less than 0.05 were considered significant and are indicated by the numerical value on the respective graphs. g, UMAP with FlowSOM overlay showing total myeloid compartment (as shown in e, dark blue cluster) of young adult and aged VAT. h, Violin plots (n = 8 young, n = 7 aged) depicting frequencies of FlowSOM-generated immune cell clusters shown in g. Statistical analysis was carried out using nonparametric Mann–Whitney–Wilcoxon tests. The P value was then adjusted for multiple comparisons using a BH test. P values of less than 0.05 were considered significant and are indicated by the numerical value on the respective graphs. Also see details in Extended Data Fig. 3.
Initial dimensionality reduction visualization of the total CD45+ cells isolated within the VAT (Fig. 3e) demonstrated again a significant reduction of the NK cell compartment (P = 0.015) as well as the tissue-resident innate lymphoid cell fraction (P = 0.015) from aged compared to young mice (Fig. 3f). We next enumerated the frequencies of the various myeloid cells present in the VAT (Fig. 3g,h). Of note, we found that while the total myeloid fraction was significantly reduced in the aging mouse (P = 0.05), the dynamics of various myeloid populations were stable within the VAT. Among myeloid cells, we did observe the emergence of a population of tissue-resident adipose tissue macrophages (termed Ly6C adipose tissue macrophages, ATMs) in the aged mice, which has previously been associated with obesity40—consistent with increased susceptibility to obesity-related comorbidities in human aging41. While we have focused on the general age-related changes within these tissues for the purpose of this article, we have provided data acquired through the validation cohort (spectral flow cytometry) and sex-related differences within Extended Data Figs. 3 and 4.
Age-induced lymphoid contraction is shared across tissues
We have applied a similar analysis pipeline—as presented for our representative organs—for all the tissues encompassed within our aging immune atlas. We assessed the frequency changes and their relative abundance in all the tissues, between sexes in aging. A summary of those features is presented within Extended Data Fig 4. We present an overview of the relative abundance of each immune population within these tissues, showcasing the richness of this resource (Fig. 4 and Extended Data Fig. 5a). We calculated the relative fold change in abundance of each identified immune population. To allow for schematic comparisons of the overall trends in lymphocyte counts between the two age groups, we employed a correlogram-style display in which the relative fold change in each population size between young and aged mice was mapped using a bubble chart, with the size of each circle representing the size of the cellular fold change (Fig. 5). The heterogeneity of the aging leukocyte trends makes it difficult to cluster tissues into their respective groups (barrier versus SLTs, among others). However, we have observed conserved trends in our aging model, presented as volcano plots (Extended Data Fig. 5b), where P values can also be visualized. In the majority of assessed tissues, we observed a sustained and significant reduction in total CD4 T cell, NK cell and pDC compartments, and a general trend towards increased granulocytes in aged mice. Furthermore, while the loss of pDCs in the aging peripheral circulation is widely accepted42, we hereby demonstrate that this change is sustained across a wide range of aged tissues. Taken together, the loss of NK cells and pDCs in the aging lung may result in reduced and/or delayed viral clearance.
Bubble chart of fold change in immune cell populations in aged relative to young mice (data averaged from analysis of n = 8 young, n = 7 aged mice). For each leukocyte subset, FlowSOM-derived frequencies of the young adult mouse were normalized to 1, and the subsequent fold change in the aged mouse was used to calculate bubble radius. Also see details in Extended Data Fig. 5b.
Aging induces loss of CD103 in naive CD8 T cells
Mass-cytometry analysis of the aging mouse has allowed for the extensive dissection of the tissue-resident myeloid compartment with a resolution thus far not achieved using conventional flow-cytometry methods. To delineate further the lymphoid compartment, we acquired additional cohorts of age-matched mice using spectral flow cytometry, using a specifically designed antibody panel. To demonstrate the synergy between the two flow-cytometry experimental batches, the effect size (ES) was computed. This is a statistical measure whereby the median expression of each measured marker was calculated for each immune population (as listed in Extended Data Fig. 6a) per tissue and per mouse, as well as the statistical significance of this feature between the two groups. The resultant plot combines both the significance as well as the median change for each feature (Fig. 6a)43. With the majority of data points largely following the R = 1 correlation, we confirm that our two datasets generated through spectral flow cytometry are highly consistent. By introducing a cut-off value for the ES as described by Cohen44, we screened for hits that were significant by setting a threshold of 2.5 on both axes, to find phenotypic changes that are associated with aging, consistent between the batches and tissue-wide (Fig. 6a). Upon screening the hits, we noted a crisp, significant reduction in CD103 expression within the naive tissue-resident CD8 T cell compartment of the majority of tissues analyzed. The feature was common among SLTs and non-lymphoid tissues alike (Fig. 6b). In tissues where this change was not significant, we observed a clear trend (Extended Data Fig. 6b). The stability of the naive CD8 T cell frequency among all the tissues analyzed (Fig. 6c) suggests that this is a phenotypic, age-related adaptation.
a, Comparison of immune features derived from each leukocyte subpopulation between experimental groups. Dot plot displaying the ES calculated between young versus old mice in validation cohort batch 1 (n = 10 young, n = 10 aged) (x axis) compared with the ES calculated in the same in validation cohort batch 2 (n = 10 young, n = 10 aged) (y axis). Each dot represents one immunological feature; colors represent individual tissues. b, Violin plots depicting the median expression of CD103 in naive CD8 T cells of young and aged mice from both validation cohorts (n = 5 young, n = 5 aged from cohort 1; n = 5 young, n = 5 middle, n = 5 aged from cohort 2). All depicted changes from young/middle to aged are significant (<0.05) with a BH false discovery rate of less than 5%. c, Violin plots depicting the frequencies of naive CD8 T cells of young and aged mice from both validation cohorts (n = 10 young, n = 10 aged from cohort 1. For cohort 2, n = 5 young, n = 5 middle, n = 5 aged for liver, spleen, VAT, lung, blood and heart. For cohort 2, n = 10 young, n = 10 middle, n = 10 aged for colon, MesLN, sdLN and spleen). d, Violin plots depicting the frequencies of naive CD4 T cells of young and aged mice from both validation cohorts (n = 10 young, n = 10 aged from cohort 1. For cohort 2, n = 5 young, n = 5 middle, n = 5 aged for liver, spleen, VAT, lung, blood and heart. For cohort 2, n = 10 young, n = 10 middle, n = 10 aged for colon, MesLN, sdLN and spleen). Statistical analysis was carried out using nonparametric Mann–Whitney–Wilcoxon tests. The P value was then adjusted for multiple comparisons using a BH test. P values of less than 0.05 were considered significant and are indicated by the numerical value on the respective graphs. Also see details in Extended Data Fig. 6.
Our validation data show that the homogeneous drop in CD4 T cell frequencies is largely driven by a sustained loss of naive CD4 T cells in aged tissues, as opposed to CD4 cell memory subsets (Fig. 6d). Altogether these findings underline the numerical and functional impairment of the naive T cell compartment in aging.
Aged bone marrow exhibits early signs of myeloid bias
One potential mechanism that could underpin many of the changes described within our resource is an altered, age-driven hematopoiesis. The myeloid lineage-commitment bias in aging45,46 is associated with declining immunocompetence and increased autoimmunity observed in the aged mouse47. We sought to investigate whether the overall tissue-wide reduction in the lymphocyte compartment was reflected in the early stages of hematopoiesis in the bone marrow of young adult and aged mice. We interrogated the hematopoietic stem and progenitor cells in the BM via spectral flow cytometry—through a 24-color surface marker panel to identify 35 clusters encompassing all known hematopoietic progenitor subsets, while also interrogating the mature CD45pos cells within the BM (Fig. 7a). We classified the cells according to their marker expression: lymphoid cells, identified using a lineage channel (Linhigh); mononuclear phagocytes (Ly6GlowCD11bhigh and/or CD11chigh); pDCs (LinhighSiglec-Hhigh); preneutrophils (preNeu, CD11bhighCD117high); neutrophils (Ly6Ghigh); eosinophils (SSChigh); basophils (FcεRhigh); and progenitors (LinlowLy6GlowCD11blowCD117high) (see Extended Data Fig. 7a-c). Notably, we found a significant decrease in lymphoid cells, complemented by a significant increase in preNeu (Extended Data Fig. 7d,e), suggestive of an increase in neutrophil output. To assess the complex cocktail of HSC subsets and progenitor frequencies in the BM, we visualized the progenitor compartment using a force-directed layout48, overlaid with FlowSOM-derived clusters (Fig. 7b). This strategy allowed us to identify, on the basis of the median expression of all markers depicted in the heatmap (Fig. 7c), 19 diverse progenitor subsets including long-term hematopoietic stem cell (LT-HSC) and short-term HSC subsets; multipotent progenitors found in the so-called LSK fraction (defined by their c-kit and Sca-1 expression and by the absence of lineage marker expression); common lymphoid progenitors (CLPs); megakaryocyte and erythrocyte progenitors; common myeloid progenitors; granulocyte and monocyte progenitors (GMPs); monocyte and DC progenitors (Fig. 7c).
a, Modified scaffold map of the total CD45pos compartment of BM. b, Force-directed layout of combined young and aged progenitor clusters identified in part a. Colors depict FlowSOM-generated clusters. c, Heatmap depicting median marker expression in FlowSOM-derived progenitor subsets of the bone marrow. d, Violin plots (n = 10 young, n = 10 aged) depicting frequencies among total CD45pos cells of FlowSOM-generated immune cell clusters shown in c. Statistical analysis was carried out using nonparametric Mann–Whitney–Wilcoxon tests. The P value was then adjusted for multiple comparisons using a BH test. P values of less than 0.05 were considered significant and are indicated by the numerical value on the respective graphs. e, Violin plots (n = 10 young, n = 10 aged) depicting frequencies derived from manual gating of CLPs and downstream precursor subsets of the BM, subdivided on the basis of Siglec-H and Ly6D expression. Statistical analysis was carried out using nonparametric Mann–Whitney–Wilcoxon tests. The P value was then adjusted for multiple comparisons using a BH test. P values of less than 0.05 were considered significant and are indicated by the numerical value on the respective graphs. Also see details in Extended Data Fig. 7.
Among the LSK subsets, we observed a significant increase in LT-HSC frequency (P = 0.005) in BM from aged compared to young mice (Fig. 7d). While we observed only a trend towards common myeloid progenitors and GMPs, we measured a significant increase in GMP-committed progenitor fractions, namely proNeu1 and cMoP49 (P < 0.05), paralleled by a clear trend towards CLP reduction in aged compared to young mice (Fig. 7d). To characterize the impairment and specific age-related alteration in CLP commitment, as well as to increase the resolution on these rare progenitors, we used manual gating and we further focused on Lin−CD117lowSca-1lowCD135highCD127high CLP. Here, using two markers, we could characterize Siglec-HlowLy6Dlow T/NK cell, Siglec-HlowLy6Dhigh B cell and Siglec-HhighLy6Dhigh pDC bona fide committed progenitors50 (Extended Data Fig. 7f). In line with our unbiased high-dimensional analysis showing a general impairment of lymphopoiesis, we found a significant reduction of all three lineage-committed CLP fractions in aged mice (Fig. 7e, T/NK precursors P < 0.05; B precursors P < 0.0001; pDC precursors P < 0.0001). This, together with the increase in myeloid-committed progenitors, provides strong evidence for a skewing towards myelopoiesis during aging at the cost of a contracting lymphopoiesis. Similarly, the loss of pDCs is a feature observed across most of the other tissues analyzed in our study, which may be explained by the unique ontogeny of these cells, emerging, at least in part, from the reduced Siglec-HhighLy6Dhigh fraction of the CLP50.
Our second spectral flow-cytometry cohort validated the above-described age-related changes to lineage commitment and was used to power a more statistics-driven, unbiased analysis.
LT-HSC contraction correlates with aged tissue-resident lymphocytes
To uncover rare age-associated immune features independent of traditional leukocyte nomenclature, we carried out an indepth analysis of BM progenitors from two independent spectral flow cohorts using supervised representation learning (CellCNN) approaches followed by FlowSOM-based validation51. This allowed the convergent identification of a small cellular subset of Lin−SCA1+CD48−CD150+ LT-HSCs, which was expanded in the aging BM (Fig. 8a and Extended Data Fig. 7). The FlowSOM-identified matching LT-HSC cluster from cohort 1 showed an overlapping signature and comparable frequency among progenitors, being significantly enriched in aging (Fig. 8b and Extended Data Fig. 7). We observed that the median expression of CD34 was significantly decreased in the old group (Fig. 8c), suggestive of reduced mobilizing and repopulating activity of aged LT-HSC52,53.
a, Histogram showing the expression pattern of CellCNN-selected BM progenitor signature (left panel). Median expression has been normalized from 0 to 1. Frequency of CellCNN-selected cluster among BM progenitors (right panel). Box plot, the center is the median, lower and upper hinges depict the first and third quartiles and the whiskers stretch to 1.5 times the interquartile range from the corresponding hinge. Statistics derived from n = 10 young and n = 10 aged independent samples, P = 0.0232. Statistical analysis was carried out using nonparametric Mann–Whitney–Wilcoxon tests. The P value was then adjusted for multiple comparisons using a BH test. P values of less than 0.05 were considered significant and are indicated by the numerical value on the respective graphs. b, Violin plots (n = 10 young, n = 10 aged) depicting frequency of FlowSOM-derived LT-HSC cluster among BM progenitors. Statistical analysis was carried out using nonparametric Mann–Whitney–Wilcoxon tests. P values of less than 0.05 were considered significant and are indicated by the numerical value on the respective graphs. c, Median expression of CD34 within FlowSOM-derived LT-HSC cluster (n = 10 young, n = 10 aged). The box plot visualizes the median, two hinges and two whiskers. The box plot represents the 25th to 75th percentiles, the whiskers are plotted following the Tukey method. All the original data points are overlaid. Statistics derived from n = 10 young and n = 10 aged independent samples, P = 0.0068. Statistical analysis was carried out using nonparametric Mann–Whitney–Wilcoxon tests. P values of less than 0.05 were considered significant and are indicated by the numerical value on the respective graphs. d, Circle correlation plots showing the multiple correlation matrix between leukocyte frequencies in the lung versus BM progenitors in young and aged mice (summarized from n = 10 young, n = 10 aged). Results are plotted to display statistical significance (P < 0.05). Here, correlation coefficients (R) higher than 0.6 are represented in red, and those lower than −0.6 in blue. e, Circle correlation plots showing the multiple correlation matrix between leukocyte frequencies in the sdLN versus BM progenitors in young and aged mice (summarized from n = 10 young, n = 10 aged). Results are plotted to display statistical significance (P < 0.05). Here, correlation coefficients (R) higher than 0.6 are represented in red, and those lower than −0.6 in blue.
We then computed the correlation between age-affected BM progenitor subsets and their mature lymphoid counterparts, by considering the frequencies of each subset as per each tissue analyzed (Fig. 8d,e). We observed widespread and significant age-induced changes in the relationship between HSCs and lymphoid-committed progenitors with the peripheral adaptive immune populations. In particular, as exemplified by lung and sdLN lymphocytes (Fig. 8c,d) in aging, we observed a positive correlation between the BM progenitors and systemic lymphoid compartment cell frequencies. Alltogether, these observations strengthen the concept that lymphoid progenitor depauperation in the hematopoietic niche is the substrate for peripheral lymphocyte contraction during aging.
Discussion
As a consequence of our increasingly aged global population, there is a need to study the challenges faced by the immune systems of older adults. A rising number of research studies have focused on the immune landscape surrounding various age-related comorbidities. In humans, cellular analysis has often been limited to the leukocytes harvested from peripheral blood54,55 and/or focused on single organs or cell types56. Consequently, dynamic changes in the frequencies of immune populations have been largely overlooked. The recently published Tabula muris senis57,58 is a powerful tool for the interrogation of the subtle changes in the expression of various genes within aging mouse tissues. However, such a resource does not lend itself automatically to further interrogation through routine experimental procedures, for example basic flow cytometry. Our highly complex multiparameter panels have been designed specifically to capture the tissue immune compartment and to provide a rich immune atlas. Similarly, we captured the highly complex HSC compartment and its dynamic changes over two time points. The resulting immune atlas and its annotations lend themselves to further adaptation for routine lab procedures.
Here we present a comprehensive atlas of the young and aged murine immune system with the aim of highlighting patterns of change in immune cell types. We provide a major informational database, in which we have assessed the aging immune compartment of the C57/BL6 mouse, the mouse strain most commonly used in immunology-related research59. We aimed to provide a comprehensive analysis of the mouse model by assessing a diverse range of tissues and organs simultaneously, using an accessible protocol and mice commercially available to the research community. To assess the effects of aging on the immune compartment of the geriatric mouse model, we have powered our experiments adequately (with a combined total of n = 18 young versus n = 17 aged mice).
There is, unfortunately, a lack of consensus about what the right age is to define a mouse as ‘old’ from an immunological point of view. Cohorts of mice of around 20 months of age have been repeatedly utilized in many previous studies and referred to as a representative aged cohort60,61. This map is not intended to be all encompassing or, by the nature of mass cytometry, to provide a precise enumeration of all cells. Instead, we here show mean frequencies of all identifiable immune cellular subsets in young and aged tissues.
While we have focused on three representative organs for barrier, lymphoid, and soft connective tissues, the analysis pipeline was applied to all organs presented here, and the raw data are available as an extended repository (https://zenodo.org/deposit/5593273). As a prime example of a barrier tissue, we initially focused on the lung as a reference point to validate our findings62. Of note, while we observed a relative lower frequency of T cells in the lungs of young mice, we also observed a relative loss of T helper cells with aging, which is in line with previous reports32. The most notable known changes in the myeloid compartment include a reduced abundance of tissue-resident AMs37, and cDC1s, which we also saw here. While previous reports have suggested an impaired phagocytosis response in aging AMs37, the relatively higher expression of MHC-II within the total aged macrophage population that we observed suggests an interaction with T cells to promote tissue inflammation with age. The underlying driver of increased MHC expression may be cell intrinsic in nature and related to the progressive replacement of embryonic-derived AMs by Siglec-FlowMHCIIhighCCR2high monocyte-derived AMs63 occurring with aging64, or related to the increased availability of pro-inflammatory, MHC-inducing cytokines, in particular IFN-γ, in the lung microenvironment62. The loss of AMs and the resulting reduction in surfactant clearing36 has been suggested to be involved in increased mortality among older patients suffering from coronavirus 2019 (COVID-19)65. Clinical trials are currently ongoing to strengthen the AM population in severe COVID-19 infections, with stimulation of granulocyte-macrophage colony-stimulating factor, the primary growth factor for AMs (clinical trial identifiers NCT04400929 and NCT04411680)66. The current progress, as well as the risks and benefits of such treatment strategies, have recently been discussed67. We have observed certain conserved trends among the majority of aging tissues, as summarized in Fig. 5. Previous studies have alternatively reported no age-related changes in the abundance of NK cells in rodent tissues68, or the opposite69: this could be potentially explained by the lack of an universal overview of NK cell dynamics in a healthy aging model. Here, we consistently observed a reduction in the frequency of NK cells in tissues where they usually reside, including the lung, liver, small intestine and LNs. This general reduction in NK cells could have major implications in the aging mammal, with the overall loss of tissue protection to eliminate either virally infected or malignant cells. In line with this, we also observed a pan-loss in frequency of pDCs in the aged rodent. While this phenomenon has been described extensively in peripheral blood70, we conclude that the same pattern can be observed across the majority of aging rodent tissues.
Analysis of a further two validation cohorts of age-matched mice using spectral flow cytometry including an additional age group (4 months old) revealed that sexual maturity did not have a significant impact on the distribution of leukocytes across tissues, nor on the hematopoietic output.
In the context of healthy aging, it has been shown that in both humans and mice, CD8 T cell responses to new infections is decreased71,72. Several studies have also shown the functional decline of CD8 T cells in mice, within the context of lymphoid tissues16,60,71,73, yet it is still unclear at present what the exact basis of this decline is. Through a statistically driven approach, we uncovered a tissue-wide, homogeneous and significant reduction in the expression of CD103 within the naive tissue-resident CD8 T cell compartment of the aging mouse (Fig. 6a–c). Our data revealed that the frequency of these cells did not change between the time points; rather, the cells selectively lose their CD103 expression. This phenotype can be observed across both lymphoid and non-lymphoid tissues. This particular subset did not reveal any changes in other functional (for example CD73, CD69) or activation (for example KI67, Granzyme B) states. Recent studies suggest that reduced CD103 expression can be linked to increased cytotoxic function74,75.
The mechanism by which the leukocyte compartment ‘adapts’ to aging tissues is a matter of controversy, and again the role of inflammatory mediators remains unclear. To understand better whether certain age-related cellular patterns were caused by in situ tissue dysregulation, or predetermined at the stem cell level, we interrogated the hematopoietic precursor compartment using spectral flow cytometry. The widespread loss of NK cells and CD4 T cells in the aging mouse correlates with significantly reduced Ly6Dlow CLPs in the aging bone marrow. Finally, the reduced frequency and numbers of pDC-committed CLP fraction further complement the general trends we described in our aging immune atlas. Overall, we confirmed a skewing of the hematopoietic compartment towards myelopoiesis76, and a systemic impairment of adaptive immune compartment (underpinned by the reduction of CLPs) accounting for the hampered antiviral and antitumor responses associated with aging. Unbiased, algorithm-driven analyses identified a subset of hematopoietic precursors, namely LT-HSC, to expand during aging. These aged LT-HSCs lack CD34 expression, a phenotype associated with reduced differentiation potential towards lymphoid cells52,53. Our findings suggest in fact that the ‘aging phenotype’ observed in the immune compartment of aging tissues is not primarily the result of a cell-intrinsic, programmed adaptation or that the aging tissue would firmly dictate its leukocyte niche. Instead, our data support the notion that immune aging is largely the result of hematopoietic output.
In conclusion, here we introduce a comprehensive leukocyte atlas of the aging immune system, with a focus on the relative abundance of all leukocyte lineages within 12 organs. This dataset is presented as a resource, to facilitate future age-related studies with more focus on tissue-specific and tissue-resident leukocyte subsets in the naive mouse model. We present this resource to the scientific community in the hope of establishing a baseline, naive comprehensive dataset to build on for future investigative work in the C57/BL6 aging mouse model. We hope to have demonstrated its utility in drawing important conclusions about the state of the overall and tissue-specific immune systems across the murine model.
Methods
In vivo experimental details
Mice used for all experiments were C57BL/6JRj, wild-type mice acquired from Janvier laboratories, where the animals were housed in the same housing facility from birth until purchase. Then, the animals were acclimatized, together, at the animal housing facility of the University of Zurich for at least 2 weeks before experimental procedures. Mice were kept in a 12-hour light–dark cycle, with an ambient temperature range within 20–24 °C; humidity ranged from 30% to 70%.
Male and female mice from the ‘young’ group were aged approximately 7–8 weeks, ‘middle’ were 16 weeks and ‘aged’ were between 18 and 20 months.
For our initial analysis cohort, we devised four experimental groups: n = 4 young adult male, n = 4 young adult female; n = 4 aged female; and n = 3 aged male C57/BL6 mice.
For our validation cohort 1, we devised the following experimental group: n = 5 young adult male, n = 5 young adult female; n = 5 aged female; and n = 5 aged male C57/BL6 mice.
For our validation cohort 2, we procured: n = 5 juvenile male, n = 5 young adult male, n = 5 juvenile female, n = 5 young adult female; n = 5 aged female; and n = 5 aged male C57/BL6 mice.
All animal experiments performed in this study were approved by and in adherence with the Cantonal Veterinary Office Zurich.
Tissue harvesting, and single-cell suspension preparation
Tissues from mice (lung, liver, visceral adipose tissue, brown adipose tissue, small intestine, thymus, testes, MesLNs, sdLNs, spleen, blood and BM) were harvested as described previously4. Briefly, mice were killed by intra-peritoneal (i.p.) injection of pentobarbital (50 μl; at 300 mg ml−1) followed by trans-cardiac perfusion with phosphate buffered saline (PBS) and heparin (5 Units ml−1). Each organ was harvested and cut into small pieces, using scissors, inside an Eppendorf tube. Next, the samples were incubated with digestion medium (HBSS + + with 2% fetal calf serum (FCS), 2 mM HEPES, DNAse (50 μl per 10 ml) and Collagenase IV) at 37 °C while shaking. The exact medium concentrations and incubation times for each organ are described in Supplementary Table 2.
Enzymatic digestion was stopped with ethylenediamine tetraacetic acid (EDTA) 5 mM. The samples were homogenized using a syringe and a 24 G needle, and the homogenate was filtered through a 100 μM cell strainer. SLTs were subjected to Red Blood Cell lysis (Biolegend) (5 min at room temperature). The CNS cell suspensions were further enriched by Percoll gradient (30%) centrifugation (1,590g, 4 °C, 30 min, no brake)77 and thymus samples too (centrifugation at 2,750 r.p.m., 4 °C, 30 min, no brake). Spleen samples from the initial cohort were subjected to myeloid cell enrichment using negative bead selection (MACS MicroBeads and Column, Miltenyi Biotec) for ter119, CD3, CD19 and CD5. The samples were then ready for labeling with antibodies for mass or fluorescence cytometry.
Mass-cytometry analysis
Antibodies used were either acquired from Fluidigm or conjugated in-house using antibody-labeling kits purchased from Fluidigm. Following antibody conjugation, reagents were titrated and tested as part of the final panel accordingly. As described previously78, CD45 antibodies conjugated to six palladium metal isotopes (104 Pd, 105 Pd, 106 Pd, 108 Pd and 110 Pd; all from Trace Sciences International) were used for live immune cell barcoding. All individual tissue samples were labeled with a combination of three different barcoding antibodies for 30 min at 37 °C on a rotary shaker. Then, following two wash steps (with PBS + 2% FCS), all samples from the same organ were combined together. Combined samples were labeled with the primary antibodies for 30 min at 37 °C, then, following a wash step, the cells were labeled with the secondary antibodies for 20 min at 4 °C. To identify dead cells, 2.5 μM cisplatin (Sigma-Aldrich) was added for 2 min on ice. After washing, samples were fixed with 1.6% paraformaldehyde (PFA; Electron Microscopy Sciences) in PBS for up to 4 days at 4 °C. On the day of acquisition, samples were incubated with intercalating solution (Iridium (Sigma) in MaxPar Fix/Perm buffer (Fluidigm)) for 1–3 h) at 4 °C. Shortly before acquisition, the samples were washed twice with FACS buffer and once with Maxpar water (Fluidigm). Barcoded samples were acquired on a Helios mass cytometer (Fluidigm), using CyTOF Software v.6.7. Quality control and tuning processes on the Helios were performed on a daily basis, as well as before and during acquisition as required. Data from different days and across acquisition time were normalized by adding five element beads to the sample immediately before acquisition and using the MATLAB-based normalization software, as described previously79.
For mass cytometry analysis, we used the antibodies listed in Supplementary Table 3.
Flow-cytometry analysis
All reagents were tested and titrated before use in experiments. To discern live cells, all individual samples were incubated with LIVE/DEAD fixable blue dead cell kit (Thermofisher) in PBS, at a concentration of 1:800, for 30 min on ice. Following a wash step, the cells were then incubated with CD16/32 antibody at a concentration of 1:400 (the purified form (Thermofisher) for lymphoid panel samples, and CD16/32 BV605 for myeloid panel samples) in PBS for 20 min on ice.
All subsequent antibody incubations were carried out in PBS supplemented with 2% FCS and 20% Brilliant Stain Buffer (BD Biosciences). Cells were incubated with the primary antibody cocktail for 30 min at 4 °C. Following another wash step, the cells were incubated in the secondary antibody cocktail for 20 min at 4 °C.
When intracellular labeling was required, cells were permeabilized using Foxp3/Transcription Factor Staining Buffer Set (eBioscience) according to the manufacturer’s instructions, for 45 min at 4 °C. Subsequently, the sample was washed once in Perm/Wash buffer (eBioscience) and incubated in the antibody mixture in Perm/Wash buffer overnight at 4 °C. The samples were washed once in Perm/Wash buffer (eBioscience) and centrifuged to pellet the cells before resuspension in PBS before flow-cytometry data acquisition. Samples were acquired on an Aurora spectral analyzer (Cytek Biosciences) using SpectroFlo Software (v.2.0) following daily quality control procedures as instructed by the manufacturer.
For spectral flow cytometry of the lymphoid and myeloid compartments of 11 organs, we used the antibodies described in Supplementary Table 4.
For spectral flow cytometry of the hematopoietic compartment in the bone marrow, we used the antibodies described in Supplementary Table 5.
High-dimensional data preprocessing and statistical analysis
From the raw data acquired by mass cytometry, using FlowJo software v.10.6.2 and 10.7.1 (TreeStar), live cells were identified using manual gating on event length, DNA (191Ir and 193Ir) and live cells (195Pt). Debarcoding of each sample was achieved using Boolean gating. For flow-cytometry data, the compensation matrix was corrected in FlowJo (TreeStar) by pregating on live immune cells, and the total fraction of live, singlet immune cells was exported. Data were then transformed with an inverse hyperbolic sine (arcsinh) function (cofactors ranging between 5 and 18,000) then imported into the R environment (v.3.6.1) for subsequent analysis80.
The high-dimensional analysis was carried out using the R environment, based on the workflow described previously by Hartmann et al.20. Briefly, UMAPs were generated using the package umap v.0.2.7.0 (ref. 21), and FlowSOM clustering was overlaid on the dimensionality reduction maps19. Frequency plots were generated using the ggplot2 package v.3.3.5, and heatmaps were generated using the pheatmap package v.1.0.12.
Force-directed layouts were generated by sampling 1,500 cells from each mouse sample, and using the ForceAtlas2 algorithm48 integrated in the VorteX graphical clustering environment creating unweighted edges between the nodes based on the ten nearest neighbors81. Resulting graphs were further modified using the Gephi Toolkit v.0.9.2.
Scaffold networks were computed using the improved version of the initial Scaffold package consisting of grappolo, vite and panorama82. For the construction of lymphocyte and myeloid cell networks, resulting graphs were rearranged in the igraph framework using the graphopt algorithm (as described in http://www.schmuhl.org/graphopt/).
CellCNN-based analysis was performed by training a randomly selected subset of cells. Default values were used for all CellCNN hyperparameters51. Default values were used for most CellCNN hyperparameters, except for the following: ncell equals 3,000;—no_arcsinh;—no_scale;—filter_response_thres 0.3;—train_perc 0.5. multidimensional scaling plots were generated using the stats package v.4.2.0 and displayed using ggscatter v.0.4.0. Correlation matrices were calculated using Hmisc v.4.5-0 and generated using circlize v.0.4.13 in conjunction with corrplot v. 0.90. All other plots were generated using ggplot2.
Statistical analysis was carried out using nonparametric Mann–Whitney–Wilcoxon tests. The P value was then adjusted for multiple comparisons using a Benjamini–Hochberg (BH) test83 using the R package rstatix v.0.7.0. P values of less than 0.05 were considered significant and are indicated by an asterisk (*) or the numerical value on the respective graphs.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
FCS files of the mass and spectral flow-cytometry data as well as extended data relating to cell counts are available for download through the following repository link: https://zenodo.org/deposit/5593273.
Code availability
An R-based script for data analysis is available through the following repository link: https://zenodo.org/deposit/5593273.
References
Pawelec, G. & Solana, R. Immunosenescence. Immunol. Today 18, 514–516 (1997).
Franceschi, C. et al. Inflamm‐aging: an evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 908, 244–254 (2000).
Adlung, L., Amit, I. & Elinav, E. Embrace the fat when getting old. Aging 11, 8730–8732 (2019).
Mrdjen, D. et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48, 380–395.e6 (2018).
Reichert, T., Chowell, G. & McCullers, J. A. The age distribution of mortality due to influenza: pandemic and peri-pandemic. BMC Med. 10, 162 (2012).
Clayton, D. & Schifflers, E. Models for temporal variation in cancer rates. II: Age–period–cohort models. Stat. Med. 6, 469–481 (1987).
Ridker, P. M., Rifai, N., Stampfer, M. J. & Hennekens, C. H. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 101, 1767–1772 (2000).
Brunet, A. & Berger, S. L. Epigenetics of aging and aging-related disease. Journals Gerontol. Ser. A Biomed. Sci. Med. Sci 69, S17–S20 (2014).
Gustafson, C. E., Kim, C., Weyand, C. M. & Goronzy, J. J. Influence of immune aging on vaccine responses. J. Allergy Clin. Immunol. 145, 1309–1321 (2020).
Nikolich-Žugich, J. The twilight of immunity: emerging concepts in aging of the immune system. Nat. Immunol. 19, 10 (2018).
Alam, M. S. et al. Oral exposure to Listeria monocytogenes in aged IL-17RKO mice: apossible murine model to study listeriosis in susceptible populations. Microb. Pathog. 99, 236–246 (2016).
Mekker, A. et al. Immune senescence: relative contributions of age and cytomegalovirus infection. PLoS Pathog. 8, e1002850 (2012).
Lin, Y. et al. Changes in blood lymphocyte numbers with age in vivo and their association with the levels of cytokines/cytokine receptors. Immun. Ageing 13, 24 (2016).
Wertheimer, A. M. et al. Aging and cytomegalovirus infection differentially and jointly affect distinct circulating T cell subsets in humans. J. Immunol. 192, 2143–2155 (2014).
Deshpande, N. R., Parrish, H. L. & Kuhns, M. S. Self-recognition drives the preferential accumulation of promiscuous CD4(+) T-cells in aged mice. eLife 4, e05949 (2015).
Decman, V. et al. Defective CD8 T cell responses in aged mice are due to quantitative and qualitative changes in virus-specific precursors. J. Immunol. 188, 1933–1941 (2012).
Montgomery, R. R. & Shaw, A. C. Paradoxical changes in innate immunity in aging: recent progress and new directions. J. Leukoc. Biol. 98, 937–943 (2015).
Schulte, H., Mühlfeld, C. & Brandenberger, C. Age-related structural and functional changes in the mouse lung. Front. Physiol. 10, 1466 (2019).
Van Gassen, S. et al. FlowSOM: Using self‐organizing maps for visualization and interpretation of cytometry data. Cytom. Part A 87, 636–645 (2015).
Hartmann, F. J. et al. High-dimensional single-cell analysis reveals the immune signature of narcolepsy. J. Exp. Med. https://doi.org/10.1084/jem.20160897 (2016).
McInnes, et al. UMAP: Uniform Manifold Approximation and Projection. J. Open Source Softw. 3, 861 (2018).
Junker, F., Gordon, J. & Qureshi, O. Fc gamma receptors and their role in antigen uptake, presentation, and T cell activation. Front. Immunol. 11, 1393 (2020).
Sheng, J. et al. A discrete subset of monocyte-derived cells among typical conventional type 2 dendritic cells can efficiently cross-present. Cell Rep. 21, 1203–1214 (2017).
Balan, S., Saxena, M. & Bhardwaj, N. in International Review of Cell and Molecular Biology (eds Galluzzi, L. & Lhuillier, C.) Vol. 348, 1–68 (Elsevier Inc., 2019).
Dress, R. J. et al. Plasmacytoid dendritic cells develop from Ly6D+ lymphoid progenitors distinct from the myeloid lineage. Nat. Immunol. 20, 852–864 (2019).
Buja, A. et al. Data visualization with multidimensional scaling. J. Comput. Graph. Stat. 17, 444–472 (2012).
M., N. et al. CyTOF workflow: differential discovery in high-throughput high-dimensional cytometry datasets. F1000Research 6, 748 (2017).
Lu, R. J. et al. Multi-omic profiling of primary mouse neutrophils predicts a pattern of sex and age-related functional regulation. Nat. Aging 1, 715–733 (2021).
Zaynagetdinov, R. et al. Identification of myeloid cell subsets in murine lungs using flow cytometry. Am. J. Respir. Cell Mol. Biol. 49, 180–189 (2013).
Ji, J. J. & Fan, J. Discovering myeloid cell heterogeneity in the lung by means of next generation sequencing. Military Med. Res. 6, 33 (2019).
Meiners, S., Eickelberg, O. & Königshoff, M. Hallmarks of the ageing lung. Eur. Respir. J. 45, 807–827 (2015).
Toapanta, F. R. & Ross, T. M. Impaired immune responses in the lungs of aged mice following influenza infection. Respir. Res. https://doi.org/10.1186/1465-9921-10-112 (2009).
Wyburn, K. R., Jose, M. D., Wu, H., Atkins, R. C. & Chadban, S. J. The role of macrophages in allograft rejection. Transplantation 80, 1641–1647 (2005).
Cho, K. W. et al. An MHC II-dependent activation loop between adipose tissue macrophages and CD4+ T cells controls obesity-induced inflammation. Cell Rep. 9, 605–617 (2014).
Fulton, S. A. et al. Inhibition of major histocompatibility complex II expression and antigen processing in murine alveolar macrophages by Mycobacterium bovis BCG and the 19-kilodalton mycobacterial lipoprotein. Infect. Immun. 72, 2101–2110 (2004).
Yao, Y. et al. Induction of autonomous memory alveolar macrophages requires T cell help and is critical to trained immunity. Cell 175, 1634–1650.e17 (2018).
Wong, C. K. et al. Aging impairs alveolar macrophage phagocytosis and increases influenza-induced mortality in Mice. J. Immunol. 199, 1060–1068 (2017).
Della Bella, S. et al. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin. Immunol. 122, 220–228 (2007).
Turner, V. M. & Mabbott, N. A. Structural and functional changes to lymph nodes in ageing mice. Immunology 151, 239–247 (2017).
Hill, D. A. et al. Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc. Natl Acad. Sci. USA 115, E5096–E5105 (2018).
Dye, L., Boyle, N. B., Champ, C. & Lawton, C. The relationship between obesity and cognitive health and decline. Proc. Nutr. Soc. 76, 443–454 (2017).
van Splunter, M. et al. Plasmacytoid dendritic cell and myeloid dendritic cell function in ageing: a comparison between elderly and young adult women. PLoS ONE14, e0225825 (2019).
Cohen, J. in Statistical Power Analysis for the Behavioral Sciences xiii–xv (Elsevier, 1977); https://doi.org/10.1016/B978-0-12-179060-8.50005-0
Cohen, J. A power primer. Psychol. Bull. 112, 155–159 (1992).
Min, H., Montecino-Rodriguez, E. & Dorshkind, K. Effects of aging on the common lymphoid progenitor to pro-B cell transition. J. Immunol. 176, 1007–1012 (2006).
Rossi, D. J. et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl Acad. Sci. USA 102, 9194–9199 (2005).
Grover, A. et al. Single-cell RNA sequencing reveals molecular and functional platelet bias of aged haematopoietic stem cells. Nat. Commun. 7, 11075–11075 (2016).
Jacomy, M., Venturini, T., Heymann, S. & Bastian, M. ForceAtlas2, a continuous graph layout algorithm for handy network visualization designed for the Gephi software. PLoS ONE https://doi.org/10.1371/journal.pone.0098679 (2014).
Kwok, I. et al. Combinatorial single-cell analyses of granulocyte-monocyte progenitor heterogeneity reveals an early uni-potent neutrophil progenitor. J. Clean. Prod. 53, 303–318.e5 (2020).
Rodrigues, P. F. et al. Distinct progenitor lineages contribute to the heterogeneity of plasmacytoid dendritic cells. Nat. Immunol. 19, 711–722 (2018).
Arvaniti, E. & Claassen, M. Sensitive detection of rare disease-associated cell subsets via representation learning. Nat. Commun. 8, 14825 (2017).
Rossi, D. J. et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl Acad. Sci. USA 102, 9194–9199 (2005).
Sudo, K., Ema, H., Morita, Y. & Nakauchi, H. Age-associated characteristics of murine hematopoietic stern cells. J. Exp. Med. 192, 1273–1280 (2000).
Prather, A. A. et al. Associations between chronic caregiving stress and T cell markers implicated in immunosenescence. Brain. Behav. Immun. 73, 546–549 (2018).
Akbar, A. N., Henson, S. M. & Lanna, A. Senescence of T lymphocytes: implications for enhancing human immunity. Trends Immunol. 37, 866–876 (2016).
Szabo, P. A. et al. Single-cell transcriptomics of human T cells reveals tissue and activation signatures in health and disease. Nat Commun. 10, 4706 (2019).
Schaum, N. et al. Single-cell transcriptomics of 20 mouse organs creates a Tabula muris. Nature 562, 367–372 (2018).
Almanzar, N. et al. A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature 583, 590–595 (2020).
Johnson, M. Laboratory mice and rats. Mater. Methods 2, (2012).
Quinn, K. M. et al. Age-related decline in primary CD8+ T cell responses is associated with the development of senescence in virtual memory CD8+ T cells. Cell Rep. 23, 3512–3524 (2018).
Leins, H. et al. Aged murine hematopoietic stem cells drive aging-associated immune remodeling. Blood 132, 565–576 (2018).
Angelidis, I. et al. An atlas of the aging lung mapped by single cell transcriptomics and deep tissue proteomics. Nat Commun. 10, 963 (2019).
Machiels, B. et al. A gammaherpesvirus provides protection against allergic asthma by inducing the replacement of resident alveolar macrophages with regulatory monocytes. Nat. Immunol. 18, 1310–1320 (2017).
Liu, Z. et al. Fate mapping via Ms4a3-expression history traces monocyte-derived cells. Cell 178, 1509–1525.e19 (2019).
Zviran, A. et al. Genome-wide cell-free DNA mutational integration enables ultra-sensitive cancer monitoring. Nat. Med. 26, 1114–1124 (2020).
Bosteels, C. et al. Sargramostim to treat patients with acute hypoxic respiratory failure due to COVID-19 (SARPAC): a structured summary of a study protocol for a randomised controlled trial. Trials 21, 491 (2020).
Lang, F. M., Lee, K. M. C., Teijaro, J. R., Becher, B. & Hamilton, J. A. GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches. Nat. Rev. Immunol. 20, 507–514 (2020).
Nogusa, S., Ritz, B. W., Kassim, S. H., Jennings, S. R. & Gardner, E. M. Characterization of age-related changes in natural killer cells during primary influenza infection in mice. Mech. Ageing Dev. 129, 223–230 (2008).
Beli, E. et al. Natural killer cell development and maturation in aged mice. Mech. Ageing Dev. 135, 33–40 (2014).
Jing, Y. et al. Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in human peripheral blood. Hum. Immunol. 70, 777–784 (2009).
Briceño, O. et al. Reduced naïve CD8+ T-cell priming efficacy in elderly adults. Aging Cell 15, 14–21 (2016).
Jiang, J., Fisher, E. M. & Murasko, D. M. Intrinsic defects in CD8 T cells with aging contribute to impaired primary antiviral responses. Exp. Gerontol. 48, 579–586 (2013).
Renkema, K. R., Li, G., Wu, A., Smithey, M. J. & Nikolich-Žugich, J. Two separate defects affecting true naive or virtual memory T cell precursors combine to reduce naive T cell responses with aging. J. Immunol. 192, 151–159 (2014).
Lardy, M. et al. T Cells + regulatory CD8 CD103 is a marker for alloantigen-induced. J. Immunol. https://doi.org/10.4049/jimmunol.177.5.2775 (2021).
Uss, E. et al. CD103 is a marker for alloantigen-induced regulatory CD8 + T cells. J. Immunol. 177, 2775–2783 (2006).
Yamamoto, R. et al. Large-scale clonal analysis resolves aging of the mouse hematopoietic stem cell compartment. Cell Stem Cell 22, 600–607.e4 (2018).
Mundt, S. et al. Conventional DCs sample and present myelin antigens in the healthy CNS and allow parenchymal T cell entry to initiate neuroinflammation. Sci. Immunol 4, 8380 (2019).
Mei, H. E., Leipold, M. D., Schulz, A. R., Chester, C. & Maecker, H. T. Barcoding of live human peripheral blood mononuclear cells for multiplexed mass cytometry. J. Immunol. 194, 2022–2031 (2015).
Finck, R. et al. Normalization of mass cytometry data with bead standards. Cytom. Part A 83A, 483–494 (2013).
Bendall, S. C. et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science (80) 332, 687–696 (2011).
Samusik, N., Good, Z., Spitzer, M. H., Davis, K. L. & Nolan, G. P. Automated mapping of phenotype space with single-cell data. Nat. Methods https://doi.org/10.1038/nmeth.3863 (2016).
Spitzer, M. H. et al. An interactive reference framework for modeling a dynamic immune system. Science (80). 349, 1259425 (2015).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B https://doi.org/10.1111/j.2517-6161.1995.tb02031.x (1995).
Acknowledgements
We thank L. Ducimetière, E. Galli, J. Gschwend, J. Komuczki, I. Lelios, A. Rita Liuzzi, W. Mildenberger and S. Tuzlak for experimental support during the harvest and processing of various mouse tissues for mass-cytometry experiments; the Cytometry Facility (University of Zurich) for technical assistance; and the Bodenmiller lab (University of Zurich) for sharing access to their CyTOF facility during a technical issue. We thank L. Robinson from Insight Editing London for critical review and editing of the manuscript. Cartoon images used in Fig. 1, depicting mice, internal organs, containers and cells were created with and adapted from smart.servier.com. This work was funded by grants from the Swiss National Science Foundation (grant number 310030_188450) and European Research Council AdG (IMPACT, 882424), both awarded to B.B.
Author information
Authors and Affiliations
Contributions
S.K. conceptualized, designed and performed all the cytometry experiments, as well as the analysis of the data. F.I. and E.F. helped with all cytometry experiments and data analysis. Both F.I. and E.F. have the right to list their name first in their CV. D.C., A.A., M.L., S.M. and F.R. provided technical support with both mass and spectral flow-cytometry experiments. M.A., D.B., G.L., M.M., C.S., S.S., C.U., S.U., M.V. and P.Z. provided technical support with spectral flow-cytometry experiments. M.G. and S.T. provided intellectual input. D.D.F. analyzed the hematopoiesis datasets and helped review the manuscript. S.K. and B.B. wrote the manuscript. B.B. supervised and funded the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Aging and the authors of this article thank the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Summary of experimental groups related to Fig. 1.
Summary of experimental groups.
Extended Data Fig. 2 MDS analysis reveals sex dimorphism within the progenitor compartment.
a: MDS plot showing the lymphoid compartment across sdLNs. The experimental groups (age, sex) are indicated by individual colors (left panel). Heatmap of the mean frequency of all lymphoid populations, calculated within the total CD45 compartment (right panel). b: MDS plot showing the myeloid compartment across sdLNs. The experimental groups (age, sex) are indicated by individual colors (left panel). Heatmap of the mean frequency of all myeloid populations, calculated within the total CD45 compartment (right panel). c: A: MDS plot showing the progenitor compartment across BM. The experimental groups (age, sex) are indicated by individual colors (left panel). Heatmap of the mean frequency of all BM populations, calculated within the total CD45 compartment (right panel). Violin plots depicting frequencies of sex-dimorphic populations in the BM (bottom panel).
Extended Data Fig. 3 Marker expression profile of leukocyte subsets in murine lung, related to Fig. 2.
a: Heatmap depicting median marker expression in FlowSOM-derived lymphoid subsets of young adult and aged lung. b: Heatmap depicting median marker expression in FlowSOM-derived myeloid subsets of young adult and aged lung. c: Violin plots depicting frequencies of FlowSOM-generated immune cell clusters in ageing, comparing male and female mice. D: UMAP visualization with FlowSOM overlay depicting the total lymphoid fraction, further characterized in the validation cohort (left panel). Violin plots depicting frequencies of specialized T cell clusters in ageing.
Extended Data Fig. 4 Marker expression profile of leukocyte subsets in murine sdLN and VAT, related to Fig. 3.
a: Heatmap depicting median marker expression in FlowSOM-derived lymphoid subsets of young adult and aged sdLN (left panel). Heatmap depicting median marker expression in FlowSOM-derived myeloid subsets of young adult and aged sdLN (right panel). b: Violin plots depicting frequencies of FlowSOM-generated immune cell clusters in ageing, comparing male and female mice. c: Heatmap depicting median marker expression in FlowSOM-derived lymphoid subsets of young adult and aged VAT (left panel). Heatmap depicting median marker expression in FlowSOM-derived myeloid subsets of young adult and aged VAT (right panel). d: Violin plots depicting frequencies of FlowSOM-generated immune cell clusters in ageing, comparing male and female mice.
Extended Data Fig. 5 Conserved trends in leukocyte abundance in aged versus young mice, related to Figs. 4–5.
a: Violin plots depicting frequencies of selective FlowSOM-generated immune cell clusters in ageing, across all tissues included in the immune atlas. b: Volcano plots comparing Log10 (fold change) of aged vs young adult leukocyte populations. Dotted horizontal line depicts cut-off for significant change (p < 0.05). Lymphoid populations are calculated as a fraction of total CD45 cells, and myeloid subsets are calculated as a fraction amongst total myeloid cells. Statistical analysis was carried out using non−paired student’s t test. P values of less than 0.05 were considered significant and are indicated by the numerical value on the respective graphs.
Extended Data Fig. 6 CD103 expression is downregulated within the naïve CD8 T cell compartment of all aged mouse tissues, related to Fig. 6.
a: gating strategy of the lymphoid compartment of the spectral flow-cytometry Validation Cohorts. b: Histograms showing CD103 expression within the naïve CD8 T cells of 4 tissues. Representative young and aged mouse.
Extended Data Fig. 7 Marker expression profile of main hematopoietic cell subsets in murine bone marrow and quantification of committed-CLP subsets in aged bone marrow, related to Fig. 6.
a: Heatmap depicting median marker expression in FlowSOM-derived total CD45 subsets in young and aged bone marrow. b: UMAP with FlowSOM overlay showing total CD45pos compartment of young adult and aged BM. c: Heatmap depicting median marker expression in FlowSOM-derived cluster. d: Violin plots depicting frequencies of immune cells among total CD45pos cells of FlowSOM- generated immune cell clusters. Statistical analysis was carried out using non−parametric Mann-Whitney-Wilcoxon tests. The p value was then adjusted for multiple comparisons using a Benjamini-Hochberg test. P values of less than 0.05 were considered significant and are indicated by the numerical value on the respective graphs. e: Violin plots depicting frequencies among total CD45pos cells of FlowSOM- generated progenitor cell clusters. f: Manual gating strategy for CLPs and downstream precursor subsets of the bone marrow, subdivided on the basis of Siglec-H and Ly6D expression.
Supplementary information
Supplementary Information
Supplementary Tables 1–5.
Rights and permissions
About this article
Cite this article
Krishnarajah, S., Ingelfinger, F., Friebel, E. et al. Single-cell profiling of immune system alterations in lymphoid, barrier and solid tissues in aged mice. Nat Aging 2, 74–89 (2022). https://doi.org/10.1038/s43587-021-00148-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-021-00148-x
This article is cited by
-
Low dose post-transplant cyclophosphamide and sirolimus induce mixed chimerism with CTLA4-Ig or lymphocyte depletion in an MHC-mismatched murine allotransplantation model
Bone Marrow Transplantation (2024)
-
Locally sourced: site-specific immune barriers to metastasis
Nature Reviews Immunology (2023)
-
Skull bone marrow channels as immune gateways to the central nervous system
Nature Neuroscience (2023)
-
Nuclear envelope disruption triggers hallmarks of aging in lung alveolar macrophages
Nature Aging (2023)
-
Long-term evaluation of safety and biological effects of Korean Red Ginseng (Panax Ginseng): a long-term in vivo study
BMC Complementary Medicine and Therapies (2022)