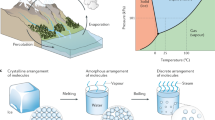
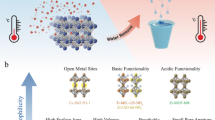
Abstract
The rising demand for freshwater and the challenge of energy scarcity have fueled interest in solar interfacial water evaporation technology, which harnesses solar energy to produce clean water. Attaining high performance with this technology necessitates the development of highly efficient photothermal materials, heat management optimization, and the resolution of salt deposition issues to ensure equipment longevity. Metal-organic frameworks (MOFs) possess large specific surface areas and high porosity, making them ideal for various water treatment applications. In recent years, MOFs have been extensively employed for solar-driven desalination. Here, we review recent developments in the functionalization of MOFs thin films, composites and MOFs-based derivatives and strategies for achieving efficient seawater desalination with MOFs while preventing salt deposition. Furthermore, desalination systems that integrate pollutant degradation and power generation are discussed, which further expand the application scenarios of solar-driven interfacial water evaporation desalination technologies.
Similar content being viewed by others
Introduction
The prevalent issue of water pollution and the increasing demand for fresh water have elevated the scarcity of freshwater resources to a significant global challenge1,2. This has prompted the exploration and development of environmentally friendly and energy-efficient desalination technologies. Given the abundance of seawater, seawater desalination (SD) is considered a valuable solution for freshwater production3,4,5. Currently, SD technologies are primarily categorized into membrane-based methods, thermal methods, and solar-driven interfacial water evaporation (SDIWE)6. Membrane-based techniques utilize selective porous membranes to separate solutes and water from seawater driven by pressure, electric potential, or concentration difference. These methods offer advantages such as low energy consumption, no phase change or chemical reactions, and ambient temperature operation7,8. Reverse osmosis technology is the mainstream membrane-based method for seawater desalination. It applies high pressure on one side of the seawater to force water through a semi-permeable membrane, leaving behind the salts9. Thermal methods, or distillation, involve heating seawater to vaporize it and then condensing the steam to obtain distilled water. These methods have lower requirements for the water quality of the feed seawater, can handle high salt concentrations, and produce high-quality water. Notable thermal desalination techniques include multi-effect distillation and multiple-stage flash distillation10,11. However, they are more complex and energy-exhaustive12. Conversely, SDIWE technology has received widespread attention in recent years due to its ability to harness sunlight exclusively to purify water from seawater or waste streams13,14,15,16,17. In this system, photothermal materials absorb solar energy and convert it into thermal energy, which in turn heats up the water, driving its evaporation. Upon condensing the resulting water vapor, clean water can be collected18,19,20. The effectiveness of SDIWE systems largely depends on the properties of the photothermal materials, thrusting their research and development into the scientific spotlight21.
In the past decades, various photothermal materials have been used in the field of SDIWE, such as metal nanoparticles22,23,24, semiconductors25,26, polymers27,28,29, and carbon materials30,31,32,33. However, these photothermal materials still suffer prohibitive costs, salt aggregation issues, narrow light absorption range, stability concerns, and production inefficiencies etc. in practical applications34,35,36. Therefore, it is necessary to develop new photothermal materials to promote the development of SDIWE systems. The new photothermal materials should possess the following characteristics: 1) the ability to capture a significant amount of solar radiation across the entire solar spectrum; 2) an efficient photothermal conversion ability and low thermal conductivity; 3) a good hydrophilicity to provide sufficient water supply for the evaporation process; 4) rapid steam generation capability due to their porous structure; 5) an ability to resist salt deposition and maintain stable evaporation rates over a long period of time21,23,37,38,39,40.
With the continuous rapid development of this field, many emerging materials, such as organic small molecules, metal-organic frameworks (MOFs), and polyphenolic coordination compounds, have also been developed, leading new directions41,42,43,44,45,46,47. Recently, there has been a report on an organometallic polymer PffBTPt, which involves the coupling of Pt(N,N)(O,O) and ffBT-2ET-2DT in the field of SDIWE48. The construction of one-dimensional photothermal materials by introducing metal complexes and organic groups demonstrates excellent light absorption capabilities, along with advantages such as simplicity in preparation and strong flexibility. There will be more research based on this material in the future. Notably, MOFs, as two-dimensional (2D) or three-dimensional (3D) metal-organic materials, characterized by their expansive surface areas and high porosity, are positioning themselves as potential candidates49,50,51. Tailoring MOFs structures or combining them with other photothermal agents, can substantially enhance light absorption and photothermal efficiency52,53,54. Furthermore, the functional frameworks of MOFs, coupled with their high specific surface areas, tunable pore sizes and diverse topologies, contribute to a broad absorption spectrum and excellent hydrophilic properties. Therefore, MOFs-based materials can be applied in SDIWE technology for solar-driven seawater desalination, making it a promising approach. Figure 1 depicts six typical MOFs in this review.
This review summarizes the latest research progress on MOFs-based solar desalination materials, focusing primarily on mechanisms, materials, and applications. The second section delves into the heat management, photothermal conversion mechanisms, and the stability of MOFs in interfacial water evaporation processes. Subsequently, the emphasis is placed on the design of three specific MOFs-based materials and summarizes the performance of different designs currently in use. Furthermore, it explores three system design approaches to prevent salt deposition. Section 4 also highlights the multi-functional applications of MOFs-based evaporators. Finally, a concise summary is provided, followed by our insights and perspective for the future developments in this field.
Mechanisms for SDIWE
SDIWE technology utilizes the renewable energy of the sun to convert seawater or wastewater into fresh water, which is achieved by a solar evaporator. It is strategically placed at the air-water interface, maximising the concentration of heat energy derived from the sun. As the solar energy is absorbed by the evaporator, it transfers the heat to the water molecules at the interface. This transfer of energy increases the kinetic energy of the water molecules, causing them to evaporate and form vapor. The following subsections delve into the heat distribution within the thermal conversion process, exploring the underlying mechanisms of photothermal conversion and the various factors that influence the stability of this process.
Thermal management in SDIWE
SDIWE involves positioning the photothermal material at the water-air interface to achieve localized heat utilization. The resulting heat specifically targets the water at the interface, promoting rapid evaporation under natural sunlight and significantly enhancing solar-to-steam conversion efficiency. Figure 2 illustrates a typical SDIWE system, where solar energy (Qsun) serves as the energy input, partially absorbed to drive water evaporation (Qevap). However, heat losses are an inevitable part of the process encompassing heat conduction (Qcond), heat convection (Qconv), and radiation (Qrad). The energy equilibrium during evaporation can be expressed by Eq. (1)55,56:
To ensure an efficient solar-steam conversion, it is essential to maximize Qsun while simultaneously minimizing Qcond, Qconv, and Qrad, thereby obtaining a larger Qevap. High-performance SDIWE systems typically need to satisfy the following requirements37,38,57,58: The absorber should minimize reflection and transmission of incident sunlight, ensuring high sunlight absorption. Moreover, absorbed light should be effectively converted into thermal energy to enhance both light absorption and photothermal conversion capabilities. During the evaporation process, maintaining a continuous and abundant water supply, efficient steam dissipation, material durability, and effective thermal insulation is also crucial. To minimize the convective energy loss of bulk water, there is usually insulation material such as foam between the MOFs and the water body59.
Photothermal conversion mechanisms
To optimize the utilization and conversion of solar energy into heat, photothermal materials should be engineered to absorb light efficiently across a broad spectrum, specifically within the wavelength range of 200-2500 nm. Various materials have been investigated for SDIWE applications, which possess different photothermal mechanisms57,60,61.
In carbon-based materials, an abundance of conjugated π bonds facilitates the excitation of electrons across a broad spectrum of solar light wavelengths, leading to various π-π* transitions that contribute to their characteristic black appearance. Upon illumination, these materials facilitate the transition of light-absorbing electrons from the ground state (HOMO) to a higher energy orbital (LUMO), as illustrated in Fig. 3a. Subsequently, the excited electrons undergo relaxation via electron-phonon coupling, wherein the absorbed light energy is transferred from the excited electrons to the vibrational mode of the entire atomic lattice. This process releases heat in the form of thermal vibration, resulting in a macroscopic temperature increase of the material62.
a Carbon-based materials. b Semiconductors. c Plasmonic metal nanomaterials. Reprinted with permission from ref. 120. (Copyright 2019 Royal Society of Chemistry).
The photothermal properties of semiconductors stem from the production of electron-hole pairs when exposed to light58. Under light exposure, electrons undergo transitions to the conduction band, forming electron-hole pairs. Subsequently, these photo-induced electrons and holes relax to the bottom of the conduction band and the top of the valence band before recombination, releasing excess energy in the form of heat. The energy difference between the conduction and valence band is crucial for photothermal conversion, and regulating the bandgap through doping or disordering is a common method to enhance light absorption and increase carrier relaxation rates. (Fig. 3b)
Meanwhile, plasmonic metal nanomaterials, including Au, Ag, Pd, Al, Cu, Ni, and In nanoparticles, represent another category of photothermal materials23,63,64,65. These materials exhibit a localized surface plasmon resonance (LSPR) phenomenon when the incident photon frequency matches the vibration frequency of the free electrons on their surface. The absorbed energy is released as heat, producing a photothermal effect. Unlike carbon-based materials, these metal nanomaterials have a narrow absorption range, requiring careful regulation of morphology, size, composition, medium, and assembly form to effectively collect incident sunlight. (Fig. 3c).
Solar-vapor efficiency
A general metric used to characterize SDIWE performance is the solar-vapor efficiency (ηs-v, %), which can be defined using the following equation:
where \(\dot{m}\) is the water evaporation rate that removes the evaporation rate under dark conditions, hLV represents the total vaporization enthalpy, Copt is the optical concentration, and qi denotes the nominal direct solar irradiation of 1 kW m–2 66,67,68,69.
hLV can be calculated with Eq. (3), where T refers to the average temperature of the SDIWE surface during evaporation70.
For 3D evaporators, the efficiency can be calculated by the following equations:
where Ein is the energy input of the incident light, Q is the sensible heat of water, c is the specific heat of water (4.2 J kg −1), T1 is the initial temperature of water71.
Stability of MOFs
Researching and developing highly stable MOFs structures is crucial for their applications in SDIWE-based solar-powered desalination. Stable MOFs structures can resist the corrosion caused by salt and other harsh conditions, ensuring the reliability and efficiency of the materials during long-term use. One key criterion for determining whether MOFs structure remains stable in water stability tests is comparing the typical chemical characteristics of exposed samples with those of the original samples. These chemical characteristics include powder X-ray diffraction patterns and BET surface area based on gas adsorption, which effectively indicate whether MOFs lose crystallinity or structural porosity after exposure to a watery environment72,73. MOFs structures with high stability typically require good thermodynamic and kinetic stability to prevent detrimental hydrolysis reactions of metal-ligand bonds51. In terms of thermodynamic stability, an essential structural feature is the presence of inert metal clusters that make them less prone to irreversible hydrolysis reactions. MOFs exhibit higher thermodynamic stability when the bond strength between metal and ligand and the stability of the metal center towards water are high. Kinetic stability relies on the presence of a higher activation energy barrier (Ea), which depends not only on the state of the reactants and products but also on the specific reaction pathway and transition states involved. By introducing hydrophobic groups on ligands, water molecules approaching the metal center can be prevented, thereby inhibiting hydrolysis reactions. Additionally, high coordination numbers of metals lead to steric crowding effects, preventing the formation of water clusters near the metal center. For example, Omary and colleagues developed a series of fluorinated MOFs that exhibit superhydrophobicity and significant water stability74,75.
To achieve solar-driven interfacial water evaporation, the active materials have to be processed into freestanding forms instead of being dispersed in solutions.
Stability of evaporators
The stability of MOFs structures plays a significant role in ensuring the stability of evaporators in seawater desalination applications. Additionally, the long-term resistance to salt also reflects the stability of the evaporator. In the process of SDIWE-based desalination, excessive salt deposition on the surface of the evaporator not only causes severe reflection of incident sunlight but also blocks water transport channels, leading to insufficient replenishment of water within the evaporator76. This significantly reduces the photothermal conversion efficiency and evaporation rate, thereby limiting the lifespan of the evaporator. Therefore, maintaining the stability of the evaporator is crucial, and a series of measures should be taken to effectively prevent salt deposition and ensure its long-term operation for efficient seawater treatment, which will be further discussed in Section 4.
Functions of MOFs in SDIWE
For SDIWE, MOFs typically possess the following functions: 1) Light absorption and conversion: The key to converting water into steam is to efficiently absorb sunlight and transform it to heat energy. By structurally tuning MOFs, for instance, through the introduction of organic groups or cocatalysts, their light absorption and charge separation efficiency can be enhanced, thereby improving the efficiency of water evaporation59. 2) Water transport: MOFs have a highly ordered porous structure and a large surface area, allowing water molecules to freely transport within them. Inside MOFs, water molecules can move through the pores and be subjected to heat energy during transport, facilitating the evaporation of water molecules from seawater. This ordered porous structure also helps to reduce the transport resistance of water molecules, enhancing the efficiency of seawater desalination. 3) Adsorption: The cavities, geometric shapes, and specific adsorption sites of MOFs enable them to remove heavy metal ions or organic pollutants through surface adsorption. By adjusting the pore size of MOFs, the surface chemistry, and the selection of functional groups, efficient adsorption and removal of different types of pollutants can be achieved77.
Materials in SDIWE
Materials design
Developing photothermal materials with high spectral absorption across the entire solar spectrum is the first step in designing high-performance SDIWE devices. Therefore, it is crucial for photothermal materials to efficiently absorb incident sunlight while minimizing light reflection and scattering. Although most MOFs exhibit poor photothermal performance, there are still a few MOFs that demonstrate excellent photothermal properties. Cu-CAT-1 is an example of a MOFs that exhibits broad light absorption characteristics. The ligand 2,3,6,7,10,11-Triphenylenehexol (HHTP) (Fig. 4a) has a large π-conjugation plane, allowing it to form coordination compounds with Cu2+ by complexing neighboring substituted hydroxyl groups. This conjugate system contributes to a high extinction coefficient, enabling the capture of sunlight across the full spectrum. Researchers have utilized Cu-CAT-1 as a basis for developing various materials. For instance, Ma et al. designed a hierarchical structure using Cu-CAT-1 as the foundational building block78. Initially, Cu(OH)2 nanowires were grown on the surface of Cu wires, which served as a foundation for synthesizing Cu-CAT-1 MOF nanorods (Fig. 4b). The resulting Cu-CAT-1 Metal-Organic Hierarchical Structures (MHSs) achieved superior light absorption of over 97.6% within the range of solar radiation. The device made by MHS was also tested in oil-contaminated water to evaluate its performance in oil-contaminated water. Figure 4c shows that MHS also maintains a high evaporation rate about 1.2 kg m−2 h−1 in both layered and emulsified oil, indicating its excellent anti-oil-fouling ability. Besides, real seawater desalination experiments were performed under natural sunlight conditions. The results presented that after evaporation, the concentration of metal ions and organic contaminations in the water is significantly reduced compared to the initial concentration, as Fig. 4d shows. Similarly, Wang et al. also proposed a double-sided evaporator from constructing Cu-CAT-1 on copper foam (Fig. 4e)79. The generated Cu–Cu(OH)2-MOF has pine needle-like hierarchical structure, which not only enables the incident light to be fully absorbed, but also facilitates the water supply. The bifacial evaporator achieves an evaporation rate as high as 3.27 kg m−2 h−1 under one sun illumination, significantly surpassing that of pure water and single-facial evaporators. Furthermore, when cooled at a flow rate of 5.8 m s−1 in seawater below ambient temperature, its evaporation rate reaches a remarkable 11.58 kg m−2 h−1, offering broad application prospects, as Fig. 4f shows.
a Structural formula of HHTP. b Schematic illustration of preparation of MHS. c Time-dependent mass change of water using the device fabricated by MHS strip in clean water, oil layer-covered water, and oil-in-water emulsion under 1 sun illumination. d Comparison of the concentrations of ions and organics in real seawater and the evaporated water. Reprinted with permission from ref. 78. (Copyright 2019 John Wiley and Sons). e Cu–Cu(OH)2-MOF foam with pine needle-like hierarchical structures was fabricated using a simple two-step solvent method. f Evaporation rates of single-face and biface-75° evaporators when coupled with air flows of 0, 1, 2, 3, 4, 5, and 5.8 m s–1. Reprinted with permission from ref. 79. (Copyright 2022 American Chemical Society).
More designs employ MOFs as template scaffolds to composite with photothermal materials, such as carbides and polymers, to obtain MOFs-based composites with high-efficiency photothermal conversion. For instance, Han et al. fabricated a nanohybrid known as zeolitic imidazolate framework-isolated graphene (G@ZIF) by applying a ZIF layer onto graphene (Fig. 5a). The G@ZIF film achieved an impressive solar-to-vapor conversion efficiency of nearly 96%. This exceptional performance is attributed to the insulating properties of the ZIF layer and the nanocavity at the G@ZIF interface80. Through the application of the G@ZIF-based photothermal steam evaporator, the process of desalinating seawater has been successfully carried out. The condensed water obtained from this process exhibits a significantly reduced salt content compared to the original seawater, as clearly depicted in Fig. 5b. Moreover, Li et al. developed a copper-BTC MOFs [Cu2(OH)(BTC)(H2O)]n·2H2O (CBA) nanorod arrays coated by polyaniline (CBAP) membrane comprising Cu-BTC complex nanorod arrays coated with polyaniline (PANI) on a porous polyvinylidene fluoride (PVDF) substrate (Fig. 5c). Through extended polymerization time, broadband adsorption and enhanced surface temperature were observed. This is because PANI is an excellent photothermal material, owing to its mechanism of molecular thermal vibration. Furthermore, when compared with PVDF and CBA membranes, the CBAP membrane demonstrates the highest evaporation rate of 1.442 kg m−2 h−1 under identical conditions (Fig. 5d). This outstanding performance establishes CBAP as an ideal material for solar-driven desalination81. Furthermore, Wang et al. prepared a Cu-MOF photothermal textile (CMPT) by immobilizing Cu-MOF on a commercial textile using a sputtered Cu film as a bonding layer (Fig. 5e). The hierarchical structure of CMPT effectively reduces light diffuse reflectance, resulting in an impressive light absorption rate of 95.9% across the solar irradiation range82.
a Scheme depicting the structure of G@ZIF. b Salinities of simulated seawater samples before and after desalination. Reprinted with permission from ref. 80. (Copyright 2021 John Wiley and Sons). c Scheme depicting the formation of CBAP membrane. d Mass change of water over time of CBA, CBAP, PVDF membranes, and without any absorbers under 1 sun illumination, respectively. Reprinted with permission from ref. 81. (Copyright 2021 John Wiley and Sons). e Scheme depicting the formation of CMPT. Reprinted with permission from ref. 82. (Copyright 2021 Elsevier).
Another design approach is fabricating MOFs-derived materials. When MOFs undergo pyrolysis, they can acquire properties resembling graphene or carbon nanotubes, or form metal nanoparticles. Through this method, the composite materials exhibit molecular thermal vibration effects similar to carbon-based materials or LSPR effects of metal nanoparticles, which enhances the photothermal effect. In the study by Chen et al., porous carbon materials derived from Cu-benzenedicarboxylate MOFs (Cu-BDC) (Fig. 6a) exhibited varying photothermal conversion efficiencies depending on the pyrolysis temperature, denoted as CBC-T. The sample pyrolyzed at 500 °C demonstrated the highest efficiency of 64.42%. As the pyrolysis temperature increased, an increase in surface defects hindered heat transfer and decreased the photothermal conversion efficiency. When the CBC-500 content in the evaporation device varies from 0 to 2 wt%, there is an observed increase in light absorption (Fig. 6b). This indicates that the inclusion of CBC-500 enhances the device’s ability to absorb light and convert it into heat. Furthermore, despite the change in CBC-500 content, the evaporation rate remains consistently high, suggesting a stable and reliable stability of the system (Fig. 6c)83. He et al. developed a solar evaporator that utilized a surface absorber made from Ni(dca)2pyz-derived porous carbon combined with a wood substrate (SUC-x@W) (Fig. 6d). Under pyrolysis temperature at 700 °C, SUC-700@W displayed not only excellent broad-spectrum light absorption and a high absorptivity, but also a high evaporation rate. This remarkable performance can be attributed to the sea urchin-like morphology of MOFs, which generates hierarchical porous structures capable of enhancing light trapping. Additionally, the abundant channels within the wood substrate facilitate efficient water transport84. Furthermore, Jiang et al. fabricated a plasmonic nanostructure known as Bi-C/CF through the calcination of Bi-MOF/CF. The Bi-C/CF structure exhibited a remarkable broad absorption spectrum and achieved a high absorption efficiency of 92.9% owing to the LSPR effect generated by Bi nanoparticles. Additionally, the presence of a porous structure within the carbon fibers facilitated the multi-scattering of incident light, further promoting the comprehensive utilization of light in the system. With the synergistic effect of plasma Bi nanoparticles, carbon nanosheets, and carbon fiber, the Bi-C/CF demonstrates an evaporation rate of 1.50 kg m−2 h−1, together with an outstanding evaporation efficiency of 91.9%. These results surpass the performance of most previously reported plasmonic metal-based evaporation systems85.
a Schematic illustration of the preparation of the photothermal evaporator CBC-T with polydimethylsiloxane as a substrate. b UV–Vis absorbance spectra of the evaporation device with different Cu-BDC-500 contents from 0% to 2%. c The corresponding efficiency for the solar-to-vapor generation of the evaporation with different masses of CBC-500 loading under 1 sun. Reprinted with permission from ref. 83. (Copyright 2021 Royal Society of Chemistry). d Scheme for the preparation of SUC-x@W from Ni-MOF and wood for interfacial solar evaporation. Reprinted with permission from ref. 84. (Copyright 2021 Elsevier).
Updated Performance
Table 1 encapsulates the advancements in the performance of MOFs-based materials for SDIWE based desalination in recent years, encompassing their light absorption capacity, evaporation rate, and efficiency. The data in the table reveal that the majority of MOFs-based evaporators exhibit light absorption rates exceeding 90%, thus harnessing solar energy effectively. Overall, materials that are composites or derivatives display higher rates of evaporation and greater efficiency compared to their pure MOF counterparts. Moreover, the amount of materials designed for these composites and derivatives far exceeds that of pure MOFs, suggesting that desalination utilizing these advanced materials is poised to become the predominant trend moving forward.
Design of SDIWE systems
Structuring the evaporators
The excellent performance of the SDIWE system also depends on the structure design of the evaporators. Macroscopically, the evaporators can be divided into zero-dimensional (0D), 2D and 3D according to different dimensions. The 0D materials, when directly dispersed in water, undergoes evaporation through bulk heating of the water, resulting in a lower efficiency compared to the 2D evaporation achieved through interfacial heating, Fig. 7a. The 2D evaporator relies on evaporation in flat materials floating on water. To achieve SDIWE, the active materials must be processed into free-standing forms floating on the water’s surface to further constitute the SDIWE system. Common strategies include growing directly on a substrate (such as carbon felt, bamboo, etc.)85,86, coating the surface of the substrate80,81,87, or preparing it as a gel88. An important feature of this evaporator is that the projected area along the normal incidence direction is equal to the evaporation area, so as to confine heat in the evaporation surface58. However, a conventional 2D planar SDIWE device has reached its performance limit48. Hence, in recent years, there has been an increasement in research on 3D evaporators. The 3D topography of the evaporator’s surface expands the evaporation area and enhances light capture (Fig. 7b), thereby further enhancing the performance of SDIWE systems. Ma et al. developed a paper-based Cu-CAT-1 MOF nanorod/gelatin (PCG) membrane and constructed evaporators featuring both 2D and 3D structures utilizing it. The 3D evaporators include two devices: a 45° positioned PCG strip and an umbrella-shaped PCG membrane (Fig. 7c). The evaporation rate of these two is 2.15 kg m−2 h−1 and 2.5 kg m−2 h−1, respectively, which are superior to that of 2D evaporator’s 1.51 kg m−2 h−1 under identical conditions (Fig. 7d)55. Therefore, the optimization of structure has a significant impact on the improvement of evaporator performance.
a Evaporation rate and energy efficiency of different 2D and 3D evaporators. Reprinted with permission from ref. 59. (Copyright 2023 John Wiley and Sons). b Methods to reduce reflection loss by multiple reflections with a cone-shaped absorber. Reprinted with permission from ref. 55. (Copyright 2021 Royal Society of Chemistry). c Schematic diagram of self-made SDIWE devices; d Time-dependent mass change curves of the PCG membrane in different SDIWE devices in pure water. Reprinted with permission from ref. 116. (Copyright 2021 Royal Society of Chemistry).
Anti-salt accumulation
Salt deposition poses a significant challenge in the application of SDIWE devices for seawater desalination. Typically, photothermal materials come into direct contact with saltwater and convert solar energy into heat to facilitate water evaporation at the interface. However, the rapid evaporation leads to a sharp increase in local ion concentration, eventually reaching supersaturation and resulting in crystallization. Salt deposition has several detrimental effects on the performance of the device. It can block internal channels within the porous evaporator, hindering water transmission. Additionally, the presence of salt on the surface of photothermal materials enhances sunlight reflection, thereby diminishing the evaporation performance of the device and potentially causing irreversible damage. To ensure long-term, efficient, and stable operation of evaporation devices, it is crucial to prevent salt deposition during the desalination process. Traditional methods to resolve this issue involve mechanical removal techniques such as hand washing, brushing, or ultrasonic treatment89,90,91,92,93. However, these approaches interrupt continuous device operation, increase operating costs, reduce production efficiency, and may even damage the photothermal materials. Furthermore, they place higher demands on the durability and mechanical properties of the photothermal materials. In practical applications, there is an urgent need to design new photothermal materials with anti-fouling properties or develop evaporation devices with built-in mechanisms to prevent salt deposition. Such advancements would greatly enhance the performance and longevity of SDIWE devices, ensuring their effectiveness in seawater desalination operations.
Salt ion diffused reflux
The rapid evaporation of water leads to an increase in local salt ion concentration, causing supersaturation and subsequent salt precipitation. To prevent the crystallization and precipitation of salt, it is essential to maintain an unsaturated salt concentration at the evaporation interface94. The current mechanism of salt ion diffusion can be categorized into two distinct processes: daytime reflux and nighttime reflux95. Daytime reflux can be achieved through the design of a super-hydrophilic channel, which ensures sufficient water supply and prevents salt ion saturation at the photothermal evaporation interface96. These evaporation devices typically consist of two components: a hydrophilic photothermal layer on top and a super-hydrophilic porous layer at the bottom. The presence of a super-hydrophilic porous layer plays a vital role in diluting the high concentration of salt ions locally and directing them back into the water body. Most units also incorporate an adiabatic foam layer, which not only provides buoyancy to the photothermal layer but also minimizes heat loss. For example, Ma et al. designed a unique MOFs-based bilayer film structure consisting of a multi-level porous carbon film derived from MOF/single-walled carbon nanotube (SWCNT) and a polystyrene sulfonate-modified PSS@HKUST-1 MOF/SWCNT (Fig. 8a). In the HKUST-1 MOFs-based bilayer film structure, the upper layer of hierarchical porous carbon matrix film possesses excellent sunlight absorption capability due to its layered multi-level pore structure. The presence of SWCNTs within the hierarchical porous carbon membrane (HPCM) enhances its anisotropic thermal conductivity by penetrating and intertwining with the porous carbon polyhedra, enabling efficient heat utilization for in-situ water evaporation. Additionally, the lower layer of PSS-modified hybrid material film has a high porosity and abundant hydrophilic channels, facilitating rapid water transport while selectively retarding the diffusion of salt ions97. This effectively prevents salt deposition on the sunlight absorption layer and ensures the longevity of the device.
a The bilayer structure of HPCM. Reprinted with permission from ref. 97. (Copyright 2019 John Wiley and Sons) b The structure of the J-MOF boat. Reprinted with permission from ref. 101. (Copyright 2023 American Chemical Society). c The working principle of salt extraction of the MAS. Reprinted with permission from ref. 102. (Copyright 2021 Elsevier).
As for night diffused reflux, researchers found that when the evaporation device is in the dark, water evaporation nearly stops, causing any salt crystals or concentrated salt ions that have precipitated at the photothermal interface to dissolve, diffuse, and return to the water body. This helps equalize the concentration gradient of salt ions. Additionally, due to the different densities of high-salt and low-salt solutions, gravity accelerates the diffusion and reflux of salt ions at the evaporation interface. Furthermore, the higher porosity and better hydrophilicity of photothermal materials are also conducive to the nocturnal transportation of salt. Therefore, salt deposition can be alleviated by a nighttime diffused reflux for a certain time. He et al. successfully fabricated a double-layer solar evaporator coated with MOF-derived porous carbon on wood SUC-700@W. The micro-channels present in the wood, combined with the porous structure of the carbon material, enable the complete dissolution of precipitated salt within 100 min when kept in the dark after continuous irradiation for 3 hours84. This process allows the salt to return to the water body.
Block salt ion transport
Hydrophobic materials not only have the ability to naturally float at the water-air interface but also act as a barrier against the penetration of saltwater, thereby preventing the accumulation of salt in pores and on the surface. While this hydrophobic film successfully inhibits salt deposition, it simultaneously hinders the infiltration of both water and salt ions, resulting in a significant reduction in the water evaporation rate. In recent years, the field of membrane materials and separation has witnessed a new research direction with the advent of Janus membranes. These membranes exhibit an asymmetric structure and possess distinct properties on both sides of their surface, offering a multitude of functionalities98,99,100. Notably, Janus membranes have found practical applications in seawater desalination. For instance, Selvam et al. developed a Janus-MOF boat with a dual-layered structure101. The upper layer features a phase-induced Ni-doped HKUST-1 (as-2 (Cal)) coated polymethyl methacrylate surface, while the lower layer consists of a hydrophobic agarose gel layer (Fig. 8b). The hydrophobic porous upper surface exhibits the ability to effectively absorb sunlight, harnessing its energy to facilitate water evaporation. Furthermore, it serves as a channel for the generated steam to escape. On the other hand, the hydrophilic lower surface maintains direct contact with the water body, ensuring a continuous supply of water onto the upper surface. Notably, this hydrophilic layer mimics the natural behavior of mangroves, allowing capillary action to repel salt ions and prevent their entry into the hydrophilic layer through the boat’s porous framework. By effectively trapping salt ions at the interface between the hydrophilic and hydrophobic layers, Selvam et al. successfully prevent salt deposition within the hydrophilic layer. This innovative approach has promising implications for practical applications in solar-driven water evaporation systems.
Salt deposition location regulation
Even though salt deposition on the surface of photothermal evaporation devices can affect their performance and service life. Salt in seawater is a valuable mineral resource. By adjusting the position of salt deposition, it is possible to separate the region where salt accumulates from the photothermal evaporation surface. This not only solves the problem of salt deposition while allowing for the extraction and but also collection of salt from seawater. Ma et al. introduced an innovative MOFs-based solar interface evaporator, MAS, which incorporates a Cu(TCNQ) nanorod array102. The MAS system enables the simultaneous production of clean water and salt from seawater (Fig. 8c). Through precise device design, the salt concentration can be controlled along the flow direction gradient. This approach effectively separates the salt crystals from the evaporation zone, allowing for salt extraction even in the absence of solar radiation and prolonging the lifespan of the evaporator. Remarkably, the extraction rate reached an impressive 47.0 g m−2 h−1. Moreover, the MAS system achieved zero liquid emissions, making it environmentally friendly.
Anti-bacteria
The microorganisms present in the water can also impact the lifetime of the evaporators. Therefore, it is necessary to develop SDIWE systems with antibacterial property. Common antibacterial mechanisms include: physical interactions between MOFs materials and bacteria; the release of metal ions or organic ligands with antibacterial effects from the crystal structure; and the loading of antibiotics into the gas phase. By adjusting the size, the pore size, and the coordination environment of the active sites on MOFs, as well as by synthesizing MOF-based composites, the antibacterial properties can be improved103. Hayes et al. have innovatively developed a nanocomposite foam system Ag/PANI/GO@MIL-125, Ag-PMG, incorporating plasmonic Ag and polyaniline, thereby enhancing the material’s desalination performance and solar-thermal conversion efficiency. The study demonstrated that Ag-PMG exhibits notable antibacterial photoactivity against B. subtilis and E. coli. Specifically, reduced graphene oxide (rGO) demonstrated antibacterial properties attributed to hydrophobic interactions with membrane phospholipids. The synergistic electrostatic adsorption between Ag and polyaniline molecules and bacterial cells, combined with the bactericidal action of silver nanoparticles, resulted in high antibacterial activity against both Gram-positive and Gram-negative bacteria. This highlights the significant enhancement of antibacterial activity in samples containing rGO, PANI, and Ag104.
Multifunctional systems
In recent years, a new research direction aims to develop a multi-functional evaporation device that extends the application scenarios of SDIWE technology beyond seawater desalination. During the process of SDIWE, various forms of mass or energy transfer and transformation occur. This technology not only converts impure water sources like seawater and sewage into clean water but also enables the conversion of solar energy into different forms such as thermal energy, chemical energy, electrical energy, and mechanical energy. The collection and storage of these energies hold potential value105,106. Apart from desalination, this section explores other application scenarios, including sewage treatment, electricity generation, and so on.
Sewage treatment
With the continuous discharge of domestic sewage, industrial wastewater, and agricultural runoff, a wide range of pollutants, from traditional heavy metals and dyes to emerging micro-pollutants, are increasingly contaminating groundwater and seawater. Recently, MOFs have gained significant attention for their promising application in pollutant degradation, with mechanisms primarily involving catalysis, adsorption, and photocatalysis107. In the process of catalytic degradation, the metal ions within MOFs act as active sites, effectively interacting with organic dye molecules108. Additionally, MOFs can exhibit excellent adsorption properties through electrostatic interactions, acid-base neutralization, the formation of hydrogen bonds, and the utilization of unsaturated coordination, and synergies with catalysis109,110. In photocatalytic processes, catalysts under light irradiation can generate highly reactive radicals such as ·OH and ·O2−, which can effectively decompose organic dyes into inorganic ions111. The advantages of MOFs lie in their tunable pore structures, significant specific surface areas, and immense potential for functionalization. These attributes allow MOFs to outperform other traditional pollutant removal technology in terms of recyclability, selectivity, and adsorption capacity. Moreover, MOFs can be further optimized for functionality through post-synthetic modifications to meet specific pollutants degradation needs112. Given this, integrating adsorption water purification function into SDIWE technology enables the evaporation device to be utilized for sewage treatment. For instance, Guo et al. developed hybrid hydrogel evaporators (HHEs) by in-situ co-gelation of polyvinyl alcohol and konjac glucomannan (KGM), incorporating carbonized Fe-MOF nanoparticles as solar-absorbing additives (Fig. 9a). The presence of biomass KGM enhances the hydration ability of hydrogel for water activation, while the hydroxyl groups provided by KGM form hydrogen and chelating bonds to aid in pollutant removal (Fig. 9b). Additionally, the application of magnet-assisted fabrication enhances the heat localization of the Fe-MOF absorber. Consequently, the HHEs show potential for desalination, heavy metal ion removal, and wastewater purification processes88. Similarly, Zhu et al. prepared a hybrid ZIF-8/CNTs@PCMVIMBr-20 (ZCP-20) film, where ZIF-8 acts as a water transmission channel, integrated with self-crosslinking poly (1-cyanomethyl-3-vinylimidazolium bromide) (PCMVIMBr) and CNTs to enable efficient solar absorption. The ZCP-20 film demonstrated effective sewage purification capabilities. After evaporation, no peaks of Methylene Blue (MB) and Rhodamine B (RhB) were observed in water contaminated with organic dyes (Fig. 9c). When applied to oil-water emulsions, the presence of oil droplets in the condensate was nearly imperceptible (Fig. 9d-f). Additionally, the evaporation rate of ZCP-20 remained consistently above 2.0 kg m−2 h−1 in various pH solutions, suggesting that ZCP-20 exhibits high performance in both seawater desalination and lake water treatment113. Moreover, Chen et al. synthesized a composite evaporator MOF@B-C by in-situ composition of bamboo and Fe-Ni-MOF-74, followed by a carbonization process. Both Fe-Ni-MOF-74 and bamboo possess hydrophilic properties, facilitating rapid water transfer86. The bimetallic coordination structure, combined with carbonization, contributes to the photothermal effect. The 3D layered network structure offers an expanded contact space and more adsorption sites for metal ions, leading to a substantial reduction in the concentration of heavy metal ions upon adsorption. The MOF@B-C evaporator demonstrates its potential applications in desalination and wastewater treatment, effectively addressing challenges related to heavy metal ions and dyes.
a Fe-based MOF-derived absorbers are located at the top of the evaporator to convert solar energy to heat and in situ generate purified vapor. b Heavy metal ions and organic dyes can be removed via simultaneous adsorption and solar distillation using HHEs. Reprinted with permission from ref. 88. (Copyright 2019 John Wiley and Sons). c The UV–Vis spectra and the colors comparison (inset) of RhB or MB-polluted water and freshwater. d Tyndall effect of oil/water emulsion. Optical images of (e) the oil/water emulsion and (f) freshwater collected from the emulsion. Reprinted with permission from ref. 113. (Copyright 2023 Royal Society of Chemistry).
Electricity generation
From an energy conversion perspective, the SDIWE process serves as an efficient technology for collecting solar energy and converting it into heat, which is stored in the form of water114. However, a significant amount of energy is lost between the solar input and the collected condensate. Making rational use of solar energy during the water evaporation process presents opportunities to address the scarcity of freshwater resources and energy shortages. Li et al. fabricated a polyaniline-coated MOFs nanorod arrays CBAP membrane, which can achieve seawater desalination and electricity generation simultaneously (Fig. 10a). Electricity is generated through the pyroelectric effect of the PVDF membrane. The dynamic thermomechanical response induced by water vapor enables the collection of piezo-pyroelectric voltage, reaching a maximum open-circuit voltage of 0.7 V81. In another study, Zhu et al. designed a solar evaporator that employs wood as the substrate, incorporating Cu-CAT MOF for enhanced performance. The maximum open circuit voltage was also 0.7 V, generated through temperature fluctuations in PVDF membranes. By placing a PVDF membrane on the evaporator surface, sustained electricity output is achieved. As water droplets evaporate, the PVDF temperature decreases, initiating a heating-cooling cycle that generates current flow in the opposite direction115. Ma et al. prepared a flexible Cu-CAT-1 MOF nanorod array membrane (PCG) (Fig. 10b), which exhibits exceptional solar desalination performance and reliable all-weather electricity generation. The electricity output of the device relies on two key factors: the capillary channels within the nanomaterials, which possess a high zeta potential and form electrical double layers, and the highly hydrophilic nature of the device that ensures continuous water flow. Figure 10c illustrates the I–V curve of the evaporator based on PCG membranes under both light and dark conditions. Notably, the power generated by this device array at night is sufficient to illuminate the LED, indicating its favorable power generation performance during nighttime as well116.
a Mechanism of evaporation-driven electricity generated by the CBAP membrane. Reprinted with permission from ref. 81. (Copyright 2021 John Wiley and Sons). b Schematic diagram of the self-made SDIWE device integrating solar desalination and evaporation-induced electricity generation. c I–V curves of the device array under dark conditions or 1 sun irradiation. Reprinted with permission from ref. 116. (Copyright 2021 Royal Society of Chemistry).
Others
Mechanical actuation
Interestingly, the process of converting solar energy into mechanical energy can be carried out simultaneously with solar-powered desalination. For example, Zhou et al prepared a film comprised hybrid nanofibers of MOFs layered on cellulose (MC film), which exhibit high solar thermal energy conversion efficiency and low coefficient of heat expansion at the same time. When exposed to light, the thermal expansion discrepancy between the MC film and polyimide layer initiates dynamic expansion and contraction within the bilayer. This phenomenon propels the deformation of the double-layer structure, facilitating the conversion of solar energy into mechanical energy. Such an innovation opens up new possibilities for solar-powered artificial muscles, boasting a specific power output 2.5 times higher than that of human muscles. The potential applications extend to fields such as mechanical automation and biomedical engineering, among others117.
Outlook
MOFs, as a class of porous crystalline materials characterized by their high specific surface area, considerable porosity, intricate structures, and multifaceted functions, stand out as a promising candidate for solar-powered desalination. This review delves into the design concepts of MOFs-based materials, which can be synergistically enhanced through the integration with photothermal materials or through carbonization processes to augment their light absorption and photothermal conversion capabilities. Additionally, three effective methods for preventing salt deposition are introduced, thereby extending the durability of evaporators. Furthermore, we overviewed the multifunctional applications of materials beyond seawater desalination, including sewage treatment and electricity generation etc. This review will help to draw more attention and research interests, leading to greater innovation and breakthroughs in the field of MOFs-based materials for solar-powered desalination. Despite certain achievements and prospects in this field, there are still several issues that need to be addressed in practical applications:
1. At the current stage, the production cost of MOFs is still relatively high, which limits their application in large-scale desalination. Although certain companies, such as BASF and Decco118, have achieved the commercialization of specific MOFs, the challenge of large-scale production at a low cost remains the primary obstacle to their industrialization. Consequently, the current research focus is on finding low-cost alternatives or increasing yield to indirectly save costs. Furthermore, the widespread use of organic reagents is bound to pose a threat to the environment and human health, making the exploration of environmentally friendly materials an aspect that cannot be overlooked119. In the future, with the improvement of production techniques and the expansion of production scale, the cost of MOFs materials is expected to be further reduced, thereby expanding their application in the field of SDIWE.
2. Significant research efforts are currently being directed towards the development of high-performance 3D evaporators, yet the application of MOFs-based materials in this field is still relatively rare. 3D evaporators offer a larger surface area for evaporation, and by precisely controlling their shape, it is possible to enhance the utilization of light energy and improve evaporation performance. In the future, this field is expected to receive greater attention and research.
3. Various other applications and technologies related to SDIWE are emerging and merit further investigation, including electro-assisted solar evaporation technology. By harnessing the Joule thermal effect of the conductive MOFs, a synergistic performance boost can be achieved when combined with photothermal methods. This approach not only enables all-weather evaporation but also mitigates the impact of insufficient weather or light conditions. It has the potential to transcend current limitations on evaporation rates and efficiency. Furthermore, this technology can be integrated with renewable energy sources like wind and hydropower to provide auxiliary power for evaporation process, thereby promoting sustainable development87.
References
Bernauer, T. & Böhmelt, T. International conflict and cooperation over freshwater resources. Nat. Sustain. 3, 350–356 (2020).
Goh, P. S. & Ismail, A. F. A review on inorganic membranes for desalination and wastewater treatment. Desalination 434, 60–80 (2018).
Kunjaram, U. P. U. et al. A self-salt-cleaning architecture in cold vapor generation system for hypersaline brines. EcoMat 4, e12168 (2022).
Wang, X. et al. Construction of a three-dimensional interpenetrating network sponge for high-efficiency and cavity-enhanced solar-driven wastewater treatment. ACS Appl. Mater. Interfaces 13, 10902–10915 (2021).
Wang, H. et al. Comprehensive analysis of a hybrid FO-NF-RO process for seawater desalination: With an NF-like FO membrane. Desalination 515, 115203 (2021).
Cai, Y., Wu, J., Shi, S. Q., Li, J. & Kim, K.-H. Advances in desalination technology and its environmental and economic assessment. J. Clean. Prod. 397, 136498 (2023).
Amy, G. et al. Membrane-based seawater desalination: present and future prospects. Desalination 401, 16–21 (2017).
Deshmukh, A. et al. Membrane distillation at the water-energy nexus: limits, opportunities, and challenges. Energy Environ. Sci. 11, 1177–1196 (2018).
Fritzmann, C., Löwenberg, J., Wintgens, T. & Melin, T. State-of-the-art of reverse osmosis desalination. Desalination 216, 1–76 (2007).
Tareemi, A. A. & Sharshir, S. W. A state-of-art overview of multi-stage flash desalination and water treatment: Principles, challenges, and heat recovery in hybrid systems. Sol. Energy 266, 112157 (2023).
Elsaid, K. et al. Environmental impact of desalination technologies: a review. Sci. Total Environ. 748, 141528 (2020).
Al-Sahali, M. & Ettouney, H. Developments in thermal desalination processes: design, energy, and costing aspects. Desalination 214, 227–240 (2007).
Menon, A. K., Haechler, I., Kaur, S., Lubner, S. & Prasher, R. S. Enhanced solar evaporation using a photo-thermal umbrella for wastewater management. Nat. Sustain. 3, 144–151 (2020).
Zhang, Y. & Tan, S. C. Best practices for solar water production technologies. Nat. Sustain. 5, 554–556 (2022).
Sun, Z. et al. Plasmon based double-Layer hydrogel device for a highly efficient solar vapor generation. Adv. Funct. Mater. 29, 1901312 (2019).
Wilson, H. M., Rahman, A. R. S., Parab, A. E. & Jha, N. Ultra-low cost cotton based solar evaporation device for seawater desalination and waste water purification to produce drinkable water. Desalination 456, 85–96 (2019).
Zhang, Y., Nandakumar, D. K. & Tan, S. C. Digestion of ambient humidity for energy generation. Joule 4, 2532–2536 (2020).
Shang, W. & Deng, T. Solar steam generation: Steam by thermal concentration. Nat. Energy 1, 16133 (2016).
Liu, G., Xu, J. & Wang, K. Solar water evaporation by black photothermal sheets. Nano Energy 41, 269–284 (2017).
Tao, P. et al. Solar-driven interfacial evaporation. Nat. Energy 3, 1031–1041 (2018). An overview of the development and applications of the solar-driven interfacial evaporation systems, and measurements to increase efficiency.
Ma, C., Wang, W., Jia, Z., Zhang, J. & Wang, C. Recent progress on emerging porous materials for solar-driven interfacial water evaporation. Energy Technol. 11, 2300263 (2023).
Zhu, M. et al. Plasmonic wood for high-efficiency solar steam generation. Adv. Energy Mater. 8, 1701028 (2018).
Zhou, L. et al. 3D self-assembly of aluminium nanoparticles for plasmon-enhanced solar desalination. Nat. Photonics 10, 393–398 (2016).
Huang, S. et al. Multifunctional molybdenum oxide for solar-driven water evaporation and charged dyes adsorption. Appl. Surf. Sci. 491, 328–334 (2019).
Wu, X. et al. A flexible photothermal cotton-CuS nanocage-agarose aerogel towards portable solar steam generation. Nano Energy 56, 708–715 (2019).
Liu, H. et al. Narrow bandgap semiconductor decorated wood membrane for high-efficiency solar-assisted water purification. J. Mater. Chem. A 6, 18839–18846 (2018).
Xu, Y. et al. All-in-one polymer sponge composite 3D evaporators for simultaneous high-flux solar-thermal desalination and electricity generation. Nano Energy 93, 106882 (2022).
Li, C. et al. Scalable and robust bilayer polymer foams for highly efficient and stable solar desalination. Nano Energy 60, 841–849 (2019).
Wang, Y. et al. Improved light-harvesting and thermal management for efficient solar-driven water evaporation using 3D photothermal cones. J. Mater. Chem. A 6, 9874–9881 (2018).
Hou, Y., Wang, M., Chen, X. & Hou, X. Continuous water-water hydrogen bonding network across the rim of carbon nanotubes facilitating water transport for desalination. Nano Res. 14, 2171–2178 (2021).
Zhuang, P. et al. Free-standing reduced graphene oxide (rGO) membrane for salt-rejecting solar desalination via size effect. Nanophotonics 9, 4601–4608 (2020).
Li, L. et al. GO/CNT-silica Janus nanofibrous membrane for solar-driven interfacial steam generation and desalination. J. Taiwan Inst. Chem. Eng. 111, 191–197 (2020).
Yang, H. C. et al. Chinese Ink: A powerful photothermal material for solar steam generation. Adv. Mater. Interfaces 6, 1801252 (2019).
Ma, S., Qarony, W., Hossain, M. I., Yip, C. T. & Tsang, Y. H. Metal-organic framework derived porous carbon of light trapping structures for efficient solar steam generation. Sol. Energy Mater. Sol. Cells 196, 36–42 (2019).
Zheng, L., Han, S., Liu, H., Yu, P. & Fang, X. Hierarchical MoS2 nanosheet@TiO2 nanotube array composites with enhanced photocatalytic and photocurrent performances. Small 12, 1527–1536 (2016).
Ibrahim, I., Seo, D. H., McDonagh, A. M., Shon, H. K. & Tijing, L. Semiconductor photothermal materials enabling efficient solar steam generation toward desalination and wastewater treatment. Desalination 500, 114853 (2021).
Zhu, L., Gao, M., Peh, C. K. N., Wang, X. & Ho, G. W. Self-contained monolithic carbon sponges for solar-driven interfacial water evaporation distillation and electricity generation. Adv. Energy Mater. 8, 1702149 (2018).
Hu, X. et al. Tailoring graphene oxide-based aerogels for efficient solar steam generation under one sun. Adv. Mater. 29, 1604031 (2017).
Ni, G. et al. Steam generation under one sun enabled by a floating structure with thermal concentration. Nat. Energy 1, 16126 (2016).
Zhao, F. et al. Highly efficient solar vapour generation via hierarchically nanostructured gels. Nat. Nanotechnol. 13, 489–495 (2018).
He, F., Wu, X., Gao, J. & Wang, Z. Solar-driven interfacial evaporation toward clean water production: burgeoning materials, concepts and technologies. J. Mater. Chem. A 9, 27121–27139 (2021).
Chen, G. et al. Biradical-featured stable organic-small-molecule photothermal materials for highly efficient solar-driven water evaporation. Adv. Mater. 32, 1908537 (2020).
Tian, S. et al. Manipulating interfacial charge-transfer absorption of cocrystal absorber for efficient solar seawater desalination and water purification. ACS Energy Lett. 5, 2698–2705 (2020).
Ma, X. et al. A photothermal and Fenton active MOF-based membrane for high-efficiency solar water evaporation and clean water production. J. Mater. Chem. A 8, 22728–22735 (2020).
Su, J. et al. Persistent radical tetrathiafulvalene-based 2D metal-organic frameworks and their application in efficient photothermal conversion. Angew. Chem. Inter. Ed. 60, 4789–4795 (2021).
He, F. et al. A simple, mild and versatile method for preparation of photothermal woods toward highly efficient solar steam generation. Nano Energy 71, 104650 (2020).
Wang, Z. et al. Versatile coating with multifunctional performance for solar steam generation. Nano Energy 74, 104886 (2020).
Zhang, M. et al. A platinum-based photothermal polymer with intermolecular/ligand-to-ligand charge transfer for efficient and sustainable solar-powered desalination. J. Mater. Chem. A 12, 9055–9065 (2024).
Abdullah, N., Yusof, N., Ismail, A. F. & Lau, W. J. Insights into metal-organic frameworks-integrated membranes for desalination process: a review. Desalination 500, 114867 (2021).
Xuan, W., Zhu, C., Liu, Y. & Cui, Y. Mesoporous metal-organic framework materials. Chem. Soc. Rev. 41, 1677–1695 (2012).
Canivet, J., Fateeva, A., Guo, Y., Coasne, B. & Farrusseng, D. Water adsorption in MOFs: fundamentals and applications. Chem. Soc. Rev. 43, 5594–5617 (2014).
Dang, S., Zhu, Q. L. & Xu, Q. Nanomaterials derived from metal-organic frameworks. Nat. Rev. Mater. 3, 17075 (2017).
Zuo, L. et al. Polydopamine modified ZIF-L/sodium alginate composites as the highly efficient photothermal membrane for solar steam generation. Sep. Purif. Technol. 308, 122859 (2023).
Bai, H. et al. Waste-treating-waste: Upcycling discarded polyester into metal-organic framework nanorod for synergistic interfacial solar evaporation and sulfate-based advanced oxidation process. Chem. Eng. J. 456, 140994 (2023).
Xia, Y. et al. Rational designs of interfacial-heating solar-thermal desalination devices: recent progress and remaining challenges. J. Mater. Chem. A 9, 6612–6633 (2021).
Wang, P. Emerging investigator series: the rise of nano-enabled photothermal materials for water evaporation and clean water production by sunlight. Environ. Sci. Nano 5, 1078–1089 (2018).
Pang, Y. et al. Solar-thermal water evaporation: A review. ACS Energy Lett. 5, 437–456 (2020).
Zhu, L., Gao, M., Peh, C. K. N. & Ho, G. W. Solar-driven photothermal nanostructured materials designs and prerequisites for evaporation and catalysis applications. Mater. Horiz. 5, 323–343 (2018). An overview of photothermal conversion mechanism and structure design for photothermal materials.
Wang, H., Zhang, C. & Du, A. Smart strategies for light and thermal management in high-efficiency solar steam generation. Sol. RRL 7, 2201128 (2023). An overview of light and thermal management strategies to improve evaporation efficiency based on structural design.
Guo, Y. et al. Hydrogels and hydrogel-derived materials for energy and water sustainability. Chem. Rev. 120, 7642–7707 (2020).
Min, X., Zhu, B., Li, B., Li, J. & Zhu, J. Interfacial solar vapor generation: materials and structural design. Acc. Mater. 2, 198–209 (2021).
Vélez-Cordero, J. R. & Hernández-Cordero, J. Heat generation and conduction in PDMS-carbon nanoparticle membranes irradiated with optical fibers. Int. J. Therm. Sci. 96, 12–22 (2015).
Zhou, L. et al. Self-assembly of highly efficient, broadband plasmonic absorbers for solar steam generation. Sci. Adv. 2, e1501227 (2016).
Zhou, L. et al. Self-assembled spectrum selective plasmonic absorbers with tunable bandwidth for solar energy conversion. Nano Energy 32, 195–200 (2017).
Bae, K. et al. Flexible thin-film black gold membranes with ultrabroadband plasmonic nanofocusing for efficient solar vapour generation. Nat. Commun. 6, 10103 (2015).
Yang, J. et al. Functionalized graphene enables highly efficient solar thermal steam generation. ACS Nano 11, 5510–5518 (2017).
Chen, C. et al. Highly flexible and efficient solar steam generation device. Adv. Mater. 29, 1701756 (2017).
Cao, S., Thomas, A. & Li, C. Emerging materials for interfacial solar-driven water purification. Angew. Chem. Inter. Ed. 62, e202214391 (2023). An overview of the basic concepts and principles for solar evaporation systems and on the development of novel photothermal materials.
Li, X. et al. Measuring conversion efficiency of solar vapor generation. Joule 3, 1798–1803 (2019).
Ivan, M. N. A. S. et al. Progress in interfacial solar steam generation using low-dimensional and biomass-derived materials. Nano Energy 120, 109176 (2024).
Shao, B. et al. Stackable nickel-cobalt@polydopamine nanosheet based photothermal sponges for highly efficient solar steam generation. J. Mater. Chem. A 8, 11665–11673 (2020).
Kang, I. J., Khan, N. A., Haque, E. & Jhung, S. H. Chemical and thermal stability of isotypic metal-organic frameworks: effect of metal ions. Chem. Eur. J. 17, 6437–6442 (2011).
Burtch, N. C., Jasuja, H. & Walton, K. S. Water stability and adsorption in metal-organic frameworks. Chem. Rev. 114, 10575–10612 (2014).
Yang, C. et al. Fluorous metal-organic frameworks with superior adsorption and hydrophobic properties toward oil spill cleanup and hydrocarbon storage. J. Am. Chem. Soc. 133, 18094–18097 (2011).
Nijem, N. et al. Water cluster confinement and methane adsorption in the hydrophobic cavities of a fluorinated metal-organic framework. J. Am. Chem. Soc. 135, 12615–12626 (2013).
Morciano, M., Fasano, M., Boriskina, S. V., Chiavazzo, E. & Asinari, P. Solar passive distiller with high productivity and Marangoni effect-driven salt rejection. Energy Environ. Sci. 13, 3646–3655 (2020).
Abdollahi, N., Moussavi, G. & Giannakis, S. A review of heavy metals’ removal from aqueous Matrices By Metal-organic Frameworks (MOFs): state-of-the art and recent advances. J. Environ. Chem. Eng. 10, 107394 (2022).
Ma, Q. et al. MOF-based hierarchical structures for solar-thermal clean water production. Adv. Mater. 31, 1808249 (2019). The first article on the application of MOFs-based materials in solar-driven water evaporation.
Wang, H. et al. Over 11 kg m–2 h–1 evaporation rate achieved by cooling metal–organic framework foam with pine needle-like hierarchical structures to subambient temperature. ACS Appl. Mater. Interfaces 14, 10257–10266 (2022).
Han, X. et al. Intensifying heat using MOF-isolated graphene for solar-driven seawater desalination at 98% solar-to-thermal efficiency. Adv. Funct. Mater. 31, 2008904 (2021).
Li, Z. et al. Polyaniline-coated MOFs nanorod arrays for efficient evaporation-driven electricity generation and solar steam desalination. Adv. Sci. 8, 2004552 (2021).
Wang, J. et al. A salt-free superhydrophilic metal-organic framework photothermal textile for portable and efficient solar evaporator. Sol. Energy Mater. Sol. Cells 231, 111329 (2021).
Chen, G. et al. Cu-based MOF-derived porous carbon with highly efficient photothermal conversion performance for solar steam evaporation. J. Mater. Chem. A 9, 16805–16813 (2021).
He, P. et al. Controllable synthesis of sea urchin-like carbon from metal-organic frameworks for advanced solar vapor generators. Chem. Eng. J. 423, 130268 (2021).
Jiang, J. et al. Bi-MOF-derived plasmonic Bi-C on carbon felt for efficient solar evaporation, water purification and salt-resistant desalination. Desalination 560, 116680 (2023).
Chen, Y. et al. A Fe-Ni-MOF-74@bamboo photothermal evaporator for efficient solar steam generation. Desalination 570, 117091 (2024).
Qian, Y., Xue, G., Chen, L., Xu, G. & Wang, G. E. Conductive metal-organic framework nanosheets constructed hierarchical water transport biological channel for high-performance interfacial seawater evaporation. Adv. Mater. 36, 2310795 (2023).
Guo, Y. et al. Biomass-derived hybrid hydrogel evaporators for cost-effective solar water purification. Adv. Mater. 32, 1907061 (2020).
Kou, H. et al. Recyclable CNT-coupled cotton fabrics for low-cost and efficient desalination of seawater under sunlight. Desalination 462, 29–38 (2019).
Zhu, B. et al. Flexible and washable CNT-embedded PAN nonwoven fabrics for solar-enabled evaporation and desalination of seawater. ACS Appl. Mater. Interfaces 11, 35005–35014 (2019).
Jin, Y. et al. A highly flexible and washable nonwoven photothermal cloth for efficient and practical solar steam generation. J. Mater. Chem. A 6, 7942–7949 (2018).
Finnerty, C., Zhang, L., Sedlak, D. L., Nelson, K. L. & Mi, B. Synthetic graphene oxide leaf for solar desalination with zero liquid discharge. Environ. Sci. Technol. 51, 11701–11709 (2017).
Shi, L. et al. SiC-C Composite as a Highly stable and easily regenerable photothermal material for practical water evaporation. ACS Sustain. Chem. Eng. 6, 8192–8200 (2018).
Wu, S.-L. et al. Solar-driven evaporators for water treatment: challenges and opportunities. Environ. Sci. Water Res. Technol. 7, 24–39 (2021).
Xu, K., Wang, C., Li, Z., Wu, S. & Wang, J. Salt mitigation strategies of solar-driven interfacial desalination. Adv. Funct. Mater. 31, 2007855 (2021). An overview of mechanisms and strategies for salt mitigation on photothermal evaporation.
Wang, Y. et al. Wettable photothermal hollow fibers arrays for efficient solar-driven desalination under omnidirectional illumination without salt precipitation. Mater. Today Energy 16, 100391 (2020).
Ma, X. et al. Hierarchical porous SWCNT stringed carbon polyhedrons and PSS threaded MOF bilayer membrane for efficient solar vapor generation. Small 15, 1900354 (2019).
Li, H.-N. et al. A self-descaling Janus nanofibrous evaporator enabled by a “moving interface” for durable solar-driven desalination of hypersaline water. J. Mater. Chem. A 10, 20856–20865 (2022).
Yang, H. C. et al. Janus membranes: creating asymmetry for energy efficiency. Adv. Mater. 30, 1801495 (2018).
Yang, H. C., Hou, J., Chen, V. & Xu, Z.-K. Janus membranes: exploring duality for advanced separation. Angew. Chem. Inter. Ed. 55, 13398–13407 (2016).
Selvam, A. et al. Emergence of the Janus-MOF (J-MOF) boat as a nascent amalgamation in the arena of photothermal desalination. ACS Appl. Mater. Interfaces 15, 26918–26927 (2023).
Ma, X. et al. Orientational seawater transportation through Cu(TCNQ) nanorod arrays for efficient solar desalination and salt production. Desalination 522, 115399 (2022).
Zhang, X., Peng, F. & Wang, D. MOFs and MOF-derived materials for antibacterial application. J. Func. Biomater. 13 (2022).
Hayes, O. R. et al. Solar-driven seawater desalination via plasmonic hybrid MOF/polymer and its antibacterial activity. RSC Adv. 13, 18525–18537 (2023).
Chen, C., Kuang, Y. & Hu, L. Challenges and opportunities for solar evaporation. Joule 3, 683–718 (2019).
Zhou, X., Zhao, F., Zhang, P. & Yu, G. Solar water evaporation toward water purification and beyond. ACS Mater. Lett. 3, 1112–1129 (2021).
Wei, J. et al. Recent advances in metal organic frameworks for the catalytic degradation of organic pollutants. Collagen Leather 5, 33 (2023).
Yarahmadi, H., Salamah, S. K. & Kheimi, M. Synthesis of an efficient MOF catalyst for the degradation of OPDs using TPA derived from PET waste bottles. Sci. Rep. 13, 19136 (2023).
Lin, G. et al. A systematic review of metal organic frameworks materials for heavy metal removal: Synthesis, applications and mechanism. Chem. Eng. J. 460, 141710 (2023).
Zadehahmadi, F. et al. Removal of metals from water using MOF-based composite adsorbents. Environ. Sci. Water Res. Technol. 9, 1305–1330 (2023).
Khan, M. S. et al. A review of metal-organic framework (MOF) materials as an effective photocatalyst for degradation of organic pollutants. Nanoscale Adv. 5, 6318–6348 (2023).
He, W., Lv, D., Guan, Y. & Yu, S. Post-synthesis modification of metal-organic frameworks: synthesis, characteristics, and applications. J. Mater. Chem. A 11, 24519–24550 (2023).
Zhu, Y. et al. Composite membranes based on self-crosslinking polyelectrolyte-wrapped ZIF-8/CNT nanoparticles for solar steam evaporation. Mater. Adv. 4, 1628–1636 (2023).
Zhou, S., Qiu, Z., Strømme, M. & Xu, C. Solar-driven ionic power generation via a film of nanocellulose @ conductive metal-organic framework. Energy Environ. Sci. 14, 900–905 (2021).
Zhu, X., Li, M., Song, L., Zhang, X. F. & Yao, J. Metal organic framework enabled wood evaporator for solar-driven water purification. Sep. Purif. Technol. 281, 119912 (2022).
Ma, X. et al. Efficiently cogenerating drinkable water and electricity from seawater via flexible MOF nanorod arrays. J. Mater. Chem. A 9, 9048–9055 (2021). This paper designs 2D/3D MOFs-based evaporators for water-electricity generation from seawater.
Zhou, S., Kong, X., Strømme, M. & Xu, C. Efficient solar thermal energy conversion and utilization by a film of conductive metal-organic framework layered on nanocellulose. ACS Mater. Lett. 4, 1058–1064 (2022).
Frameworks for commercial success. Nat. Chem. 8, 987–987 (2016).
Severino, M. I., Gkaniatsou, E., Nouar, F., Pinto, M. L. & Serre, C. MOFs industrialization: a complete assessment of production costs. Faraday Discuss 231, 326–341 (2021).
Gao, M., Zhu, L., Peh, C. K. & Ho, G. W. Solar absorber material and system designs for photothermal water vaporization towards clean water and energy production. Energy Environ. Sci. 12, 841–864 (2019).
Wang, J. et al. Universal strategy to prepare a flexible photothermal absorber based on hierarchical Fe-MOF-74 toward highly efficient solar interfacial seawater desalination. ACS Appl. Mater. Interfaces 13, 45944–45956 (2021).
Wang, J., Wang, W., Mu, X., Li, Z. & Wang, C. Hexagonal cluster Mn-MOF nanoflowers with super-hydrophilic properties for efficient and continuous solar-driven clean water production. Sustain. Energy Fuels 5, 1995–2002 (2021).
Zhao, X. et al. Photothermal-photocatalytic route of MOF-based membrane with nanosheet array structures for solar-driven water purification. Chem. Eng. J. 475, 146268 (2023).
Zhao, X., Ma, X. & Peng, X. Carbon nanofiber stringed hierarchical porous carbon polyhedrons flexible thin films for solar vapor generation. Appl. Phys. A 125, 537 (2019).
Chen, B. et al. Upcycling waste poly(ethylene terephthalate) into a porous carbon cuboid through a MOF-derived Carbonization strategy for interfacial solar-driven water-thermoelectricity cogeneration. ACS Sustain. Chem. Eng. 10, 16427–16439 (2022).
He, P. et al. Controllable synthesis of N/Co-doped carbon from metal-organic frameworks for integrated solar vapor generation and advanced oxidation processes. J. Mater. Chem. A 10, 13378–13392 (2022).
Yin, X., Zhang, Y., Xu, X. & Wang, Y. Bilayer fiber membrane electrospun from MOF derived Co3S4 and PAN for solar steam generation induced sea water desalination. J. Solid State Chem. 303, 122423 (2021).
Acknowledgements
This work was supported by the National Key R&D Program of China (2023YFB3810900).
Author information
Authors and Affiliations
Contributions
P.Z. contributed to initial literature collection, original writing, editing and reviewing; Y.H. contributed to ideation and reviewing; B.Y. contributed to ideation; J.G. contributed to initial literature collection; Z.Y. contributed to supervision, writing and reviewing, founding collection; and X.P. contributed to supervision, writing and reviewing, founding collection.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Materials thanks Jiang Gong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: John Plummer.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, P., Hu, Y., Yao, B. et al. Metal-organic frameworks for solar-driven desalination. Commun Mater 5, 92 (2024). https://doi.org/10.1038/s43246-024-00534-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43246-024-00534-z