Abstract
Metabolism is an indispensable part of T cell proliferation, activation and exhaustion, yet the metabolism of chimeric antigen receptor (CAR)-T cells remains incompletely understood. CARs are composed of extracellular domains—often single-chain variable fragments (scFvs)—that determine ligand specificity and intracellular domains that trigger signalling following antigen binding. Here, we show that CARs differing only in the scFv variously reprogramme T cell metabolism. Even without exposure to antigens, some CARs increase proliferation and nutrient uptake in T cells. Using stable isotope tracers and mass spectrometry, we observed basal metabolic fluxes through glycolysis doubling and amino acid uptake overtaking anaplerosis in CAR-T cells harbouring a rituximab scFv, unlike other similar anti-CD20 scFvs. Disparate rituximab and 14G2a-based anti-GD2 CAR-T cells are similarly hypermetabolic and channel excess nutrients to nitrogen overflow metabolism. Modest overflow metabolism of CAR-T cells and metabolic compatibility between cancer cells and CAR-T cells are identified as features of efficacious CAR-T cell therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Source data for Figs. 1–6 are provided in Supplementary Tables 1–23 and via GitHub at https://github.com/aliyalakhani/CAR-T-Cell-Metabolism.
Raw MS data and search results for phosphopeptides are available in the MassIVE public repository (project ID MSV000093637).
Raw RNA-seq data for transcriptomics are available in the National Center for Biotechnology Information Sequence Read Archive (accession no. PRJNA1085526).
The sequence and structure of the 14G2a mAb are provided through the Protein Data Bank (entry no. 4TUJ).
Source data are provided with this paper.
Code availability
The code for metabolic flux analysis, nutrient uptake measurement, by-product secretion measurement and statistical analysis is available via GitHub at https://github.com/aliyalakhani/CAR-T-Cell-Metabolism.
References
Ho, P. & Chen, Y. Y. Synthetic biology in immunotherapy and stem cell therapy engineering. in Synthetic Biology (eds Smolke, C. et al.) 349–372 (Wiley-VCH, 2018); https://doi.org/10.1002/9783527688104.ch17
Brudno, J. N. et al. T cells genetically modified to express an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J. Clin. Oncol. 36, 2267–2280 (2018).
Cohen, A. D. et al. Safety and efficacy of B-cell maturation antigen (BCMA)-specific chimeric antigen receptor T cells (CART-BCMA) with cyclophosphamide conditioning for refractory multiple myeloma (MM). Blood 130, 505 (2017).
Majzner, R. G. & Mackall, C. L. Clinical lessons learned from the first leg of the CAR T cell journey. Nat. Med. 25, 1341–1355 (2019).
Rafiq, S., Hackett, C. S. & Brentjens, R. J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 17, 147–167 (2020).
Geiger, R. et al. l-Arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 167, 829–842 (2016).
Can, E. et al. Noninvasive rapid detection of metabolic adaptation in activated human T lymphocytes by hyperpolarized 13C magnetic resonance. Sci. Rep. 10, 200 (2020).
Levine, L. S. et al. Single-cell analysis by mass cytometry reveals metabolic states of early-activated CD8+ T cells during the primary immune response. Immunity 54, 829–844 (2021).
Wang, R. et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity https://doi.org/10.1016/j.immuni.2011.09.021 (2011).
Blagih, J. et al. The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity 42, 41–54 (2015).
Ron-Harel, N. et al. Mitochondrial biogenesis and proteome remodeling promote one-carbon metabolism for T cell activation. Cell Metab. 24, 104–117 (2016).
Klein Geltink, R. I. et al. Metabolic conditioning of CD8+ effector T cells for adoptive cell therapy. Nat. Metab. 2, 703–716 (2020).
Bantug, G. R., Galluzzi, L., Kroemer, G. & Hess, C. The spectrum of T cell metabolism in health and disease. Nat. Rev. Immunol. 18, 19–34 (2018).
Vardhana, S. A. et al. Impaired mitochondrial oxidative phosphorylation limits the self-renewal of T cells exposed to persistent antigen. Nat. Immunol. 21, 1022–1033 (2020).
Liu, X. et al. Affinity-tuned ErbB2 or EGFR chimeric antigen receptor T cells exhibit an increased therapeutic index against tumors in mice. Cancer Res. 75, 3596–3607 (2015).
Lu, J. & Jiang, G. The journey of CAR-T therapy in hematological malignancies. Mol. Cancer 21, 194 (2022).
Majzner, R. G. & Mackall, C. L. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 8, 1219–1226 (2018).
Horna, P., Nowakowski, G., Endell, J. & Boxhammer, R. Comparative assessment of surface CD19 and CD20 expression on B-cell lymphomas from clinical biopsies: implications for targeted therapies. Blood 134, 5345 (2019).
Till, B. G. et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood 119, 3940–3950 (2012).
Larson, S. M. et al. CD19/CD20 bispecific chimeric antigen receptor (CAR) in naive/memory T cells for the treatment of relapsed or refractory non-Hodgkin lymphoma. Cancer Discov. 13, 580–597 (2023).
Salles, G. et al. Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience. Adv. Ther. 34, 2232–2273 (2017).
Chen, X. et al. Rational protein design yields a CD20 CAR with superior antitumor efficacy compared with CD19 CAR. Cancer Immunol. Res. https://doi.org/10.1158/2326-6066.CIR-22-0504 (2023).
Long, A. H. et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 21, 581–590 (2015).
Mössner, E. et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 115, 4393–4402 (2010).
Uchiyama, S. et al. Development of novel humanized anti-CD20 antibodies based on affinity constant and epitope. Cancer Sci. 101, 201–209 (2010).
Tanner, L. B. et al. Four key steps control glycolytic flux in mammalian cells. Cell Syst. 7, 49–62 (2018).
Hosios, A. M. et al. Amino acids rather than glucose account for the majority of cell mass in proliferating mammalian cells. Dev. Cell 36, 540–549 (2016).
Kelly, B. & Pearce, E. L. Amino assets: how amino acids support immunity. Cell Metab. 32, 154–175 (2020).
Hope, H. C. et al. Coordination of asparagine uptake and asparagine synthetase expression modulates CD8+ T cell activation. JCI Insight 6, e137761 (2021).
Ron-Harel, N. et al. T cell activation depends on extracellular alanine. Cell Rep. 28, 3011–3021 (2019).
Kaymak, I. et al. Carbon source availability drives nutrient utilization in CD8+ T cells. Cell Metab. 34, 1298–1311 (2022).
Scherer, S. et al. Pyrimidine de novo synthesis inhibition selectively blocks effector but not memory T cell development. Nat. Immunol. 24, 501–515 (2023).
Wellen, K. E. & Thompson, C. B. Cellular metabolic stress: considering how cells respond to nutrient excess. Mol. Cell 40, 323–332 (2010).
Bond, M. R. & Hanover, J. A. A little sugar goes a long way: the cell biology of O-GlcNAc. J. Cell Biol. 208, 869–880 (2015).
Butkinaree, C., Park, K. & Hart, G. W. O-linked β-N-acetylglucosamine (O-GlcNAc): extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochim. Biophys. Acta 1800, 96–106 (2010).
Swamy, M. et al. Glucose and glutamine fuel protein O-GlcNAcylation to control T cell self-renewal and malignancy. Nat. Immunol. 17, 712–720 (2016).
Bell, H. N. et al. Microenvironmental ammonia enhances T cell exhaustion in colorectal cancer. Cell Metab. 35, 134–149 (2023).
Spinelli, J. B. et al. Metabolic recycling of ammonia via glutamate dehydrogenase supports breast cancer biomass. Science 358, 941–946 (2017).
Tessem, M.-B. et al. Evaluation of lactate and alanine as metabolic biomarkers of prostate cancer using 1H HR-MAS spectroscopy of biopsy tissues. Magn. Reson. Med. 60, 510–516 (2008).
Quinn, W. J. 3rd et al. Lactate limits T cell proliferation via the NAD(H) redox state. Cell Rep. 33, 108500 (2020).
Angelin, A. et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 25, 1282–1293 (2017).
Park, J. O. et al. Near-equilibrium glycolysis supports metabolic homeostasis and energy yield. Nat. Chem. Biol. 15, 1001–1008 (2019).
Fell, D. A. Metabolic control analysis. in Metabolic Engineering: Concepts and Applications Vol. 13b (eds Nielsen, J. et al.) 171–211 (Wiley-VCH, 2021); https://doi.org/10.1002/9783527823468.ch6
Lindner, S. E., Johnson, S. M., Brown, C. E. & Wang, L. D. Chimeric antigen receptor signaling: functional consequences and design implications. Sci. Adv. 6, eaaz3223 (2020).
Moeller, M. et al. A functional role for CD28 costimulation in tumor recognition by single-chain receptor-modified T cells. Cancer Gene Ther. 11, 371–379 (2004).
Chang, Z. L. et al. Rewiring T-cell responses to soluble factors with chimeric antigen receptors. Nat. Chem. Biol. 14, 317–324 (2018).
Zhao, Y. et al. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J. Immunol. 183, 5563–5574 (2009).
Chen, J. et al. Tuning charge density of chimeric antigen receptor optimizes tonic signaling and CAR-T cell fitness. Cell Res. 33, 341–354 (2023).
Zhang, X., Zhu, L., Zhang, H., Chen, S. & Xiao, Y. CAR-T cell therapy in hematological malignancies: current opportunities and challenges. Front. Immunol. 13, 927153 (2022).
Subklewe, M., von Bergwelt-Baildon, M. & Humpe, A. Chimeric antigen receptor T cells: a race to revolutionize cancer therapy. Transfus. Med. Hemother. 46, 15–24 (2019).
Kawalekar, O. U. et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity 44, 380–390 (2016).
Hirabayashi, K. et al. Dual targeting CAR-T cells with optimal costimulation and metabolic fitness enhance antitumor activity and prevent escape in solid tumors. Nat. Cancer 2, 904–918 (2021).
Hudecek, M. et al. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol. Res 3, 125–135 (2015).
Reaves, M. L., Young, B. D., Hosios, A. M., Xu, Y.-F. & Rabinowitz, J. D. Pyrimidine homeostasis is accomplished by directed overflow metabolism. Nature 500, 237–241 (2013).
Kurmi, K. & Haigis, M. C. Nitrogen metabolism in cancer and immunity. Trends Cell Biol. 30, 408–424 (2020).
Chae, W.-J. et al. Qualitatively differential regulation of T cell activation and apoptosis by T cell receptor ζ chain ITAMs and their tyrosine residues. Int. Immunol. 16, 1225–1236 (2004).
Nicholson, I. C. et al. Construction and characterisation of a functional CD19 specific single chain Fv fragment for immunotherapy of B lineage leukaemia and lymphoma. Mol. Immunol. 34, 1157–1165 (1997).
Zettlitz, K. A. et al. ImmunoPET of malignant and normal B cells with 89Zr- and 124I-labeled obinutuzumab antibody fragments reveals differential CD20 internalization in vivo. Clin. Cancer Res. 23, 7242–7252 (2017).
Jensen, M., Tan, G., Forman, S., Wu, A. M. & Raubitschek, A. CD20 is a molecular target for scFvFc:ζ receptor redirected T cells: implications for cellular immunotherapy of CD20+ malignancy. Biol. Blood Marrow Transplant. 4, 75–83 (1998).
Madeira, F. et al. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res. 50, W276–W279 (2022).
Mitchell, D. M., Ravkov, E. V. & Williams, M. A. Distinct roles for IL-2 and IL-15 in the differentiation and survival of CD8+ effector and memory T cells. J. Immunol. 184, 6719–6730 (2010).
Van Acker, H. H. et al. Interleukin-15 enhances the proliferation, stimulatory phenotype, and antitumor effector functions of human gamma delta T cells. J. Hematol. Oncol. 9, 101 (2016).
Park, J. O. et al. Metabolite concentrations, fluxes and free energies imply efficient enzyme usage. Nat. Chem. Biol. 12, 482–489 (2016).
Wang, L. et al. Peak annotation and verification engine for untargeted LC–MS metabolomics. Anal. Chem. 91, 1838–1846 (2019).
Seitzer, P., Bennett, B. & Melamud, E. MAVEN2: an updated open-source mass spectrometry exploration platform. Metabolites 12, 684 (2022).
Antoniewicz, M. R., Kelleher, J. K. & Stephanopoulos, G. Elementary metabolite units (EMU): a novel framework for modeling isotopic distributions. Metab. Eng. 9, 68–86 (2007).
Antoniewicz, M. R., Kelleher, J. K. & Stephanopoulos, G. Determination of confidence intervals of metabolic fluxes estimated from stable isotope measurements. Metab. Eng. 8, 324–337 (2006).
Andrews, S. FastQC: a quality control tool for high throughput sequence data. www.bioinformatics.babraham.ac.uk/projects/fastqc/ (2010).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Kim, D. et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36 (2013).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Beber, M. E. et al. eQuilibrator 3.0: a database solution for thermodynamic constant estimation. Nucleic Acids Res. 50, D603–D609 (2022).
Waterhouse, A. et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303 (2018).
Hebditch, M. & Warwicker, J. Web-based display of protein surface and pH-dependent properties for assessing the developability of biotherapeutics. Sci. Rep. 9, 1969 (2019).
Paz, I., Kligun, E., Bengad, B. & Mandel-Gutfreund, Y. BindUP: a web server for non-homology-based prediction of DNA and RNA binding proteins. Nucleic Acids Res. 44, W568–W574 (2016).
Acknowledgements
We would like to thank the members of the Park laboratory, the UCLA Molecular Instrumentation Center, the UCLA Metabolomics Center and the UCLA Proteome Research Center, as well as M. Nofal, L.B. Tanner, Y. Song, A.M. Wu, M.C. Jensen, S. Rosenberg and S. Feldman. This work was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award no. R35GM143127 (J.O.P.), the National Institutes of Health Instrumentation grant no. 1S10OD016387-01, the National Institutes of Health Biotechnology Training Grant under award no. T32GM067555 (A.L.), the Alliance for Cancer Gene Therapy Young Investigator Award (Y.Y.C.), the Cancer Research Institute Lloyd J. Old STAR Award (Y.Y.C.) and the UCLA Jonsson Comprehensive Cancer Center Intramural Award (Y.Y.C. and J.O.P.).
Author information
Authors and Affiliations
Contributions
A.L. and J.O.P. designed the study and wrote the paper, with feedback from Y.Y.C. A.L., X.C., L.C.C., M.H., M.K., Y.C. and J.O.P. carried out the experiments. X.C., L.C.C., M.H. and M.K. generated CAR-T cells. A.L. measured nutrient uptake and by-product secretion rates. X.C. and M.H. measured exhaustion and activation markers. L.C.C. performed RNA-seq. X.C. conducted in vivo experiments. A.L. and Y.C. performed phosphopeptide measurements. A.L. and J.O.P. performed metabolomics and metabolic flux analysis. A.L. and J.O.P. performed integral analysis of metabolomics, transcriptomics and functional data using thermodynamic and metabolic control frameworks.
Corresponding author
Ethics declarations
Competing interests
X.C. and Y.Y.C. are inventors of a patent whose value may be affected by the publication of this work. Y.Y.C. holds several patent applications in CAR-T cell therapy. Y.Y.C. is a founder of, holds equity in and receives consulting fees from ImmPACT Bio. Y.Y.C. is a member of the scientific advisory board of and holds equity in Catamaran Bio, Notch Therapeutics, Pluto Immunotherapeutics, Prime Medicine, Sonoma Biotherapeutics and Waypoint Bio.
Peer review
Peer review information
Nature Metabolism thanks Sidi Chen, Joseph Melenhorst and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alfredo Giménez-Cassina, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
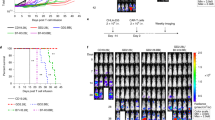
Extended Data Fig. 1 CAR-T cell transduction efficiencies, growth, and subtypes.
(a) Gating strategy for counting viable cells and quantifying their viability. Cell populations were gated for viable cells based on side-scatter area (SSC-A) vs. forward-scatter area (FSC-A) profiles. Viable cells were then gated for single cells based on FSC height (FSC-H) vs. FSC-A profiles. (b) CAR-T cells are viable up to 20 days post-retroviral transduction for donor 6. Proliferation curves of CAR-T cells from days 17 to 20 post-transduction (left) and viability of CAR-T cells on day 20 post-transduction (right) are shown. (c) Antibody staining of EGFRt (transduction marker), IgG4 extracellular spacer (Fc), and N-terminal HA tag was used to quantify transduction efficiencies and CAR surface expression by flow cytometry. The CD19 CAR lacks the CH2-CH3 domain of the IgG4 and cannot be detected via Fc staining. The CD19 CAR and Leu16 CAR lack the N-terminal HA tag. Each box shows the three quartiles (with the center line representing the median), and the whiskers extend to the minimum and maximum values that are within 1.5-fold of the interquartile range (n = 2-6 biologically independent samples). (d) CAR-T cell phenotypes for donor 2 (left) and donor 5 (right). The subtypes of CAR-T cells in in vitro culture were determined by CD4, CD8, CD45RA, and CD62L staining in the absence of antigen stimulation. (e) Growth curves of CAR-T cells generated using primary human T cells from healthy donors showed that most CAR-T cells and EGFRt control T cells grew after a complete medium change to RPMI medium supplemented with dFBS, IL-2, and IL-15 on day 0. Cell counts between day 1 and the last time point for each donor were used to calculate proliferation rates. Panels (b) and (d) show the mean ± s.e.m. with n = 3 biological replicates. Statistical significance in panel (c) was determined using two-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test to compare the pairwise differences in EGFRt, FC (anti-CD20 CAR-T cells only), and HA (rituximab, RFR-LCDR, rituximab.AA, and RFR-LCDR.AA) staining.
Extended Data Fig. 2 The metabolome of EGFRt control T cells and CAR-T cells showed CAR-dependent metabolic profiles.
To account for batch effects, each metabolomics sample was normalized to its median ion count. Within each row, yellow and blue colors indicate higher and lower levels of a metabolite compared to the row mean of the respective donor CAR-T cells.
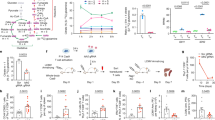
Extended Data Fig. 3 Central carbon metabolites, co-factor ratios, and metabolite secretion.
(a) Fructose-1,6-bisphosphate (FBP) levels were higher in the four rituximab-based CAR-T cells than in EGFRt control T cells. (b) TCA cycle metabolites were higher in rituximab CAR-T cells than in EGFRt control T cells. (c) ATP/ADP was significantly lower in rituximab CAR-T cells, while glutathione/glutathione disulfide (GSH/GSSG) and NADH/NAD+ ratios showed no statistical difference. (d) Higher fractions of glucose-derived carbons were secreted as lactate by the four rituximab-based CAR-T cells than by EGFRt control T cells. The lactate-to-glucose carbon flux ratio represents fermentative glycolytic activity. (e) In anti-CD19 and rituximab-based CAR-T cells, higher fractions of glutamine were diverted to glutamate secretion than in EGFRt control T cells. (f) Even in the presence of alanine in the medium, rituximab-based CAR-T cells secreted alanine at substantial rates unlike EGFRt control T cells, anti-CD19 CAR-T cells, and Leu16 anti-CD20 CAR-T cells, which consumed alanine. Each box in panels (a)-(e) shows the three quartiles (with the center line representing the median), and the whiskers extend to the minimum and maximum values that are within 1.5-fold of the interquartile range (n = 3-15 biological replicates from up to five donors). Metabolite levels in (a)-(c) are normalized to EGFRt control T cells, and the ratios are taken from the normalized metabolite levels. Statistical significance in panels (a)-(e) was determined by two-tailed t tests for a linear mixed-effects model (Methods) in reference to EGFRt control T cells (†). Panel (f) shows the mean ± s.e.m. with n = 3 biological replicates. Statistical significance in panel (f) was determined by two-tailed t test in reference to EGFRt control T cells. n.s. indicates no statistical significance.
Extended Data Fig. 4 The TCA cycle activity in CAR-T cells.
(a) [U-13C6]glucose isotope tracing shows expected labeling patterns in the TCA cycle intermediates. (b) [U-13C5]glutamine isotope tracing shows expected labeling patterns in the TCA cycle intermediates. (c) The contribution of glucose to α-ketoglutarate was lower in rituximab anti-CD20 CAR-T cells compared to that of EGFRt control T cells based on the significantly higher M + 0 fraction in the former. EGFRt T cells and CAR-T cells from donors 1 and 4 were labeled for 72 h in medium containing [U-13C6]glucose. Labeling fractions were corrected for natural isotope abundance and impurities. (d) Glutamine contribution to α-ketoglutarate was significantly higher in rituximab anti-CD20 CAR-T cells than in EGFRt control T cells. EGFRt T cells and CAR-T cells from donor 3 were labeled for 48 h in medium containing 50% [U-13C5]glutamine. Labeling fractions were corrected for natural isotope abundance and impurities. (e) The relative contributions of glucose and glutamine on a carbon basis to the TCA cycle intermediates in the CAR-T cell panel indicated that citrate was derived mainly from glucose while the downstream TCA cycle metabolites were derived mainly from glutamine. Rituximab CAR-T cells displayed higher glutamine-to-glucose contribution ratios compared to EGFRt control T cells. The carbon contributions of glucose and glutamine were obtained by measuring the fractions of the total carbons (12C + 13C) of individual metabolites that were 13C and accounting for the enrichment fractions of the respective 13C tracers (Supplementary Note 5). Panel (c) shows the mean ± s.e.m. with n = 6 biological replicates. Panel (d) shows the mean ± s.e.m. with n = 3 biological replicates. Statistical significance in panels (c) and (d) was determined by two-tailed t tests in reference to EGFRt control T cells (†) for M + 0 labeling. Panel (e) shows the mean ± propagated error, and statistical significance was determined by bootstrapping (Supplementary Note 5). n.s. indicates no statistical significance.
Extended Data Fig. 5 Tracing nitrogen and carbon reveals nucleotide turnover.
(a) EGFRt T cells and CAR-T cells from donor 4 were cultured in medium containing 50% [γ-15N]glutamine and 50% unlabeled glutamine for 72 h. Adenosine diphosphate and monophosphate were labeled more in all CAR-T cells than in EGFRt control T cells. Uridine diphosphate in anti-CD20 CAR-T cells and many of the nucleotides in rituximab CAR-T cells were significantly more labeled than those of EGFRt control T cells. (b) EGFRt T cells and CAR-T cells from donor 5 were cultured in medium containing [1,2-13C2]glucose for 48 h. Nucleotide diphosphates and monophosphates were significantly more labeled in rituximab and RFR-LCDR CAR-T cells than in EGFRt control T cells. The greater labeling fractions in the same (48- and 72-hour) time periods indicated faster nucleotide turnover in rituximab and RFR-LCDR CAR-T cells. The signals of 13C-labeled CDP, CMP, and GMP were too low to be reliable (n.d.). 13C-labeling fractions were corrected for natural isotope abundance and impurities. Plots show the mean ± s.e.m. with n = 3 biological replicates. Statistical significance was determined by two-tailed t test in reference to EGFRt control T cells (†) for M + 0 labeling. n.s. indicates no statistical significance.
Extended Data Fig. 6 Overflow metabolism and in vivo efficacy of CAR-T cells.
(a) Ammonia can be incorporated into metabolism via glutamate, glutamine, and carbamoyl phosphate. (b-d) EGFRt T cells and CAR-T cells from donor 4 were cultured in medium containing 800 µM 15NH4Cl for 72 h. (b) Incorporation of 15N from the labeled ammonia (15NH3) into glutamate was minimal in all T cells but significantly higher for anti-CD19 and rituximab-based anti-CD20 CAR-T cells compared with EGFRt control T cells. (c) Incorporation of 15N from 15NH3 into carbamoyl phosphate was minimal in all T cells but significantly lower for rituximab CAR-T cells compared with EGFRt T cells. (d) Incorporation of 15N from 15NH3 into glutamine was significantly lower for anti-CD19 and rituximab-based anti-CD20 CAR-T cells compared with EGFRt control T cells. (e) EGFRt T cells and CAR-T cells secreted thymine, uracil, and xanthine. (f-g) Raji and Ramos lymphoma cells were cultured for 48 h. (f) Proliferation rates were determined based on the changes in cell numbers between day 1 and day 2. (g) Nutrient uptake and byproduct secretion rates were compared between Raji and Ramos cells. (h) Kaplan-Meier survival curve against Ramos cancer cells. NOD/SCID/IL-2Rγnull (NSG) mice (n = 6 animals) were injected intravenously with Ramos cells and subsequently treated with control (EGFRt), anti-CD19, or anti-CD20 CAR T cells. In panels (b)-(e), plots show the mean ± s.e.m. with n = 3 biological replicates, and statistical significance was determined by two-tailed t tests in reference to EGFRt control T cells (†). Panels (f)-(g) show the mean ± s.e.m. with n = 3 biological replicates, and statistical significance was determined by two-tailed t tests. Statistical significance in panel (h) was determined by multiple log-rank (Mantel-Cox) tests with adjustment using the Benjamini-Hochberg FDR controlling procedure.
Extended Data Fig. 7 Comparison of metabolic fluxes across CAR-T cell variants.
(a) Anti-GD2 CAR-T cells were more similar to rituximab CAR-T cells than any other CAR-T cell variants in terms of nutrient uptake rates (black) and byproduct secretion rates (red) (cf. Figure 6b). SSD is the sum of squared differences, and L is the total distance between individual points and the line of unity. Plot shows the mean ± s.e.m. with n = 12 biological replicates for EGFRt control T cells and anti-CD20 CAR-T cells and n = 6 biological replicates for anti-GD2 CAR-T cells. (b) Metabolite labeling patterns in CAR-T cells, which were fed 50% [γ-15N]glutamine, [U-13C6]glucose, and [U-13C5]glutamine, showed the greatest similarity between 14G2a-based anti-GD2 CAR-T cells and rituximab CAR-T cells (cf. Figure 6c). (c-d) EGFRt T cells and CAR-T cells from donor 4 were cultured in medium containing 50% [γ-15N]glutamine for 72 h. 15N-labeled pyrimidine and purine nucleobases were measured from medium samples collected each day. Ion counts for xanthine represent the sum of M + 1 and M + 2 15N-labeled ions. (c) Rituximab anti-CD20 and 14G2a anti-GD2 CAR-T cells displayed similarly high accumulation of purine and pyrimidine nucleobases. (d) The four rituximab-based CAR-T cells had faster nucleotide degradation than EGFRt control T cells. Alanine insertions in the non-signaling intracellular domains of CARs resulted in significant differences in thymine secretion. Panels (c) and (d) show the mean ± s.e.m. with n = 3 biological replicates, and statistical significance was determined by two-tailed t tests in reference to the day-3 medium sample from EGFRt control T cells (†). Further statistical tests were conducted between rituximab and anti-GD2 CAR-T cells, rituximab and rituximab.AA CAR-T cells, and RFR-LCDR and RFR-LCDR.AA CAR-T cells for day-3 nucleobase measurements. n.s. indicates no statistical significance.
Extended Data Fig. 8 Tracing nitrogen and carbon in nucleotide and hexosamine biosynthesis in CAR-T cells with two-alanine insertions.
(a) EGFRt T cells and CAR-T cells from donor 4 were cultured in medium containing 50% [γ-15N]glutamine for 72 h. Many nucleotides were labeled more in rituximab-based CAR-T cells than in EGFRt control T cells. Alanine insertions resulted in minimal differences. (b-c) EGFRt T cells and CAR-T cells from donor 5 were cultured in medium containing [1,2-13C2]glucose for 48 h. (b) Many nucleotides were labeled more in rituximab-based CAR-T cells than in the EGFRt control T cells. Alanine insertions resulted in minimal differences. The signals of 13C-labeled CDP, CMP, and GMP were too low to be reliable (n.d.). (c) N-acetylglucosamine-1/6-phosphate (GlcNAc-1/6 P) and UDP-N-acetylglucosamine (UDP-GlcNAc) were labeled more in rituximab-based CAR-T cells than in EGFRt control T cells. Rituximab.AA and RFR-LCDR.AA CAR-T cells with alanine insertions increased M + 0 fractions of UDP-GlcNAc compared with rituximab and RFR-LCDR CAR-T cells, respectively. 13C-labeling fractions were corrected for natural isotope abundance and impurities. Plots show the mean ± s.e.m. with n = 3 biological replicates. Statistical significance of the observed M + 0 labeling fractions was determined by two-tailed t test in reference to EGFRt control T cells (†), between rituximab and rituximab.AA CAR-T cells, and between RFR-LCDR and RFR-LCDR.AA CAR-T cells. n.s. indicates no statistical significance.
Extended Data Fig. 9 CAR-T cells were cultured in the absence of antigen stimulation for RNA-seq.
Across three donors, genes associated with (a) glycolysis, (b) the pentose phosphate pathway, (c) the TCA cycle, (d) nucleotide biosynthesis, (e) hexosamine biosynthesis, (f) pyrimidine degradation, (g) purine degradation, and (h) glutamine metabolism are shown. Each row indicates a donor (donor 2, 5, or 6). Within each row, yellow and blue colors indicate higher and lower levels of the transcript compared with EGFRt control T cells. Arrow colors represent the standard Gibbs free energy of reaction ΔrG’°, which approximates the extent of metabolic flux control based on how close to or far away from equilibrium the reaction is.
Extended Data Fig. 10 Electrostatic property and phosphorylation of CARs may affect T cell activation and exhaustion.
(a) Activation- (top row) and exhaustion-marker (bottom row) expression on mutant rituximab CAR-T cells were compared to EGFRt, Leu16 CAR-, and rituximab CAR-T cells (n=6 biological replicates from two donors). (b) Phosphorylation of the signaling domains of CARs including the three ITAM regions of CD3ζ was measured (n=2 biologically independent samples). Even with good peptide sequence coverage, CD28 phosphopeptide was below the limit of detection. (c) Top three largest positively charged patches (PCPs) containing continuous positive charged residues are shown in each scFv. Dark blue, largest PCP; medium blue, second largest PCP; light blue, third largest PCP. PCP score (sum of the number of residues in the three largest PCPs) and charge per amino acid are displayed under each construct. (d) Activation- (top row) and exhaustion-marker (bottom row) expression on CAR-T cells were evaluated on day 2 or 3 without antigen stimulation (n=12-18 biological replicates from up to six donors). Donors 2-2 and 5-2 represent second batches of transduced cells from donors 2 and 5, respectively. Each box in panels (a) and (d) shows the three quartiles (with the center line representing the median), and the whiskers extend to the minimum and maximum values that are within 1.5-fold of the interquartile range. Statistical significance for activation and exhaustion markers in panels (a) and (d) was determined by two-tailed t tests for a linear mixed-effects model (Methods) in reference to EGFRt control T cells (†). Statistical significance in panel (b) was determined using two-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test to compare the pairwise differences in ITAM1, ITAM2, and ITAM3 phosphorylation.
Supplementary information
Supplementary Information
Supplementary Notes 1–5, Tables 1–23 and References.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lakhani, A., Chen, X., Chen, L.C. et al. Extracellular domains of CARs reprogramme T cell metabolism without antigen stimulation. Nat Metab (2024). https://doi.org/10.1038/s42255-024-01034-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42255-024-01034-7