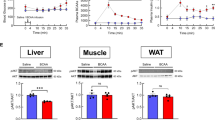
Abstract
Lactate is a circulating metabolite and a signalling molecule with pleiotropic physiological effects. Studies suggest that lactate modulates energy balance by lowering food intake, inducing adipose browning and increasing whole-body thermogenesis. Yet, like many other metabolites, lactate is often commercially produced as a counterion-bound salt and typically administered in vivo through hypertonic aqueous solutions of sodium l-lactate. Most studies have not controlled for injection osmolarity and the co-injected sodium ions. Here, we show that the anorectic and thermogenic effects of exogenous sodium l-lactate in male mice are confounded by the hypertonicity of the injected solutions. Our data reveal that this is in contrast to the antiobesity effect of orally administered disodium succinate, which is uncoupled from these confounders. Further, our studies with other counterions indicate that counterions can have confounding effects beyond lactate pharmacology. Together, these findings underscore the importance of controlling for osmotic load and counterions in metabolite research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Source data are provided with this paper.
References
He, W. et al. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature 429, 188–193 (2004).
Haas, R. et al. Intermediates of metabolism: from bystanders to signalling molecules. Trends Biochem. Sci. 41, 460–471 (2016).
Husted, A. S., Trauelsen, M., Rudenko, O., Hjorth, S. A. & Schwartz, T. W. GPCR-mediated signaling of metabolites. Cell Metab. 25, 777–796 (2017).
Ryan, D. G. et al. Coupling Krebs cycle metabolites to signalling in immunity and cancer. Nat. Metab. 1, 16–33 (2019).
Rabinowitz, J. D. & Enerback, S. Lactate: the ugly duckling of energy metabolism. Nat. Metab. 2, 566–571 (2020).
Brooks, G. A. et al. Lactate in contemporary biology: a phoenix risen. J. Physiol. 600, 1229–1251 (2022).
Brooks, G. A. The science and translation of lactate shuttle theory. Cell Metab. 27, 757–785 (2018).
Ferguson, B. S. et al. Lactate metabolism: historical context, prior misinterpretations, and current understanding. Eur. J. Appl. Physiol. 118, 691–728 (2018).
Barros, L. F. Metabolic signaling by lactate in the brain. Trends Neurosci. 36, 396–404 (2013).
Hui, S. et al. Glucose feeds the TCA cycle via circulating lactate. Nature 551, 115–118 (2017).
Magistretti, P. J. & Allaman, I. Lactate in the brain: from metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 19, 235–249 (2018).
Sun, S., Li, H., Chen, J. & Qian, Q. Lactic acid: no longer an inert and end-product of glycolysis. Physiology 32, 453–463 (2017).
Ahmed, K. et al. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metab. 11, 311–319 (2010).
Engelstoft, M. S. et al. Seven transmembrane G-protein-coupled receptor repertoire of gastric ghrelin cells. Mol. Metab. 2, 376–392 (2013).
Morland, C. et al. The lactate receptor, G-protein-coupled receptor 81/hydroxycarboxylic acid receptor 1: expression and action in brain. J. Neurosci. Res. 93, 1045–1055 (2015).
Cai, T. Q. et al. Role of GPR81 in lactate-mediated reduction of adipose lipolysis. Biochem. Biophys. Res. Commun. 377, 987–991 (2008).
Liu, C. et al. Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J. Biol. Chem. 284, 2811–2822 (2009).
Morville, T. et al. Divergent effects of resistance and endurance exercise on plasma bile acids, FGF19, and FGF21 in humans. JCI Insight https://doi.org/10.1172/jci.insight.122737 (2018).
Murphy, R. M., Watt, M. J. & Febbraio, M. A. Metabolic communication during exercise. Nat. Metab. 2, 805–816 (2020).
Morville, T., Sahl, R. E., Moritz, T., Helge, J. W. & Clemmensen, C. Plasma metabolome profiling of resistance exercise and endurance exercise in humans. Cell Rep. 33, 108554 (2020).
McCarthy, S. F., Islam, H. & Hazell, T. J. The emerging role of lactate as a mediator of exercise-induced appetite suppression. Am. J. Physiol. Endocrinol. Metab. 319, E814–E819 (2020).
Lehnig, A. C. & Stanford, K. I. Exercise-induced adaptations to white and brown adipose tissue. J. Exp. Biol. https://doi.org/10.1242/jeb.161570 (2018).
Lam, C. K., Chari, M., Wang, P. Y. & Lam, T. K. Central lactate metabolism regulates food intake. Am. J. Physiol. Endocrinol. Metab. 295, E491–E496 (2008).
Schultes, B. et al. Lactate infusion during euglycemia but not hypoglycemia reduces subsequent food intake in healthy men. Appetite 58, 818–821 (2012).
Ferrannini, E. et al. Metabolic and thermogenic effects of lactate infusion in humans. Am. J. Physiol. 265, E504–E512 (1993).
Chiolero, R. et al. Effects of infused sodium acetate, sodium lactate, and sodium beta-hydroxybutyrate on energy expenditure and substrate oxidation rates in lean humans. Am. J. Clin. Nutr. 58, 608–613 (1993).
Haesler, E., Schneiter, P., Temler, E., Jequier, E. & Tappy, L. Effects of lactate infusion on hepatic gluconeogenesis and glycogenolysis. Clin. Physiol. 15, 581–595 (1995).
Carriere, A. et al. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes 63, 3253–3265 (2014).
Jeanson, Y. et al. Lactate induces FGF21 expression in adipocytes through a p38–MAPK pathway. Biochem. J. 473, 685–692 (2016).
Park, S. S. et al. Lactobacillus amylovorus KU4 ameliorates diet-induced obesity in mice by promoting adipose browning through PPARγ signaling. Sci. Rep. 9, 20152 (2019).
Wang, H. J. et al. Adaptive thermogenesis enhances the life-threatening response to heat in mice with an Ryr1 mutation. Nat. Commun. 11, 5099 (2020).
Yao, Z. et al. Dietary lactate supplementation protects against obesity by promoting adipose browning in mice. J. Agric. Food Chem. 68, 14841–14849 (2020).
Lagarde, D. et al. Lactate fluxes mediated by the monocarboxylate transporter-1 are key determinants of the metabolic activity of beige adipocytes. J. Biol. Chem. 296, 100137 (2021).
Yan, S. et al. IRF3 reduces adipose thermogenesis via ISG15-mediated reprogramming of glycolysis. J. Clin. Invest. https://doi.org/10.1172/JCI144888 (2021).
Cai, H. et al. Moderate l-lactate administration suppresses adipose tissue macrophage M1 polarization to alleviate obesity-associated insulin resistance. J. Biol. Chem. 298, 101768 (2022).
Qu, Y. et al. The different effects of intramuscularly-injected lactate on white and brown adipose tissue in vivo. Mol. Biol. Rep. 49, 8507–8516 (2022).
Brooks, G. A. et al. Lactate as a major myokine and exerkine. Nat. Rev. Endocrinol. 18, 712 (2022).
Morland, C. et al. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat. Commun. 8, 15557 (2017).
Lambertus, M. et al. l-lactate induces neurogenesis in the mouse ventricular-subventricular zone via the lactate receptor HCA1. Acta Physiol. 231, e13587 (2021).
E, L., Lu, J., Selfridge, J. E., Burns, J. M. & Swerdlow, R. H. Lactate administration reproduces specific brain and liver exercise-related changes. J. Neurochem. 127, 91–100 (2013).
Cerda-Kohler, H. et al. Lactate administration activates the ERK1/2, mTORC1, and AMPK pathways differentially according to skeletal muscle type in mouse. Physiol. Rep. 6, e13800 (2018).
Takahashi, K., Kitaoka, Y., Matsunaga, Y. & Hatta, H. Effects of lactate administration on mitochondrial enzyme activity and monocarboxylate transporters in mouse skeletal muscle. Physiol. Rep. 7, e14224 (2019).
Takahashi, H. et al. TGF-β2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Nat. Metab. 1, 291–303 (2019).
Kitaoka, Y., Takeda, K., Tamura, Y. & Hatta, H. Lactate administration increases mRNA expression of PGC-1α and UCP3 in mouse skeletal muscle. Appl. Physiol. Nutr. Metab. 41, 695–698 (2016).
Tsukamoto, S., Shibasaki, A., Naka, A., Saito, H. & Iida, K. Lactate promotes myoblast differentiation and myotube hypertrophy via a pathway involving MyoD in vitro and enhances muscle regeneration in vivo. Int. J. Mol. Sci. https://doi.org/10.3390/ijms19113649 (2018).
Lundquist, A. J., Gallagher, T. J., Petzinger, G. M. & Jakowec, M. W. Exogenous l-lactate promotes astrocyte plasticity but is not sufficient for enhancing striatal synaptogenesis or motor behavior in mice. J. Neurosci. Res. 99, 1433–1447 (2021).
Cha, S. H. & Lane, M. D. Central lactate metabolism suppresses food intake via the hypothalamic AMP kinase/malonyl-CoA signaling pathway. Biochem. Biophys. Res. Commun. 386, 212–216 (2009).
van Hall, G. Lactate kinetics in human tissues at rest and during exercise. Acta Physiol. 199, 499–508 (2010).
Hart, B. L. Biological basis of the behavior of sick animals. Neurosci. Biobehav Rev. 12, 123–137 (1988).
Ewaschuk, J. B., Naylor, J. M. & Zello, G. A. d-lactate in human and ruminant metabolism. J. Nutr. 135, 1619–1625 (2005).
Garcia-Alvarez, M., Marik, P. & Bellomo, R. Stress hyperlactataemia: present understanding and controversy. Lancet Diabetes Endocrinol. 2, 339–347 (2014).
Ou, Z. et al. A GPR17–cAMP–lactate signaling axis in oligodendrocytes regulates whole-body metabolism. Cell Rep. 26, 2984–2997 (2019).
Halestrap, A. P. The SLC16 gene family—structure, role and regulation in health and disease. Mol. Asp. Med. 34, 337–349 (2013).
Carriere, A. et al. The emerging roles of lactate as a redox substrate and signaling molecule in adipose tissues. J. Physiol. Biochem. 76, 241–250 (2020).
Carey, A. L. et al. Reduced UCP-1 content in in vitro differentiated beige/brite adipocytes derived from preadipocytes of human subcutaneous white adipose tissues in obesity. PLoS ONE 9, e91997 (2014).
Chung, K. J. et al. A self-sustained loop of inflammation-driven inhibition of beige adipogenesis in obesity. Nat. Immunol. 18, 654–664 (2017).
Shin, W. et al. Impaired adrenergic agonist-dependent beige adipocyte induction in aged mice. Obesity 25, 417–423 (2017).
Rogers, N. H., Landa, A., Park, S. & Smith, R. G. Aging leads to a programmed loss of brown adipocytes in murine subcutaneous white adipose tissue. Aging Cell 11, 1074–1083 (2012).
Barreau, C. et al. Regionalization of browning revealed by whole subcutaneous adipose tissue imaging. Obesity 24, 1081–1089 (2016).
Cannon, B. & Nedergaard, J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J. Exp. Biol. 214, 242–253 (2011).
Vasan, S. K. et al. The proposed systemic thermogenic metabolites succinate and 12,13-diHOME are inversely associated with adiposity and related metabolic traits: evidence from a large human cross-sectional study. Diabetologia 62, 2079–2087 (2019).
Mills, E. L. et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 560, 102–106 (2018).
Liu, K. et al. Scd1 controls de novo beige fat biogenesis through succinate-dependent regulation of mitochondrial complex II. Proc. Natl Acad. Sci. USA 117, 2462–2472 (2020).
Laires, M. J., Monteiro, C. P. & Bicho, M. Role of cellular magnesium in health and human disease. Front. Biosci. 9, 262–276 (2004).
Shahzad, B., Mughal, M. N., Tanveer, M., Gupta, D. & Abbas, G. Is lithium biologically an important or toxic element to living organisms? An overview. Environ. Sci. Pollut. Res. Int. 24, 103–115 (2017).
Pedersen, B. K. Physical activity and muscle-brain crosstalk. Nat. Rev. Endocrinol. 15, 383–392 (2019).
MacCannell, A. D. & Roberts, L. D. Metabokines in the regulation of systemic energy metabolism. Curr. Opin. Pharmacol. 67, 102286 (2022).
Nalbandian, M. & Takeda, M. Lactate as a signaling molecule that regulates exercise-induced adaptations. Biology https://doi.org/10.3390/biology5040038 (2016).
Zhang, J. et al. Endothelial lactate controls muscle regeneration from ischemia by inducing M2-like macrophage polarization. Cell Metab. 31, 1136–1153 (2020).
Yang, J. et al. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc. Natl Acad. Sci. USA 111, 12228–12233 (2014).
Proia, P., Di Liegro, C. M., Schiera, G., Fricano, A. & Di Liegro, I. Lactate as a metabolite and a regulator in the central nervous system. Int. J. Mol. Sci. https://doi.org/10.3390/ijms17091450 (2016).
El Hayek, L. et al. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J. Neurosci. 39, 2369–2382 (2019).
Panksepp, J. The ventromedical hypothalamus and metabolic adjustments of feeding behavior. Behav. Biol. 9, 65–75 (1973).
Wilson, W. H. & Heller, H. C. Elevated blood glucose levels and satiety in the rat. Physiol. Behav. 15, 137–143 (1975).
Baile, C. A., Zinn, W. M. & Mayer, J. Effects of lactate and other metabolites on food intake of monkeys. Am. J. Physiol. 219, 1606–1613 (1970).
Racotta, R. & Russek, M. Food and water intake of rats after intraperitoneal and subcutaneous administration of glucose, glycerol and sodium lactate. Physiol. Behav. 18, 267–273 (1977).
Langhans, W., Damaske, U. & Scharrer, E. Different metabolites might reduce food intake by the mitochondrial generation of reducing equivalents. Appetite 6, 143–152 (1985).
Langhans, W., Egli, G. & Scharrer, E. Selective hepatic vagotomy eliminates the hypophagic effect of different metabolites. J. Auton. Nerv. Syst. 13, 255–262 (1985).
Nagase, H., Bray, G. A. & York, D. A. Effects of pyruvate and lactate on food intake in rat strains sensitive and resistant to dietary obesity. Physiol. Behav. 59, 555–560 (1996).
Silberbauer, C. J., Surina-Baumgartner, D. M., Arnold, M. & Langhans, W. Prandial lactate infusion inhibits spontaneous feeding in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278, R646–R653 (2000).
Kleinert, M. et al. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 14, 140–162 (2018).
Janowitz, H. D., Hanson, M. E. & Grossman, M. I. Effect of intravenously administered glucose on food intake in the dog. Am. J. Physiol. 156, 87–91 (1949).
Bellinger, L. L. & Williams, F. E. The effect of portal and jugular infused glucose, mannitol and saline on food intake in dogs. Physiol. Behav. 46, 693–698 (1989).
Farningham, D. A. & Whyte, C. C. The role of propionate and acetate in the control of food intake in sheep. Br. J. Nutr. 70, 37–46 (1993).
Pedersen, M. G. B. et al. Oral lactate slows gastric emptying and suppresses appetite in young males. Clin. Nutr. 41, 517–525 (2022).
Murack, M. & Messier, C. The impact of lactic acid and medium chain triglyceride on blood glucose, lactate and diurnal motor activity: a re-examination of a treatment of major depression using lactic acid. Physiol. Behav. 208, 112569 (2019).
Wu, Q. et al. Intestinal hypoxia-inducible factor 2α regulates lactate levels to shape the gut microbiome and alter thermogenesis. Cell Metab. 33, 1988–2003(2021).
Rupar, K. et al. Full activation of thermogenesis in brown adipocytes requires Basigin action. FEBS J. https://doi.org/10.1111/febs.16716 (2023).
Jeong, J. H., Chang, J. S. & Jo, Y. H. Intracellular glycolysis in brown adipose tissue is essential for optogenetically induced nonshivering thermogenesis in mice. Sci. Rep. 8, 6672 (2018).
Villarroya, J. et al. Lactate induces expression and secretion of fibroblast growth factor-21 by muscle cells. Endocrine 61, 165–168 (2018).
Hashimoto, T., Hussien, R., Oommen, S., Gohil, K. & Brooks, G. A. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J. 21, 2602–2612 (2007).
Blad, C. C., Tang, C. & Offermanns, S. G-protein-coupled receptors for energy metabolites as new therapeutic targets. Nat. Rev. Drug Discov. 11, 603–619 (2012).
Acknowledgements
We thank C. S. A. Svendsen, K. Racz, K. Stohlmann, N. Tiutcheva and the Rodent Metabolic Phenotyping Platform for experimental and technical assistance. We also thank members of the Z.G.-H. group and the C.C. group for scientific discussions. This work was supported by a research grant from the Danish Diabetes Academy, which is funded by the Novo Nordisk Foundation, grant number NNF17SA0031406 (PhD scholarship to J.L.), by a research grant from the European Foundation for the Study of Diabetes (EFSD) and Lilly European Diabetes Research Programme 2019, and by Center for Adipocyte Signaling (NNF15CC0018486 and NNF15SA0018346). M.K. is supported by the Deutsche Forschungsgemeinschaft (KL 3285/2-1) and the Novo Nordisk Foundation (NNF19OC0055192). C.C. is supported by research grants from the Lundbeck Foundation (Fellowship R238-2016-2859) and the Novo Nordisk Foundation (grant number NNF17OC0026114). This project has received funding from the European Research Council under the European Union’s Horizon 2020 Research and Innovation Programme (grant agreement no. 639382 to Z.G.-H.). The Novo Nordisk Foundation Center for Basic Metabolic Research is an independent Research Center, based at the University of Copenhagen, and partially funded by an unconditional donation from the Novo Nordisk Foundation (https://cbmr.ku.dk/; grant number NNF18CC0034900). Cartoon illustrations were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
J.L., Z.G.-H. and C.C. conceptualized the project. J.L., A.W.B., C.G., S.F., P.R.-R., C.V.M., A.L.B., O.S.J., N.F., C.L., T.S.N., A.B.K., T.M. and C.C. performed mouse studies. F.S., M.S.I. and J.L. performed cell studies. All authors contributed to data analysis and/or interpretation. J.L. drafted the manuscript. Z.G.-H. and C.C. edited the manuscript. Other authors provided comments and minor edits to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Joshua Rabinowitz, Evan Rosen and Daniela Cota for their contribution to the peer review of this work. Primary Handling Editor: Alfredo Giménez-Cassina, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Effects of Na-l-lactate on body weight and body composition.
a-e, DIO mice treated daily with injections of 2 g/kg Na-L-lactate or isotonic saline for one week while single-housed in metabolic cages (mouse study 1 in Methods). a, Change in absolute body weight during the study (isotonic saline n = 8 mice, Na-L-lactate n = 8 mice). b, Change in absolute body weight at day 7 (isotonic saline n = 8 mice, Na-L-lactate n = 8 mice). c, Fat mass before (pre = day -3) and after (post = day 7) the study (isotonic saline n = 8 mice, Na-L-lactate n = 8 mice). d, Lean mass before (pre = day -3) and after (post = day 7) the study (isotonic saline n = 8 mice, Na-L-lactate n = 8 mice). e, Absolute change in fat and lean mass at day 7 (isotonic saline n = 8 mice, Na-L-lactate n = 8 mice). Data shown as the mean ± SEM.
Extended Data Fig. 2 Effects of Na-l-lactate, iso-osmolar Na-D-lactate and iso-osmolar NaCl on body weight.
a-b, DIO mice treated daily with injections of 2 g/kg Na-L-lactate, 2 g/kg Na-D-lactate or isotonic saline for one week while pair/group-housed in standard cages (mouse study 2 in Methods). a, Change in absolute body weight during the study (isotonic saline n = 6 mice, Na-L-lactate n = 5 mice, Na-D-lactate n = 5 mice). b, Change in absolute body weight at day 7 (isotonic saline n = 6 mice, Na-L-lactate n = 5 mice, Na-D-lactate n = 5 mice). c-d, DIO mice treated once with an injection of 2 g/kg Na-L-lactate or PBS while single-housed in standard cages (mouse study 3 in Methods). c, Absolute body weight before (pre = 0 hours) and after (post = 24 hours) the injection (PBS n = 8 mice, Na-L-lactate n = 8 mice). d, Absolute change in body weight after 24 hours (PBS n = 8 mice, Na-L-lactate n = 8 mice). e-f, DIO mice treated once with an injection of NaCl (iso-osmolar to Na-L-lactate in mouse study 3) or PBS while single-housed in standard cages (mouse study 4 in Methods). e, Absolute body weight before (pre = 0 hours) and after (post = 24 hours) the injection (PBS n = 6 mice, NaCl n = 8 mice). f, Absolute change in body weight after 24 hours (PBS n = 6 mice, NaCl n = 8 mice). g, L-lactate levels in Dulbecco’s Modified Eagle Media measured by Statstrip Lactate-meter after addition of Na-L-lactate (20 mM) or Na-D-lactate (20 mM) (Na-L-lactate n = 3 independent solutions, Na-D-lactate n = 3 independent solutions). h, L-lactate levels in DMEM measured by Statstrip lactate-meter after addition of Na-L-lactate at concentrations of 5, 10, 15 and 20 mM (5 mM Na-L-lactate n = 2 independent solutions, 10 mM Na-L-lactate n = 2 independent solutions, 15 mM Na-L-lactate n = 2 independent solutions, 20 mM Na-L-lactate n = 2 independent solutions). Data shown as the mean ± SEM.
Extended Data Fig. 3 Effects of injection volume and treatment hypertonicity on food intake and body weight.
a-e, Lean mice injected with PBS at a volume of 5 or 40 µL per g body weight or isotonic saline at a volume of 40 µL per g body weight (mouse study 11 in Methods). a, Cumulative food intake during the study (5 µL/g PBS n = 8 mice, 40 µL/g PBS n = 8 mice, isotonic saline n = 8 mice). Dark phase is indicated by grey-shaded area. b, Total food intake after 24 hours (5 µL/g PBS n = 8 mice, 40 µL/g PBS n = 8 mice, isotonic saline n = 8 mice). c, Percentage change in body weight after 24 hours (5 µL/g PBS n = 8 mice, 40 µL/g PBS n = 8 mice, isotonic saline n = 8 mice). d, Change in absolute body weight after 24 hours (5 µL/g PBS n = 8 mice, 40 µL/g PBS n = 8 mice, isotonic saline n = 8 mice). e, Body weight before (pre = 0 hour) and after (post = 24 hours) the injections (5 µL/g PBS n = 8 mice, 40 µL/g PBS n = 8 mice, isotonic saline n = 8 mice). f-i, DIO mice treated daily for 21 days with injections of 1 g/kg Na-L-lactate or PBS (mouse study 12 in Methods). f, Percentage change in body weight during the study (PBS n = 8 mice, Na-L-lactate n = 8 mice). g, Percentage change in body weight at day 21 (PBS n = 8 mice, Na-L-lactate n = 8 mice, PBS versus Na-L-lactate p = 0.0676). h, Change in absolute body weight during the study (PBS n = 8 mice, Na-L-lactate n = 8 mice). i, Change in absolute body weight at day 21 (PBS n = 8 mice, Na-L-lactate n = 8 mice). j-k, DIO mice treated daily for 16 days with injections of 1 g/kg Na-L-lactate, iso-osmolar NaCl or PBS (mouse study 9 in Methods). j, Change in absolute body weight during the study (PBS n = 8 mice, Na-L-lactate n = 12 mice, NaCl n = 8 mice). k, Change in absolute body weight at day 16 (PBS n = 8 mice, Na-L-lactate n = 12 mice, NaCl n = 8 mice). l-m, DIO mice treated daily for 14 days with injections of 1 g/kg Na-L-lactate, iso-osmolar D-mannitol or PBS (mouse study 10 in Methods). l, Change in absolute body weight during the study (PBS n = 5 mice, Na-L-lactate n = 5 mice, D-mannitol n = 5 mice). m, Change in absolute body weight at day 14 (PBS n = 5 mice, Na-L-lactate n = 5 mice, D-mannitol n = 5 mice). n-q, DIO mice treated daily for 14 days with injections of 1 g/kg Na-L-lactate, iso-osmolar D-mannitol or PBS (mouse study 13 in Methods). n, Percentage change in body weight during the study (PBS n = 9 mice, Na-L-lactate n = 11 mice, D-mannitol n = 11 mice). o, Percentage change in body weight at day 14 (PBS n = 9 mice, Na-L-lactate n = 11 mice, D-mannitol n = 11 mice). p, Change in absolute body weight during the study (PBS n = 9 mice, Na-L-lactate n = 11 mice, D-mannitol n = 11 mice). q, Change in absolute body weight at day 14 (PBS n = 9 mice, Na-L-lactate n = 11 mice, D-mannitol n = 11 mice). r, Plasma osmolarity in lean mice in response to injection of 1 g/kg Na-L-lactate (mouse study 14, pilot experiment, in Methods) (0 min n = 2 mice, 8 min n = 3 mice, 14 min n = 3 mice, 25 min n = 3 mice, 35 min n = 3 mice, 45 min n = 2 mice). s, Plasma osmolarity in lean mice measured 25 minutes after sham injections or injections with PBS, 1 g/kg Na-L-lactate, iso-osmolar NaCl or iso-osmolar D-mannitol (mouse study 14, main experiment, in Methods) (sham n = 6 mice, PBS n = 8 mice, Na-L-lactate n = 8 mice, NaCl n = 8 mice, D-mannitol n = 8 mice, sham versus Na-L-lactate p = 0.0031, sham versus NaCl p = 0.0025, sham versus D-mannitol p = 0.0001, PBS versus Na-L-lactate p = 0.0007, PBS versus NaCl p = 0.0005, PBS versus D-mannitol p < 0.0001). t-u, DIO mice treated daily for 21 days with injections of 1 g/kg Na-L-lactate or PBS (mouse study 12 in Methods). t, Cumulative food intake during the study (PBS n = 4 cages, Na-L-lactate n = 4 cages). u, Total food intake after 21 days (PBS n = 4 cages, Na-L-lactate n = 4 cages). v-w, DIO mice treated daily for 14 days with injections of 1 g/kg Na-L-lactate, iso-osmolar D-mannitol or PBS (mouse study 13 in Methods). v, Cumulative food intake during the study (PBS n = 9 mice, Na-L-lactate n = 11 mice, D-mannitol n = 11 mice). w, Total food intake after 14 days (PBS n = 9 mice, Na-L-lactate n = 11 mice, D-mannitol n = 11 mice). Data were analysed by one-way ANOVA with a Bonferroni post hoc test (b-d, o, s and w) and unpaired two-tailed t-test (g and u). Data shown as the mean ± SEM; ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Extended Data Fig. 4 Food intake after central infusion of Na-l-lactate.
a, Cumulative food intake in lean mice after intracerebroventricular (ICV) administration of 10 nmoles of Na-L-lactate or an identical volume of isotonic saline (mouse study 15, experiment 2, in Methods) (isotonic saline n = 4 mice, Na-L-lactate n = 4 mice, treatment p = 0.9395, time x treatment p = 0.9828). Dark phase is indicated by grey-shaded area. b, Cumulative food intake in lean mice after ICV administration of 500 nmoles Na-L-lactate or an identical volume of isotonic saline (mouse study 15, experiment 3, in Methods) (isotonic saline n = 3 mice, Na-L-lactate n = 3 mice, treatment p = 0.6620, time x treatment p = 0.9743). Dark phase is indicated by grey-shaded area. c, Cumulative food intake in lean mice after ICV administration of a GLP1-R agonist or an identical volume of PBS (mouse study 15, experiment 4, in Methods) (PBS n = 3 mice, GLP1-R agonist n = 3 mice, treatment p = 0.0579, time x treatment p = 0.0117). Dark phase is indicated by grey-shaded area. Data analyzed by two-way ANOVA with a Bonferroni post hoc test (a, b, and c). Data shown as the mean ± SEM.
Extended Data Fig. 5 Effect of Na-L-lactate on body weight in GPR81 knockout and wild-type mice.
a-d, DIO GPR81 knockout mice (GPR81-KO) and wild-type control mice (GPR81-WT) treated daily with injections of 2 g/kg Na-L-lactate or iso-osmolar NaCl for 15 days while single-housed in standard cages (mouse study 16 in Methods). a, Change in absolute body weight in GPR81-KO mice during the study (Na-L-lactate n = 6 mice, NaCl n = 6 mice). b, Change in absolute body weight in GPR81-KO mice after 15 days (Na-L-lactate n = 6 mice, NaCl n = 6 mice). c, Change in absolute body weight in GPR81-WT mice during the study (Na-L-lactate n = 7 mice, NaCl n = 6 mice). d, Change in absolute body weight in GPR81-WT mice after 15 days (Na-L-lactate n = 7 mice, NaCl n = 6 mice). Data shown as the mean ± SEM.
Extended Data Fig. 6 Effects of Na-l-lactate on adipose browning and thermogenesis.
a-d, Young and lean mice treated daily with injections of 2 g/kg Na-L-lactate, iso-osmolar NaCl or PBS for 9 days (mouse study 18 in Methods). a, Hematoxylin and eosin staining of inguinal white adipose tissue. Multilocular adipocytes are indicated by black arrows. Each column in the three groups correspond to one mouse (PBS n = 6 mice, Na-L-lactate n = 7 mice, NaCl n = 7 mice). b, Whole-body oxygen consumption in anaesthetized mice before and after administration of norepinephrine (NE) (PBS n = 6 mice, Na-L-lactate n = 7 mice, NaCl n = 7 mice). c, Body weight at the day of the experiment shown in Extended Data Fig. 6b (PBS n = 6 mice, Na-L-lactate n = 7 mice, NaCl n = 7 mice). d, Whole-body oxygen consumption normalized to body weight in anaesthetized mice before and after administration of NE (PBS n = 6 mice, Na-L-lactate n = 7 mice, NaCl n = 7 mice). e, Whole-body oxygen consumption in lean anaesthetized mice placed in metabolic cages and treated first with either 2 g/kg Na-L-lactate or PBS, as indicated by the left black arrow, and subsequently with NE, as indicated by the right black arrow (mouse study 19 in Methods) (PBS n = 6 mice, Na-L-lactate n = 6 mice). f, Ucp1 expression in immortalized mouse brown adipocytes (BMC line) after 24 hours of stimulation with 25, 50 and 100 mM Na-L-lactate (data from a single experiment, data points represent technical replicates). g, Ucp1 expression in immortalized mouse brown adipocytes (BMC line) after stimulation with 25, 50 and 100 mM Na-L-lactate or similar concentrations of NaCl for 24 hours (data from a single experiment, data points represent technical replicates). h, Ucp1 expression in immortalized mouse brown adipocytes (BMC line) after stimulation with 25, 50 and 100 mM Na-L-lactate or similar concentrations of NaCl for 24 hours (data from a single experiment, data points represent technical replicates). i, Ucp1 expression in immortalized mouse brown adipocytes (WT-1 line) after stimulation with 25, 50 and 100 mM Na-L-lactate for 6 hours (data from a single experiment, data points represent technical replicates). j, Ucp1 expression in immortalized mouse brown adipocytes (WT-1 line) after stimulation with 25, 50 and 100 mM Na-L-lactate or similar concentrations of NaCl for 6 hours (data from a single experiment, data points represent technical replicates). k, Ucp1 expression in immortalized mouse brown adipocytes (WT-1 line) after stimulation with 25, 50 and 100 mM Na-L-lactate or similar concentrations of NaCl for 6 hours (data from a single experiment, data points represent technical replicates). Data analyzed by one-way ANOVA with a Bonferroni post hoc test (c) and two-way ANOVA with a Bonferroni post hoc test (b, d, e). Statistical analyses on data based on technical replicates are not shown (f-k). Data shown as the mean ± SEM.
Extended Data Fig. 7 Effects of Na-l-lactate on tissue gene expression, body weight and food intake.
a-e, Gene expression in gastrocnemius muscle and liver from DIO mice treated daily for 16 days with injections of 1 g/kg Na-L-lactate, iso-osmolar NaCl or PBS (mouse study 9 in Methods). a, Expression of selected metabolic genes in gastrocnemius (PBS n = 8 mice, Na-L-lactate n = 12 mice, NaCl n = 8 mice, Mt2 PBS versus Na-L-lactate p = 0.0208, Mt2 Na-L-lactate versus NaCl p = 0.0187). b, Expression of genes involved in hepatic glycogen synthesis (PBS n = 8 mice, Na-L-lactate n = 12 mice, NaCl n = 8 mice). c, Expression of genes involved in hepatic glycogenolysis (PBS n = 8 mice, Na-L-lactate n = 12 mice, NaCl n = 8 mice). d, Expression of genes involved in hepatic glucose transport (Glut1, Glut2) and glycolysis (Hk1, Hk2, Gck, Pfkl, Pgk1, Pkm1, and Pkm2) (PBS n = 8 mice, Na-L-lactate n = 12 mice, NaCl n = 8 mice). e, Expression of genes involved in hepatic gluconeogenesis (PBS n = 8 mice, Na-L-lactate n = 12 mice, NaCl n = 8 mice, Pck1 PBS versus Na-L-lactate p = 0.0230). f-i, Changes in body weight in DIO mice treated with daily injections of 1 g/kg Na-L-lactate or PBS for one week while single-housed in metabolic cages (mouse study 20 in Methods). f, Percentage change in body weight during the study (PBS n = 8 mice, Na-L-lactate n = 8 mice). g, Percentage change in body weight at day 7 (PBS n = 8 mice, Na-L-lactate n = 8 mice, PBS versus Na-L-lactate p = 0.0008). h, Change in absolute body weight during the study (PBS n = 8 mice, Na-L-lactate n = 8 mice). i, Change in absolute body weight at day 7 (PBS n = 8 mice, Na-L-lactate n = 8 mice). j-m, Changes in body weight in DIO mice treated with daily injections of 1 g/kg Na-L-lactate or iso-osmolar NaCl for one week while single-housed in metabolic cages (mouse study 21 in Methods). j, Percentage change in body weight during the study (NaCl n = 8 mice, Na-L-lactate n = 8 mice). k, Percentage change in body weight at day 7 (NaCl n = 8 mice, Na-L-lactate n = 8 mice). l, Change in absolute body weight during the study (NaCl n = 8 mice, Na-L-lactate n = 8 mice). m, Change in absolute body weight at day 7 (NaCl n = 8 mice, Na-L-lactate n = 8 mice). n, Cumulative food intake in response to 1 g/kg Na-L-lactate or PBS (mouse study 20 in Methods) (PBS n = 8 mice, Na-L-lactate n = 8 mice). o, Total food intake after 190 hours (mouse study 20 in Methods) (PBS n = 8 mice, Na-L-lactate n = 8 mice, PBS versus Na-L-lactate p = 0.0116). p, Cumulative food intake in response to 1 g/kg Na-L-lactate or iso-osmolar NaCl (mouse study 21 in Methods) (NaCl n = 8 mice, Na-L-lactate n = 8 mice). q, Total food intake after 190 hours (mouse study 21 in Methods) (NaCl n = 8 mice, Na-L-lactate n = 7 mice). Dashed vertical lines indicate time of injection (n and p). Dark phases are indicated by grey-shaded areas (n and p). Data analyzed by two-way ANOVA with Bonferroni post hoc test (a-e) and by unpaired two-tailed t-test (g, k, o, and q). Data shown as the mean ± SEM; * p < 0.05,*** p < 0.001.
Extended Data Fig. 8 Effects of succinate and counterion salts on body weight.
a-b, Lean mice shifted from chow to high-fat diet and with ad libitum access to either tap water, tap water with NaCl, tap water with disodium succinate or tap water with disodium fumarate for 28 days (mouse study 22 in Methods). a, Change in absolute body weight during the study (Tap water n = 10 mice, NaCl n = 10 mice, disodium succinate n = 11 mice, disodium fumarate n = 11 mice). b, Change in absolute body weight at day 28 (Tap water n = 10 mice, NaCl n = 10 mice, disodium succinate n = 11 mice, disodium fumarate n = 11 mice). c-d, DIO mice treated once with an injection of 210 mg/kg MgCl2 or PBS (mouse study 23 in Methods). c, Absolute body weight before (pre = 0 hours) and after (post = 24 hours) the injection (PBS n = 6 mice, MgCl2 n = 6 mice). d, Change in absolute body weight after 24 hours (PBS n = 6 mice, MgCl2 n = 6 mice). e-f, DIO mice treated once with an injection of 60 mg/kg LiCl or PBS (mouse study 24 in Methods). e, Absolute body weight before (pre = 0 hours) and after (post = 24 hours) the injection (PBS n = 6 mice, LiCl n = 6 mice). f, Change in absolute body weight after 24 hours (PBS n = 6 mice, LiCl n = 6 mice). g-h, DIO mice treated once with an injection of 40 mg/kg CaCl2 or isotonic saline (mouse study 25 in Methods). g, Absolute body weight before (pre = 0 hours) and after (post = 24 hours) the injection (isotonic saline n = 7 mice, CaCl2 n = 7 mice). h, Change in absolute body weight after 24 hours (isotonic saline n = 7 mice, CaCl2 n = 7 mice). Data shown as the mean ± SEM.
Supplementary information
Supplementary Tables 1–3
Supplementary Table 1: Overview of representative studies. Table 2: Data on experimental solutions (inclusive theoretical osmolarities and measured osmolalities). Table 3: Primer list.
Source data
Source Data Figs. 1–8
Source data for Figs. 1–8.
Source Data Extended Data Figs. 1–8
Source data for Extended Data Figs. 1–8.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lund, J., Breum, A.W., Gil, C. et al. The anorectic and thermogenic effects of pharmacological lactate in male mice are confounded by treatment osmolarity and co-administered counterions. Nat Metab 5, 677–698 (2023). https://doi.org/10.1038/s42255-023-00780-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00780-4
This article is cited by
-
Activation of GPR81 by lactate drives tumour-induced cachexia
Nature Metabolism (2024)
-
MCT1 helps brown fat suck up succinate
Nature Metabolism (2024)
-
Astrocytic metabolic control of orexinergic activity in the lateral hypothalamus regulates sleep and wake architecture
Nature Communications (2024)
-
Type 2 diabetes and succinate: unmasking an age-old molecule
Diabetologia (2024)
-
Lactic control of tumor growth: a new role for an old metabolite
Signal Transduction and Targeted Therapy (2023)