Abstract
Major symbiotic organisms have evolved to establish beneficial relationships with hosts. However, understanding the interactions between symbionts and insect hosts, particularly for their roles in defense against pathogens, is still limited. In a previous study, we proposed that the fungus Metarhizium anisopliae can infect the brown planthopper Nilaparvata lugens, a harmful pest for rice crops. To expand on this, we investigated changes in N. lugens’ intestinal commensal community after M. anisopliae infection and identified key gut microbiotas involved. Our results showed significant alterations in gut microbiota abundance and composition at different time points following infection with M. anisopliae. Notably, certain symbionts, like Acinetobacter baumannii, exhibited significant variations in response to the fungal infection. The decrease in these symbionts had a considerable impact on the insect host’s survival. Interestingly, reintroducing A. baumannii enhanced the host’s resistance to M. anisopliae, emphasizing its role in pathogen defense. Additionally, A. baumannii stimulated host immune responses, as evidenced by increased expression of immune genes after reintroduction. Overall, our findings highlight the significance of preserving a stable gut microbial community for the survival of insects. In specific conditions, the symbiotic microorganism A. baumannii can enhance the host’s ability to resist entomopathogenic pathogens through immune regulation.
Similar content being viewed by others
Introduction
Symbionts are widely distributed in animals and plants, and they have significant effects on various physiological activities of their hosts, including growth, reproduction, and survival1,2,3. Insects, in particular, have a diverse array of symbiotic bacteria in their gut, which play a crucial role in defending against external pathogens and interacting with other organisms4. These symbiotic bacteria can affect insect-plant interactions by digesting plant polymers and providing insects with essential nutrients5,6,7, interfering with plant defenses8, degrading plant toxins9,10,11, and modulating food provisioning from plants to insects12,13,14,15. Several studies have demonstrated that certain symbiotic bacteria or their metabolites within the insect gut can defend against pathogenic bacteria16. The depletion of gut microbiota in pyrochlorella has been shown to increase host susceptibility to pathogenic bacteria17. In Tribolium castaneum (Herbst), the composition and abundance of commensal bacteria in the gut are altered after Bacillus thuringiensis infection, leading to enhanced resistance against pathogens18. However, in the case of Beauveria bassiana infection in mosquito midguts, it promotes the proliferation of the pathogenic bacterium Serratia, thereby facilitating fungal-induced mosquito mortality19. These examples demonstrate that symbionts play vital roles in insects but can exhibit different functions depending on the specific factors involved.
Insect hosts have evolved sophisticated mechanisms, including the involvement of gut bacteria, to respond to pathogens and maintain homeostasis20,21. When Drosophila is infected by pathogenic bacteria, its gut microbiota can stimulate the Imd pathway, leading to the expression of antimicrobial peptides (AMPs) and negative regulators of the Imd pathway, which improves the insect’s survival rate22. Insect symbionts are also closely associated with the development of host resistance and can enhance defense against pathogens23. Some bacteria themselves or their metabolites have been shown to degrade pesticides effectively and play a vital role in controlling insect pests24,25,26,27. Pantoea agglomerans, a symbiont in the gut of desert locusts, produces phenolics that inhibit the growth of M. anisopliae within the locusts’ bodies28. However, the specific role of certain symbionts in the host’s body can vary in response to pathogen infection29.
Insect pathogenic fungi are naturally occurring and act as microbial insecticides, invading insect pests and potentially causing epidemic diseases in their populations30. These fungi can enter the host’s body by penetrating the cuticle. They evade host immunity through hyphal bodies in the hemolymph and secrete toxins, depleting host nutrients and disrupting tissue structure, ultimately leading to the death of the host31. However, when infected by external pathogenic fungi, insects rely on their own humoral and cellular immunity to defend against the pathogens4. Additionally, the host insect can employ various processes, including the production of antibacterial substances by symbiotic bacteria16 and modulation of competition among microbial species32,33, to enhance host resistance. The aphid intracellular bacteria such as Regiella insecticola can enhance aphid resistance against the phytophthora fungus (Erynia) while suppressing fungal spore formation on aphid carcasses. This can reduce disease transmission rates among nearby aphid populations19.
Entomopathogenic fungi are great potentials for suppressing the population of insect pests, offering advantages in reducing insect resistance and promoting plant growth34,35,36,37,38,39. Several of these fungi have been developed as effective biological control agents against pests40. For example, M. anisopliae has shown efficacy in infecting N. lugens at all stages of their life cycle41. However, the potential effects of M. anisopliae on the gut microbiota of N. lugens and the interactions between symbionts and fungal infection are still unknown. Therefore, this study aimed to investigate the changes in the gut microbiota of N. lugens after time-course infection with M. anisopliae. The study also aimed to screen key bacteria involved in this process and analyze their functions, with the goal of exploring the interaction between insect pathogenic fungi and host gut microbiota. This research could offer valuable insights into the interactions between host gut symbionts and fungi, potentially leading to the development of innovative approaches for utilizing M. anisopliae and host gut symbiotic bacteria in pest control for rice crops.
Results
Gut microbiota abundance in N. lugens after M. anisopliae infection
We first examine the M. anisopliae lethal effect to N. lugens. The number of dead N. lugens in the following time was counted by spraying 1×107 conidia/mL on the surface of the body. We found that the Median Lethal Time (LT50) of N. lugens was advanced to 6.68 days compared with the control, indicating that M. anisopliae CQMa421 was effective in infecting and killing N. lugens (Fig. 1A, B, p < 0.001; t-test).
A Survival rate of N. lugens under M. anisopliae treatment. B The LT50 of N. lugens under M. anisopliae treatment. C Colony forming units (CFU) of gut bacteria in fungus-infected and uninfected gut (n = 30) at 24, 48, and 72 hpi. D The anatomy of complete gut of N. lugens (A, foregut; M, midgut; P, posterior gut; MT, marsupial tube). E Observation of gut homogenate cultures of fungal infected and uninfected N. lugens. The error bars represent the standard errors (SE) of the mean in (B) and (C). Double asterisks represent a significant difference determined by the t-test at P < 0.01.
We assessed whether fungal infection affects the homeostasis of the N. lugens gut and observed that the total bacterial loads in the gut of N. lugens (Fig. 1C). We observed a significant decrease in bacterial loads (colony forming unit, CFU) in insect infected with M. anisopliae compared to the uninfected control group treated with 0.05% Tween 80 at 24 hpi (Fig. 1D, E, p < 0.001; t-test). As the treatment time increased, the bacterial load in the treated group remained lower than the control group at 48 hpi, although the difference was not statistically significant. However, the total bacterial load was significantly higher in the treated group compared to the uninfected control at 72 hpi (Fig. 1D, E, p < 0.001; t-test). Overall, our findings indicate that the abundance of bacteria in the host gut increases with the duration of fungal infection, from 24 hpi to 72 hpi. This suggests that fungal infection may have an impact on the dynamics of the N. lugens gut microbiota over time.
Identification of gut microbiota in N. lugens after M. anisopliae infection
At the phylum level, major five phylum, Proteobacteria, Actinobacteriota, Firmicutes, Bacteroidota, and Deinococcota, were annotated from N. lugens individuals with different treatments. At the genus level, the top 5 taxa included Acinetobacter, Arsenophonus, Pantoea, Microbacterium and Achromobacter. We also investigated the richness, diversity, and evenness of the gut microbiota in N. lugens before and after M. anisopliae infection. We observed that N. lugens infected with M. anisopliae exhibited different core Amplicon Sequence Variants (ASVs) levels compared with the control, with higher levels at 48 and 72 hpi (Fig. 2A). Further, the ACE, Shannon, Simpson, Shannoneven and Simpsoneven index were collected to evaluate the richness and diversity of the gut microbiota changes of N. lugens. The richness of gut microbiota in infected N. lugens was gradually decreased with the time of infection compared with uninfected N. lugens (Fig. 2B). However, the diversity (Fig. 2C, D) and evenness level showed an increasing trend compared with the control treatment (Fig. 2E, F). These results suggest that fungal infection might alter the host gut microbiota composition and reduce bacterial diversity at initial period.
A Changes of gut microbiota ASV in different treatments of N. lugens. B The ACE index of BPH infected and uninfected with M. anisopliae. C The Shannon index of BPH infected and uninfected with M. anisopliae. D The Simpson index of N. lugens infected and uninfected with M. anisopliae. E The Shannoneven index of N. lugens infected and uninfected with M. anisopliae. F The Simpsoneven index of BPH infected and uninfected with M. anisopliae. “CK” indicates N. lugens treated with Tween-80, and “Tr” indicates N. lugens infected with M. anisopliae. The error bars represent the standard errors (SE).
Community composition analysis showed that Proteobacteria, Actinobacteriota, Bacteroidota and Firmicutes were the dominant bacteria in the intestinal tract of N. lugens, accounting for the largest proportion (>90%) (Fig. 3A). After M. anisopliae infection, these four classes of bacteria were still dominant with the increase of infection time. However, the numbers of Firmicutes and Bacteroidota increased at 72 hpi but Actinobacteriota was reduced (Fig. 3B). At the genus level, Acinetobacter, Arsenophonus, Pantoea, Microbacterium and Achromobacter were the most abundant intestinal commensal taxa in the brown planthopper. Infection of M. anisopliae resulted in Acinetobacter increased by 3.16%, while Pantoea decreased by 2.19% (Fig. 3C). Furthermore, Arsenophonus and Pantoea was higher after 72hpi than that at 24hpi and 48hpi, and the abundance of Microbacterium, Achromobacter and Wolbachia all increased with the increase of infection time (Fig. 3D).
A Community abundance at the phylum level of M. anisopliae infected and uninfected N. lugens; B Community abundance at the phylum level of N. lugens at different times with and without M. anisopliae infection; C Community abundance at the genus level of M. anisopliae infected and uninfected N. lugens; D Community abundance at the genus level of N. lugens at different times with and without M. anisopliae infection. “CK” indicates BPH treated with TWeen-80, and “Tr” indicates BPH infected with M. anisopliae.
Alterations of N. lugens gut microbial composition after M. anisopliae infection
The results indicated that there was no significant difference in the composition of the intestinal commensal flora between brown planthopper infected with M. anisopliae and those that were not infected (Fig. 4A). Moreover, our findings revealed that 29 ASVs were consistently present in the gut microbiota across different processing times (Fig. 4B). Additionally, we identified 54, 75, and 19 specific species of intestinal microbes in N. lugens at 24, 48, and 72 hpi, respectively. In the control group, we found 30, 25, and 15 specific species at the same respective time points. Totally, the infection of N. lugens showed a decrease in species number at the ASV level. In evaluation of N. lugens, both infected and non-infected with M. anisopliae, at different time points, we observed that Acinetobacter, Arsenophonus, Pantoea, Microbacterium, and Achromobacter were the predominant bacteria present. However, we did not find any significant difference in their abundance at each time point. (Figs. 4C and S1).
A Principal Coordinates Analysis (PCoA) based on Bray–Curtis dissimilarities showing the community dissimilarity for bacterial community. B ASV Venn diagram of N. lugens post M. anisopliae infection at different times. C Comparison of the difference in mean relative abundance between different groups in genus level.
Additionally, we noticed that the main functional pathways included cellular process, environmental information processing, genetic information processing, human disease, metabolism and biological system among the intestinal commensal bacteria of N. lugens (Fig. S2). The highest abundance in the metabolic pathway was observed in all six groups of samples, indicating potential impacts on the metabolic process in the body of N. lugens. After M. anisopliae infection, the process of genetic information processing fluctuated with time, suggesting that M. anisopliae could also have effects on the genetic information processing of the host N. lugens.
Potential bacteriostasis of N. lugens gut symbionts to M. anisopliae
We identified several gut bacteria that potentially play a role in mediating M. anisopliae infection in N. lugens. Several key bacterial species, namely Acinetobacter sp. (Acinetobacter baumannii), Pantoea sp., Enterobacter sp., Bacillus sp., and Microbacterium sp were isolated from these bacterial cultures based on the sequencing results. The results showed that Acinetobacter sp., Bacillus sp., and Microbacterium sp. exhibited an inhibition circle formation with M. anisopliae, and the diameter of the circle was significantly different from that of the control group (Fig. 5A; Fig. 5C, p < 0.01), indicating their potential to inhibit the growth or activity of the fungal pathogen. However, Pantoea sp. and Enterobacter sp. did not exhibit the similar interaction with M. anisopliae (Fig. 5A, C). Additionally, we characterized the appearance of Acinetobacter baumannii colonies, which the resulting colonies appeared light yellow with regular edges on the culture media (Fig. 5B).
A Co-culture of Acinetobacter sp. with M. anisopliae showed a clear ring of inhibition. B Single colony morphology of Acinetobacter sp. C Inhibition size of Acinetobacter sp. by M. anisopliae. Three biological replicates were conducted in each treatment. The error bars represent the standard errors (SE).
Effects of A. baumannii on N. lugens susceptibility
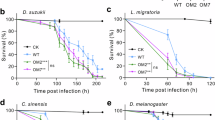
The results indicated that the survival rate of healthy brown planthoppers feeding on rice significantly decreased after treatment with M. anisopliae (Fig. 6A, C, p < 0.01; One-Way ANOVA). In order to further understand the role of intestinal commensal bacteria in host survival and defense against pathogenic fungi, we conducted antibiotic treatments without the challenge of entomopathogenic fungus (Fig. S3). The survival of N. lugens was found to significantly decrease after the antibiotic feeding when compared to those normal individuals that did not receive antibiotic treatment (Fig. 6A, B, p < 0.01; One-Way ANOVA). Additionally, the reintroduction of symbiotic bacteria A. baumannii did not enhance the survival of N. lugens (Fig. 6B), suggesting that the stability of intestinal commensal bacteria play a crucial role in maintaining the survival of N. lugens.
A Survival curves of different N. lugens strains with Tween-80 treatment. B LT50 of different N. lugens strains with Tween-80 treatment. C Survival curves of different N. lugens strains with M. anisopliae treatment. D LT50 of different BPH strains with M. anisopliae treatment. BPH, the brown planthopper Nilaparvata lugens. Error bars indicates the standard error (SE), and different letters indicates the statistical difference (p < 0.05).
Interestingly, after M. anisopliae treatment, the LT50 of antibiotic N. lugens decreased to 2.73 days, which was significantly lower than in the absence of fungal infection (Fig. 6B, p < 0.01; One-Way ANOVA). In contrast, we did not observe a significant difference in LT50 (lethal time for 50% mortality) between the control group and the treatment group when the host’s intestinal function is normal, as shown in Fig. 6B. However, when we artificially introduced A. baumannii through feeding, we observed a significantly higher LT50 in N. lugens compared to the control treatment (Fig. 6D, LT50 = 5.05 days; p < 0.05; One-Way ANOVA). The depletion of gut symbionts may aid the brown planthopper in resisting the infection of entomopathogenic fungi through the inoculation of this specific symbiont A. baumannii.
Effects of symbiont reintroduction on host immune responses
We conducted further investigations into the expression of immune-related genes in the IMD, JNK, and Toll pathways following A. baumannii inoculation. The results showed that the expressions of immune-related genes were initially decreased after 12 h of A. baumannii inoculation compared to the control group. However, these differences were not statistically significant (Fig. 7A–H). Interestingly, after 24 h of reintroducing A. baumannii, we observed a significant increase in the expressions of Duo X, JNK, p38b, cyclin-dependent STK, venom allergen 5, relish, toll, and PGRP-LB, (Fig. 7A–H) except for spaezle (p < 0.05, Fig. 7I; One-Way ANOVA). Even after 48 h of the bacteria feeding, Duo X, cyclin-dependent STK, and venom allergen 5 continued to exhibit significantly higher expression compared to the control group. Notably, Duo X showed a particularly pronounced increase in expression with prolonged reintroduction time (Fig. 7A). Based on these findings, we speculate that the elevated resistance of N. lugens to the fungus M. anisopliae after A. baumannii inoculation may be attributed to the preactivation of host immune responses.
Discussion
The brown planthopper, N. lugens, is a persistent pest that poses a significant threat to rice plants42. To address this issue in an environmentally friendly manner, various alternatives have been explored, including the use of insect pathogenic fungi43,44. Previous studies have demonstrated the effective potential of Metarhizium in infecting and eliminating insect pests, including the rice planthopper45,46,47. M. anisopliae CQMa 421 has been found to reduce the number of eggs laid by the rice planthopper and decrease the hatching rate, while having minimal impact on the population of natural enemies in paddy conditions48. In this study, we aimed to delve deeper into the interaction between these fungi and the commensal bacteria present in the gut of the brown planthopper. Our objective was to gain valuable insights into the symbiotic relationships between these organisms and pathogens, with a goal of developing new and effective biological control strategies.
The infection of the rice planthopper by the fungus triggers physiological responses in the host, such as changes in recognition, immunity, and metabolism48,49,50. Meanwhile, when insect pathogenic fungi enter the insect’s body, they inevitably interact with the host’s symbiotic bacteria51. In our study, infestation of the rice planthopper by M. anisopliae resulted in changes in both the number and species of microorganisms present in the insect’s gut. These changes were dynamic, showing an initial decrease followed by an increase in symbiotic bacteria over time. This suggests an intricate interaction between the fungi and bacteria within insect host. Indeed, studies conducted on the Asian rice gall midge have indicated that there are variations in bacterial communities during their interactions with susceptible or resistant rice varieties52. The infestation of rice by the gall midge has been found to impact the density and diversity of pseudomonas and wolbachia in the microbiome of the host plant53.
In this study, we observed an initial decrease in the population of symbiotic bacteria in the host insect, which could be attributed to the disruption caused by the growth of pathogenic fungi. However, over time, we noticed that the growth of symbiotic bacteria eventually reached an equilibrium. Interestingly, we also observed a tendency for certain symbiotic bacteria to overgrow in response to the invasion of pathogenic fungi. This finding suggests a complex dynamic between the host insect, symbiotic bacteria, and pathogenic fungi, highlighting the intricate nature of their interactions. Both the experimental groups infected with fungal infection and the uninfected groups exhibited variability in terms of the diversity and abundance of symbiotic bacteria. This suggests that factors beyond the presence of fungal infection alone can influence the composition and population of symbiotic bacteria52. However, Proteobacteria, Actinobacteriota, and Firmicutes remained dominant, indicating their important regulatory role in the insect gut and autoimmunity.
Symbiotic bacteria can play a crucial role in resisting the invasion of pathogenic bacteria54. These beneficial bacteria may produce antimicrobial compounds or compete for resources, limiting the establishment of harmful pathogens. On the other hand, certain symbiotic bacteria may have a detrimental effect on the host insect. This suggests that the role of symbiotic bacteria in host-pathogen interactions can be complex, with some bacteria providing protection while others may exacerbate the negative impact of pathogens. Furthermore, gut symbiotic bacteria also assist in the digestion and absorption of nutrients, provide essential vitamins and metabolites, and contribute to the overall health and fitness of the insect host55. Previous studies have shown that the insect pathogenic fungus B. bassiana can interact with the opportunistic pathogenic bacterium Serratia marcescens to accelerate mosquito mortality19. These studies have highlighted that symbiotic bacteria can have diverse effects on their host, with providing similar benefits or functions as the host itself. Understanding the specific roles of different symbiotic bacteria and their interactions with pathogens is crucial for future research in this field.
Through plate confrontation experiments with a wide range of gut microbiota isolates in this study, it was found that Acinetobacter sp., Bacillus sp., and Microbacterium sp. exhibited a ring of inhibition when incubated with this fungus. Previous studies have also shown that both the fermentation broth and volatiles from the gut microbiota of Melanotus cribricollis, specifically Acinetobacter gyllenbergii, strongly inhibit the germination of Metarhizum pingshaense spores56. However, the underlying mechanism behind this inhibition is not well understood. Several studies have reported that Entomobacterium fumigatus can affect nitrogen assimilation in honeybees and assist Curculio chinensis in degrading the plant toxin tea saponin to protect it from toxicity57,58. Acinetobacter has also been shown to be important for physiological stress in several insects, although specific references for this claim are needed59,60. In our study, we also noted that symbiont A. baumannii can enhance the host’s ability to resist the pathogenic fungus M. anisopilea.
It is speculated that M. anisopliae infection may suppress the immune system of the host insects, leading to changes in the gut bacteria. However, the potential interactions between host symbiotic bacteria and pathogenic fungi are yet to be fully understood. Our results showed that supplementing with Acinetobacter could decrease the mortality rate of the insect host N. lugens when they are exposed to entomopathogenic fungus M. anisopliae. However, there was no significant difference in survival if the host was not exposed to pathogenic fungi. These findings suggest that Acinetobacter, acting as a beneficial gut bacterium in N. lugens, can play a role in counteracting fungal invasion in the planthopper. Additionally, our results suggested that the reintroduction of this symbiont A. baumannii may stimulate the immune response of host N. lugens. Similar to this study, replenishing Arsenophonus in brown planthoppers reduced the infection rate of Metarhizium flavoviride by increasing the number of plasma hemocytes61. In summary, the results suggest that preserving a stable population of host symbionts is crucial for the survival and physiology of the host organism. Specifically, the presence of A. baumannii as a symbiotic bacterium appears to play a role in enhancing the host’s ability to resist infections caused by external pathogens.
Methods
Insect culture and fungus M. anisopliae
In this experiment, the N. lugens used were obtained from a breeding population maintained at the Genetic Engineering Research Center of Chongqing University in Chongqing, China. The insects were reared on rice seedlings (Oryza sativa) in transparent plastic cages (40 cm × 40 cm × 60 cm). The environmental conditions in the rearing facility were set to a 14-h light and 10-h dark cycle, with a temperature of 27 ± 1 °C and humidity of 70% ± 5%. To ensure sufficient egg laying, the adult N. lugens females and males were transferred to a new cage with fresh rice seedlings and allowed to stay for 48 h before being removed. This period allowed them to lay enough eggs. The newly emerged nymphs from these eggs were collected for use in the subsequent experiments.
The entomopathogenic fungus strain M. anisopliae CQMa421 (CGMCC No. 4607) was obtained from the Engineering Research Center for Fungal Insecticides in Chongqing, China. Before the experiment, the fungus was cultured on SDAY medium, which consisted of 18 g agar per liter of sterile water, 5 g yeast extract, 10 g glucose, and 2.5 g peptone. The fungus was grown for 14 days prior to use. For the treatment of N. lugens, the collected M. anisopliae conidia were scraped and mixed with 0.05% Tween-80. The mycelium was then removed by filtering through sterile filter paper. A concentration of 1×107 conidia/mL was prepared for the treatment group. In contrast, the control group was treated with sterile 0.05% Tween-80 solution.
Insect gut dissection and bacterial cultivation
To assess the effects of M. anisopliae on N. lugens, the following procedures were carried out in this experiment. Thirty N. lugens insects were immobilized using CO2 gas and then sterilized with 75% alcohol for 60 seconds. This sterilization process was conducted after the insects had been infected for 24, 48, and 72 h, respectively. After sterilization, the insects were immersed in sterilized water to remove any residual alcohol. This cleaning process was repeated twice. The cleaned adult insects were then transferred onto sterile dissected wax discs. The intact guts of N. lugens were carefully removed and rinsed with sterilized water. The dissected guts were placed in a 1.5 mL centrifuge tube with 1 mL of sterile PBS on ice. The guts were homogenized by grinding and then diluted to a concentration of 1000-fold after thorough shaking. The resulting gut solution was plated onto Luria-Bertani (LB) agar plates. These plates were then incubated at a constant temperature of 25 ± 1 °C for 24 h. After incubation, the bacterial colonies present in the N. lugens gut were counted using a colony-forming unit (CFU) assay. Each treatment was performed in triplicate to ensure reliable results. By following these steps, we were able to evaluate the presence and quantity of bacteria in the gut of N. lugens after infection with M. anisopliae.
16S rRNA gene amplicon sequencing analysis of N. lugens
To investigate the impact of fungus on the gut microbiota composition of N. lugens, we analyzed the dynamic changes in gut bacterial composition and diversity in both uninfected and M. anisopliae-infected N. lugens at 24, 48, and 72 hpi. The gut samples of N. lugens treated with M. anisopliae were collected and sent to Shanghai Meiji Biomedical Technology Co., LTD. (https://www.majorbio.com) for analysis. The analysis was performed using the IIIumina Miseq platform. Each treatment was performed in seven replicates to ensure reliable results. For library construction, the DNA from the gut samples was amplified using a bacterial universal primer that targets the V3 + V4 region (338 F: 5’-ACTCCTACGGGAGGCAGCA-3’; 806 R: 5’-GGACTACHVGGGTWTCTAAT-3’). The obtained double-end reads underwent quality control and filtering based on their quality scores. Then, the overlapping reads were spliced to obtain optimized data after quality control splicing.
To process the optimized data, sequence denoising methods such as DADA2 or Deblur were applied. These methods help to obtain representative sequences and abundance information of Amplicon Sequence Variants (ASVs). Non-repetitive sequences were extracted from the optimized sequences, and single sequences without repeats were removed. The remaining non-repetitive sequences, excluding the single sequences, were clustered into operational taxonomic units at a 97% similarity threshold using the USEARCH7-uparse algorithm. A representative read was selected using the QI algorithm, and it was blasted against the Silva database (silva138/16s_bacteria) using the RDP classifier (version 2.11) with a confidence threshold set at 70%. These analysis steps were performed to determine the composition and abundance of bacterial species present in the gut of N. lugens after infection with M. anisopliae.
Bioinformatic analysis of the gut microbiota was conducted using the Majorbio Cloud platform (https://cloud.majorbio.com). Based on the ASV information, rarefaction curves and alpha diversity indices such as observed ASVs, abundance-based coverage estimator (ACE) index, Shannon index, Simpson index, Shannoneven, and Simpsoneven index were calculated using Mothur v1.30.1. The similarity among microbial communities in different samples was assessed using Principal Coordinate Analysis (PCoA) based on Bray-Curtis dissimilarity, utilizing the Vegan v2.5-3 package. The PERMANOVA test, also performed with the Vegan package, was used to determine the percentage of variation explained by the treatment and its statistical significance. To identify significantly abundant taxa (phylum to genera) among different groups, linear discriminant analysis effect size (LEfSe) was employed. LEfSe was performed using the website (http://huttenhower.sph.harvard.edu/LEfSe), and taxa with an LDA score greater than 2 and a significance level of p < 0.05 were considered significant. Additionally, distance-based redundancy analysis (db-RDA) was conducted using the Vegan v2.5-3 package to investigate the effect of M. anisopliae on the bacterial community structure of insect host gut. Based on Tax4Fun function prediction, we also examined the main functional pathways among the intestinal commensal bacteria of N. lugens.
Isolation and identification of host gut bacteria
To isolate potential commensal bacteria from the gut of N. lugens, the following procedures were conducted in this experiment. Each treatment of 30 N. lugens guts was collected using the methods mentioned before. A 100 μl dilution of the gut samples was spread smoothly onto different culture media, including LB Medium, Nutrient Agar Medium (NA), Gauze’s MediumI(GS), and 1/2 LB medium. The plates were then incubated at a temperature of 28 °C for different durations (i.e., 24 and 48 h), and two separate periods of 96 h each. After the incubation period, single colonies with distinct morphology were selected from each culture medium. These colonies were streaked in three separate areas on new plates to obtain relatively pure strains. The process of selecting and streaking colonies was repeated five times to ensure the isolation of relatively pure bacterial strains.
The expanded population genomic DNA from the different bacterial isolates was isolated using PCR amplification targeting the region of the 16S rDNA gene. The primer sequences used for PCR amplification were 27 F (5’-AGAGTTTGATCCTGGCTCAG-3’) and 1492 R (5’-GGTTACCTTGTTACGACTT-3’). The amplified PCR products were sent to Shanghai Sangon Biological Engineering Co., LTD. (https://www.sangon.com/) for sequencing analysis. Then the obtained sequences were compared against the GenBank database using the BLAST algorithm to identify the bacterial species. These steps were taken to isolate and identify potential commensal bacteria from the gut of N. lugens, helping to further understand the microbial community associated with the insect.
Preparation of artificial food and sterile N. lugens culture
The artificial diet of N. lugens was prepared following previously reported methods32. In summary, amino acids, vitamins (except ascorbic acid), and trace elements (except FeCl3·6H2O) were prepared as 2 ×, 10 ×, and 100 × stock solutions, respectively, and stored at −20 °C. When used, the amino acid stock solution, sucrose, KH2PO4, MgCl2·6H2O, and FeCl3·6H2O were successively added to a beaker and stirred until completely dissolved. Then, the vitamin and trace element stock solutions, as well as the ascorbic acid solution, were added. The pH of the solution was adjusted to 6.8 using 4% KOH, and it was diluted to the working concentration with distilled water. The solution was finally filtered and sterilized using Millipore disposable filters (0.45 mm).
For feeding N. lugens, a clear glass cylinder measuring 10 cm in length and 2.5 cm in diameter was used (Fig. S4). Two Parafilm membranes were stretched evenly to enclose one end of the glass tube. Each tube contained twenty N. lugens individuals, and the opposite end was sealed with a moistened cotton ball. The glass tube was shaded with a slightly larger black paper shade, leaving one end with the artificial feed exposed to light. N. lugens accessed the diet by piercing through the internal Parafilm membrane. The artificial diet for N. lugens was refreshed daily.
To examine the effect of antibiotics on the target symbiont, tetracycline and ciprofloxacin were added to the artificial diet at a concentration of mg/100 ml. The control group was fed the antibiotic-free artificial diet. After 48 h of antibiotic treatment, five BPHs were selected from each group to evaluate the reduction of the target bacteria (Fig. S3). Total RNA extraction of N. lugens was performed using the Total RNA Isolation Kit Plus, with thorough grinding according to the manufacturer’s instructions (FOREGENE biotechnology). First-strand cDNA synthesis was carried out using the FOREGENE RT Easy TM II kit. Quantitative real-time PCR analysis was conducted using the BIOGROUND SYBRPrime qPCR cycling parameters: 95 °C for 3 min, followed by 40 cycles of 94 °C for 20 s, 94 °C for 10 s, and 60 °C for 20 s. The reference control used was β-actin (FGGACTTCGAGCAGGAAATGGC, R: CGACGTCGCACTTCATGATCGAG). Each treatment was repeated three times.
Bacteriostasis of isolated symbionts to fungus and bioassay
To investigate the potential role of intestinal commensal bacteria in the defense against pathogenic fungi, we conducted survival tests under different conditions. Firstly, we placed the isolated and purified symbionts on LB medium. In a 90 mm diameter Petri dish, three blocks of M. anisopliae were positioned around the bacterial parts using sterile blocks. The blocks were positioned 3.5 cm away from the center of the symbiont circle. Subsequently, the Petri dishes were incubated at 28 °C for 24 h. After the incubation period, we analyzed the size of the inhibitory zone to examine the inhibitory effect of the symbionts on M. anisopliae.
To assess the effect of symbiont bacteria on N. lugens infection, we performed the following steps. Acinetobacter baumannii bacteria were incubated at 28 °C for 24 h and then collected by centrifugation at 8000 × g for 5 min. The bacterial precipitate was collected, and sterile deionized water was added to prepare a bacterial suspension. The concentration of the suspension was adjusted to 0.4 at 600 nm, and 1 ml of the bacterial suspension was added to 99 ml of the artificial feed. In the control group, 1 ml of sterile deionized water was added to the artificial feed instead. N. lugens was fed for 48 h using an artificial feeding device (Fig. S4).
In the experiment, the control group was not subjected to any artificial feed or antibiotics. The sterile group was initially fed with an artificial diet supplemented with antibiotics for 48 h, followed by an artificial diet containing bacteria for another 48 h. To maintain experimental consistency, the sterile group comprised brown planthoppers that were fed an artificial diet supplemented with antibiotics for a total of 96 h. After the antibiotic or bacterial feeding, all N. lugens individuals were exposed to a spore suspension of M. anisopliae at a concentration of 1×107 conidia/mL, following the methods described above for each respective group. The planthoppers were then placed in a controlled environment of 70% ± 1% humidity and a temperature of 27 ± 1 °C. The number of living individuals in each group was recorded daily based on their performance. Each treatment was repeated three times to obtain reliable assay results.
Immune responses of host N. lugens after symbiont A. baumannii inoculation
To investigate the expression of immune genes (Table S1) in N. lugens after A. baumannii inoculation, we focused on the IMD and Toll pathways, which play crucial roles in insect innate immunity. The Toll pathway is initiated by the interaction between spaezle and Toll, while key genes in the IMD pathway include PGRP-LB, Duo X, p38b, and Relish. To carry out our study, we initially treated the planthoppers with antibiotics for 48 h to eliminate any existing bacterial infections. Subsequently, we fed them an artificial diet supplemented with A. baumannii for 12 h, 24 h, and 48 h, respectively. Total RNA was extracted using the FOREGENE Total RNA Isolation Kit, followed by first-strand cDNA synthesis using the FOREGENE RT EasyTM II kit. For gene expression analysis, cDNA samples were diluted according to the instructions provided with the BIOGROUND SYBRPRIME qPCR set (Fast HS). The diluted cDNA samples were mixed with 2×SP qPCR Mix and subjected to amplification in the Analytik jena qTOWER3G detection system. We employed a two-step method (94 °C for 20 s, 94 °C for 10 s, 60 °C for 20 s, 40 cycles) to generate melting curves. Each biological replicate was measured three times, and the results were normalized using the β-actin gene as a reference. The relative gene expression was calculated using the 2-ΔΔCT method.
Statistics and reproducibility
In the study, Sidak’s multiple tests were employed to analyze various parameters including bacterial colony-forming unit (CFU) counts, the diameter of the inhibition circle, and the results obtained from quantitative polymerase chain reaction (qPCR) analysis in both the fungi-treated and control groups. The feeding rate of brown planthoppers (BPHs) in the artificial apparatus was calculated by dividing the number of meals consumed by the number of survivors. To compare significant differences in the bioassay results and gene expression levels of brown planthoppers between different groups, a one-way analysis of variance (ANOVA) was performed. Statistical analyses were conducted using GraphPad Prism 8.0 and SPSS 26.0 software. A significance level of p < 0.05 was considered to indicate statistical significance. p < 0.05 was considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw sequence reads of all samples analyzed in this study are available at the NCBI Sequence Read Archive with Bioproject accession No. PRJNA1054509. The raw data are available online with a file of Supplementary Data.
References
Rio, R. V. M. et al. Insight into the transmission biology and species-specific functional capabilities of tsetse (Diptera: Glossinidae) obligate symbiont Wigglesworthia. Mbio 3, e00240–11 (2012).
Simon, J. C. et al. Facultative symbiont infections affect aphid reproduction. PLoS One 6, e21831 (2011).
Zhang, Y. X., Zhang, S. K. & Xu, L. T. The pivotal roles of gut microbiota in insect plant interactions for sustainable pest management. NPJ Biofilms Microbiomes 9, 66 (2023).
Ferrandon, D., Imler, J. L., Hetru, C. & Hoffmann, J. A. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat. Rev. Immunol. 7, 862–874 (2007).
Dantur, K. I., Enrique, R., Welin, B. & Castagnaro, A. P. Isolation of cellulolytic bacteria from the intestine of Diatraea saccharalis larvae and evaluation of their capacity to degrade sugarcane biomass. Amb. Express 5, 15 (2015).
Baharuddin, M. Cellulase enzyme activity of Bacillus circulans from larvae cossus cossus in lignocellulosic substrat. Am. J. Biomed. Life Sci. 4, 21–25 (2016).
Zhang, Q. et al. Enhanced biogas production by ligninolytic strain KA3 for anaerobic digestion of corn straw. Energies 14, 2990 (2021).
Giron, D. et al. Influence of microbial symbionts on plant-insect interactions. Adv. Bot. Res. 81, 225–257 (2017).
Berasategui, A. et al. The gut microbiota of the pine weevil is similar across Europe and resembles that of other conifer-feeding beetles. Mol. Ecol. 25, 4014–4031 (2016).
Zhang, S. K. et al. The gut microbiota in Camellia weevils are influenced by plant secondary metabolites and contribute to saponin degradation. mSystems 5, e00692–19 (2020).
Cheng, C. et al. Bacterial microbiota protect an invasive bark beetle from a pine defensive compound. Microbiome 6, 132 (2018).
De Vries, E. J., Vos, R. A., Jacobs, G. & Breeuwer, H. A. J. Western flower thrips (Thysanoptera: Thripidae) preference for thrips-damaged leaves over fresh leaves enables uptake of symbiotic gut bacteria. Eur. J. Entomol. 103, 779–786 (2006).
Xu, L. T. et al. Sexual variation of bacterial microbiota of Dendroctonus valens guts and frass in relation to verbenone production. J. Insect Physiol. 95, 110–117 (2016).
Lu, M., Hulcr, J. & Sun, J. H. The role of symbiotic microbes in insect invasions. Annu Rev. Ecol. Evol. S 47, 487–505 (2016).
He, M. Y. et al. Gut bacteria induce oviposition preference through ovipositor recognition in fruit fly. Commun. Biol. 5, 973 (2022).
Currie, C. R., Scott, J. A., Summerbell, R. C. & Malloch, D. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature 398, 701–704 (1999).
Landry, M. et al. Composition of the spruce budworm (Choristoneura fumiferana) midgut microbiota as affected by rearing conditions. Plos One 10, e0144077 (2015).
Futo, M., Sell, M. P., Kutzer, M. A. M. & Kurtz, J. Specificity of oral immune priming in the red flour beetle Tribolium castaneum. Biol. Lett. 13, 20170632 (2017).
Wei, G. et al. Insect pathogenic fungus interacts with the gut microbiota to accelerate mosquito mortality. Proc. Natl Acad. Sci. USA 114, 5994–5999 (2017).
Pang, X. J. et al. Mosquito C-type lectins maintain gut microbiome homeostasis. Nat. Microbiol. 1, 16023 (2016).
Lu, H. L. & St Leger, R. J. Insect immunity to entomopathogenic fungi. Adv. Genet. 94, 251–285 (2016).
Yao, Z. C. et al. Compartmentalized PGRP expression along the dipteran Bactrocera dorsalis gut forms a zone of protection for symbiotic bacteria. Cell Rep. 41, 111523 (2022).
Brownlie, J. C. & Johnson, K. N. Symbiont-mediated protection in insect hosts. Trends Microbiol. 17, 348–354 (2009).
Cheng, D. et al. Gut symbiont enhances insecticide resistance in a significant pest, the oriental fruit fly Bactrocera dorsalis (Hendel). Microbiome 5, 13 (2017).
Xia, X. F. et al. Gut microbiota mediate insecticide resistance in the diamondback Moth, Plutella xylostella (L.). Front Microbiol. 9, 25 (2018).
Xia, X. et al. DNA sequencing reveals the midgut microbiota of diamondback moth, Plutella xylostella (L.) and a possible relationship with insecticide resistance. PLoS One 8, e68852 (2013).
Ramya, S. L. et al. Detection of carboxylesterase and esterase activity in culturable gut bacterial flora isolated from diamondback moth, Plutella xylostella (Linnaeus), from India and its possible role in indoxacarb degradation. Braz. J. Microbiol. 47, 327–336 (2016).
Dillon, R. J. & Charnley, A. K. Chemical barriers to gut infection in the desert locust: In Vivo production of antimicrobial phenols associated with the bacterium Pantoea agglomerans. J. Invertebr. Pathol. 66, 72–75 (1995).
Desalegn, G., Turetschek, R., Kaul, H. P. & Wienkoop, S. Microbial symbionts affect Pisum sativum proteome and metabolome under Didymella pinodes infection. J. Proteom. 143, 173–187 (2016).
Barelli, L., Moonjely, S., Behie, S. W. & Bidochka, M. J. Fungi with multifunctional lifestyles: endophytic insect pathogenic fungi. Plant Mol. Biol. 90, 657–664 (2016).
Wang, C. S. & Wang, S. B. Insect pathogenic fungi: genomics, molecular interactions, and genetic improvements. Annu Rev. Entomol. 62, 73–90 (2017).
Dillon, R. J. & Dillon, V. M. The gut bacteria of insects: nonpathogenic interactions. Annu Rev. Entomol. 49, 71–92 (2004).
Forsgren, E., Olofsson, T. C., Vásquez, A. & Fries, I. Novel lactic acid bacteria inhibiting Paenibacillus larvaein honey bee larvae. Apidologie 41, 99–108 (2009).
Biswas, C. et al. Endophytic colonization of white jute (Corchorus capsularis) plants by different Beauveria bassiana strains for managing stem weevil (Apion corchori). Phytoparasitica 41, 17–21 (2012).
Garrido-Jurado, I. et al. Transient endophytic colonization of melon plants by entomopathogenic fungi after foliar application for the control of Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae). J. Pest Sci. 90, 319–330 (2016).
Cory, J. S. Evolution of host resistance to insect pathogens. Curr. Opin. Insect Sci. 21, 54–59 (2017).
Jaber, L. R. & Ownley, B. H. Can we use entomopathogenic fungi as endophytes for dual biological control of insect pests and plant pathogens? Biol. Control 116, 36–45 (2018).
Behie, S. W., Zelisko, P. M. & Bidochka, M. J. Endophytic insect-parasitic fungi translocate nitrogen directly from insects to plants. Science 336, 1576–1577 (2012).
Lopez, D. C. & Sword, G. A. The endophytic fungal entomopathogens Beauveria bassiana and Purpureocillium lilacinum enhance the growth of cultivated cotton (Gossypium hirsutum) and negatively affect survival of the cotton bollworm (Helicoverpa zea). Biol. Control 89, 53–60 (2015).
Roy, H. E. et al. Bizarre interactions and endgames: entomopathogenic fungi and their arthropod hosts. Annu Rev. Entomol. 51, 331–357 (2006).
Xue, J. et al. Transcriptome analysis of the brown planthopper Nilaparvata lugens. PLoS One 5, e14233 (2010).
Ling, Y. & Weilin, Z. Genetic and biochemical mechanisms of rice resistance to planthopper. Plant Cell Rep. 35, 1559–1572 (2016).
Abrol, D. & Hankar, U. Pesticides, food safety and integrated pest management. Integr. Pest Manag.: Pestic. Probl. 7, 167–199 (2014).
Tang, J. F. et al. Evaluation of Metarhizium anisopliae for rice planthopper control and its synergy with selected insecticides. Crop Prot. 121, 132–138 (2019).
Peng, G. X. et al. Field trials of Metarhizium anisopliae var. acridum (Ascomycota: Hypocreales) against oriental migratory locusts, Locusta migratoria manilensis (Meyen) in Northern China. Crop Prot. 27, 1244–1250 (2008).
Erler, F. & Ates, A. O. Potential of two entomopathogenic fungi, Beauveria bassiana and Metarhizium anisopliae (Coleoptera: Scarabaeidae), as biological control agents against the June beetle. J. Insect Sci. 15, 44 (2015).
Swami, H., Ameta, O. P. & Lekha Bioefficacy of novel insecticides against pod borer, (Helicoverpa armigera Hubner) in pigeonpea. Legume Res. 40, 756–761 (2017).
Peng, Y. F., Tang, J. F., Hong, M. S. & Xie, J. Q. Suppression of rice planthopper populations without affecting the rice microbiota by the entomopathogenic fungus Metarhizium anisopliae. Appl Environ. Microbiol. 86, e01337–20 (2020).
Jiang, W. et al. Effects of the entomopathogenic fungus Metarhizium anisopliae on the mortality and immune response of Locusta migratoria. Insects 11, 36 (2019).
Xu, Y. J. et al. Metabolomics reveals insect metabolic responses associated with fungal infection. Anal. Bioanal. Chem. 407, 4815–4821 (2015).
Deng, J. et al. Associated bacteria of a pine sawyer beetle confer resistance to entomopathogenic fungi via fungal growth inhibition. Environ. Microbiome 17, 47 (2022).
Ojha, A. et al. Bacterial community structure in the Asian rice gall midge reveals a varied microbiome rich in proteobacteria. Sci. Rep. 7, 9424 (2017).
Sinha, D. K. et al. Infestation of rice by gall midge influences density and diversity of Pseudomonas and Wolbachia in the host plant microbiome. Curr. Genomics 23, 126–136 (2022).
Del Gallo, M. et al. Inoculation of four endophytic bacteria on lycopersicon esculentum and their antagonism towards some pathogenic fungus. J. Biotechnol. 150, S494–S494 (2010).
Wiesmann, C. L. et al. Origins of symbiosis: shared mechanisms underlying microbial pathogenesis, commensalism and mutualism of plants and animals. FEMS Mricrobiol Rev. 47, 1–12 (2023).
Xin, F. L. et al. Inhibitory activity of symbiotic bacteria of Melanotus cribricollis larvae against Metarhizium anisopliae. Chin. J. Ecol. 40, 3990–3997 (2021).
Li, Z. et al. Specific enriched Acinetobacter in Camellia weevil gut facilitate the degradation of tea saponin: inferred from bacterial genomic and transcriptomic analyses. Microbiol Spectr. 10, e0227222 (2022).
Álvarez-Pérez, S. et al. Nitrogen assimilation varies among clades of nectar- and insect-associated Acinetobacters. Micro. Ecol. 83, 256 (2022).
Pietri, J. E. & Liang, D. S. The links between insect symbionts and insecticide resistance: causal relationships and physiological tradeoffs. Ann. Entomol. Soc. Am. 111, 92–97 (2018).
Fiester, S. E. & Actis, L. A. Stress responses in the opportunistic pathogen Acinetobacter baumannii. Future Microbiol. 8, 353–365 (2013).
Zhu, H. H. et al. Influence of symbiotic bacteria Arsenophonus, rice variety and temperature on the incidence rate of Nilaparvata lugens to Metarhizium flavoviride. Chin. J. Rice Sci. 31, 643–651 (2017).
Acknowledgements
We appreciate Chunfang Huang for assisting maintaining insect population. The project acquired the grants from the Natural Science Foundation of Chongqing (Project No. 2023TIAD-GPX0148), the Fundamental Research Funds for the Central Universities with project No. 2023CDJKYJH043 and 2024CDJXY016.
Author information
Authors and Affiliations
Contributions
J.X. and Y.X. conceptualized the study. C.T., X.H., J.T. and J.X. designed the experiments. C.T, J.T., L.W., X.L. and Y.P. conducted the experiments and data collection. X.H., Y.P., J.X. and Y.X. acquired the fundings. J.X. and C.T. wrote the draft. C.T., J.X., J.T., L.W., X.L., Y.P. and Y.X. reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications biology thanks Deepak Kumar Sinha, Yoshitomo Kikuchi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Dr Sridhar Mani and Dr Ophelia Bu.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tang, C., Hu, X., Tang, J. et al. The symbiont Acinetobacter baumannii enhances the insect host resistance to entomopathogenic fungus Metarhizium anisopliae. Commun Biol 7, 1184 (2024). https://doi.org/10.1038/s42003-024-06779-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-024-06779-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.