Abstract
Plasma membrane damage in vitrified oocytes is closely linked to mitochondrial dysfunction. However, the mechanism underlying mitochondria-regulated membrane stability is not elucidated. A growing body of evidence indicates that mitochondrial activity plays a pivotal role in cell adaptation. Since mitochondria work at a higher temperature than the constant external temperature of the cell, we hypothesize that suppressing mitochondrial activity would protect oocytes from extreme stimuli during vitrification. Here we show that metformin suppresses mitochondrial activity by reducing mitochondrial temperature. In addition, metformin affects the developmental potential of oocytes and improves the survival rate after vitrification. Transmission electron microscopy results show that mitochondrial abnormalities are markedly reduced in vitrified oocytes pretreated with metformin. Moreover, we find that metformin transiently inhibits mitochondrial activity. Interestingly, metformin pretreatment decreases cell membrane fluidity after vitrification. Furthermore, transcriptome results demonstrate that metformin pretreatment modulates the expression levels of genes involved in fatty acid elongation process, which is further verified by the increased long-chain saturated fatty acid contents in metformin-pretreated vitrified oocytes by lipidomic profile analysis. In summary, our study indicates that metformin alleviates cryoinjuries by reducing membrane fluidity via mitochondrial activity regulation.
Similar content being viewed by others
Introduction
Oocyte cryopreservation technology is a promising strategy to restore fertility for women with diseases, such as cancer, premature ovarian failure, and so on1. It is also an alternative approach to protect oocytes from deterioration in aged women2. In addition, oocyte cryopreservation is important for modern animal husbandry and germplasm conservation, especially for the preservation of endangered species and top-quality breeds, which greatly meet the needs of the country and society3,4.
However, a growing body of evidence demonstrates that the relatively low quality of vitrified oocytes has hampered the efficacious application of the technique5,6,7,8,9. It was reported that membrane damage was one of the main reasons for compromised oocyte developmental potential after cryopreservation10. Both osmotic mechanical stress and cryoprotectant toxicity could cause oocyte membrane and organelle membrane damage during vitrification11. Membrane fluidity is one of the physical parameters of the plasma membrane. It is involved in cell motility12, adhesion13, endocytosis14, substance secretion15, and carrier transport16. Previous study indicated that lipids, such as cholesterol and long-chain fatty acids, could decrease membrane fluidity17,18. Results showed that lower membrane fluidity would make the membrane stiffer, thereby preventing the exacerbation of membrane damage19. It was reported that cells susceptible to freeze-thaw stimuli exhibited increased cell membrane fluidity20. Right now, the membrane fluidity alterations in oocytes during vitrification have not been determined.
It is well known that mitochondria play a pivotal role in lipid synthesis and lipolysis, which meet the energy demand of oocyte development. Studies showed that mitochondrial dysfunction compromised oocyte developmental potential after cryopreservation10,21. After freezing and thawing, ruptured mitochondria and vacuoles of different sizes were observed. Moreover, oocytes were suffered from oxidative stress22. Previous studies indicated that mitochondrial injuries, including decreased mitochondrial membrane potential and reduced ATP levels, were observed in vitrified oocytes23. Moreover, mitochondrial morphology abnormalities were discovered in vitrified oocytes by transmission electron microscopy analysis24. The latest proteomics data also proved that differentially expressed proteins were enriched in mitochondrial terms in vitrified oocytes when compared with the fresh group25.
Previous studies indicated that impaired mitochondrial function and oxidative stress were associated with increased mitochondrial thermogenesis in vitrified mouse oocytes26,27. It was reported that mitochondria normally operate at high temperatures that could reach 50 °C28. Decreased mitochondrial activity was accompanied by slowed down thermogenic process29. Thus, mitochondrial temperature was used to determine the extent of mitochondrial activity. Since mitochondria are sensitive to environmental changes, it is probable that the dramatic temperature fluctuation, from 50 °C in normal physiological state to −196 °C during vitrification would disrupt the active metabolic process. Herein, we hypothesized that reducing mitochondrial activity prior to vitrification would protect mitochondria from extreme stress during oocyte vitrification.
Metformin, the first-line medication for type II diabetes treatment, also plays an important role in improving oocyte quality. Studies have shown that metformin alleviates oocytes defects induced by arecoline30, aging31, and PCOS disease32. Notably, previous studies have shown that metformin exhibits an inhibitory role on mitochondrial complex I33,34. In addition, several other compounds, such as rotenone35, oligomycin36, and UK509937 also possess the capability to suppress mitochondrial activity. However, whether mitochondrial inhibitors would protect oocytes against cryoinjuries remains largely unexplored. Therefore, in the present study, porcine oocyte was used as a prominent model to explore the effect of mitochondrial inhibitors during vitrification. And we discovered that metformin could induce mitochondria silence and improve the survival rate after thawing. Furthermore, the interrelationship between metformin-regulated mitochondrial activity and membrane fluidity as well as the mechanism underlying was revealed.
Results
Metformin attenuates cryo-damage in vitrified oocytes
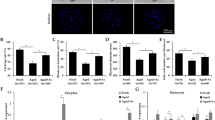
To screen suitable mitochondrial inhibitors that enable oocytes to decrease the mitochondrial temperature in a short time, we first treated MII oocytes with mitochondria-targeted inhibitors metformin (Met), rotenone (Rtn), oligomycin (OA), and UK5099 at different concentrations for 1 h. We found that 400 μM metformin (P = 0.0181), 1 μM Rtn (P = 0.0093), 0.5 μM OA (P < 0.0001), 1.5 μM OA (P < 0.0001), 2.5 μM OA (P < 0.0001), 1 μM UK5099 (P = 0.0092), and 2 μM UK5099 (P = 0.0009) could significantly reduce mitochondrial temperature (Fig. 1a, b; Fig. S1). Then the lowest effective concentration of different inhibitors was applied to treat oocytes. After parthenogenetic activation, the blastocyst formation rate was significantly reduced in the 0.5 μM OA (P = 0.0184) and 1 μM Rtn (P < 0.0001) treatment group when compared with that of the control counterparts. In contrast, neither the cleavage nor the blastocyst rate showed a significant difference after 400 μM metformin and 1 μM UK5099 treatment (Fig. 2a–c). Next, we pretreated MII oocytes with 400 μM metformin and 1 μM UK5099 before vitrification. As shown in Table 1 and Fig. 2d, compared with the vitrified group, metformin significantly increased the oocyte survival rate (P = 0.0165), whereas UK5099 had no effect on oocyte survival. Therefore, 400 μM metformin was selected as the optimal agent in reducing thermogenesis in porcine oocytes and was used in the subsequent study.
a Representative images of MTY staining in porcine oocyte treated with 0.1, 0.2, 0.4 mM metformin. Scale bar: 100 μm. b Quantification analysis of MTY fluorescence intensity in control (n = 48) as well as 0.1 mM (n = 42), 0.2 mM (n = 49), 0.4 mM (n = 42) Met treated oocytes. One-way ANOVA was utilized for statistical analyses. “n” represented the oocyte number used in this test. *P < 0.05. “ns”, non-significant (P > 0.05). Error bars indicated SEM.
a Representative images of MII oocytes, cleavage embryos, and blastocysts in control, Met, rotenone, OA, UK5099 groups. Scale bar: 100 μm. b The percentage of cleavage embryos in control (n = 300) as well as 0.4 mM Met (n = 110), 1 μM rotenone (n = 109), 0.5 μM OA (n = 104), and 1 μM UK5099 (n = 89) treated groups. c The percentage of the blastocyst in control (n = 300), 0.4 mM Met (n = 110), 1 μM rotenone (n = 109), 0.5 μM OA (n = 104), and 1 μM UK5099 (n = 89) treated groups. d Representative images of FDA staining in vitrified oocytes pretreated with different mitochondrial inhibitors. Scale bar: 100 μm. One-way ANOVA was utilized for statistical analyses. “n” represented the oocyte number used in this test. *P < 0.05; ***P < 0.001.“ns”, non-significant (P > 0.05). Error bars indicated SEM.
Moreover, the effect of metformin on ultrastructural changes in vitrified oocytes was evaluated by transmission electron microscopy analysis, and the result showed that mitochondrial number was significantly increased in vitrified oocytes after metformin pretreatment (P = 0.0079) (Fig. 3a, b). In addition, ruptured mitochondrial membrane, significantly increased mitochondrial area (P < 0.0001), and decreased electron density (P < 0.0001) were found in vitrified oocytes compared with that of the fresh group, while metformin could recover the cryo-damaged phenotype observed (P < 0.001) (Fig. 3a–d). Similarly, mitochondrial temperature was significantly decreased in warmed oocytes (P < 0.0001), and metformin pretreatment could elevate mitochondrial temperature dramatically (P = 0.0001) (Fig. 3e, f). However, metformin didn’t reverse the reduced ATP levels in vitrified oocytes (Fig. 3g). Taking together, these data suggest that metformin can alleviate cryo-damage caused by vitrification to protect mitochondrial function.
a Representative images of mitochondrial ultrastructure in fresh (F), vitrified (V), and metformin pretreatment (Met + V) groups of oocytes. Red star (*) and red arrow represented mitochondria and broken mitochondrial membrane, respectively. b Quantification analysis of the mitochondrial number/100 μm2 in F (n = 37), V (n = 34), Met + V (n = 38) groups of oocytes. c Quantification analysis of the mitochondrial electron density in F (n = 128), V (n = 126), Met + V (n = 153) groups of oocytes. d Quantification analysis of the mitochondrial area in F (n = 128), V (n = 126), Met + V (n = 153) groups of oocytes. e Representative images of MTY staining in F, V, Met + V groups. Scale bar: 100 μm. f Quantification analysis of the MTY fluorescence intensity in F (n = 48), V (n = 19), Met + V (n = 28) groups. g ATP levels analysis of oocytes in F (n = 32), V (n = 32), Met + V (n = 32) groups. One-way ANOVA was utilized for statistical analyses. “n” represented the oocyte number or mitochondria used in this test. **P < 0.01; ***P < 0.001. “ns”, non-significant (P > 0.05). Error bars indicated SEM.
Metformin represses mitochondrial activity
According to the above result, we discovered that metformin could reduce the mitochondrial temperature before vitrification and alleviate cryoinjuries in porcine oocytes. To unravel the underlying mechanism, we examine the effect of metformin on mitochondrial activity. FRAP was used to trace the intracellular mitochondrial movement. As shown in Fig. 4a, b, metformin significantly decreased the mitochondrial motion in oocytes (P < 0.05). Result showed that metformin treatment significantly reduced ATP levels in oocytes before vitrification (P = 0.0246) (Fig. 4c). In addition, Seahorse metabolic flux analyzer was used to detect mitochondrial respiration (Fig. 4d). Results showed that metformin treatment significantly reduced mitochondrial ATP production (P = 0.0058), basal respiration (P = 0.0418), and maximum respiration rate (P = 0.0358) in oocytes (Fig. 4e–g). These results indicate that metformin can repress mitochondrial activity before vitrification.
a Representative images of mitochondria FRAP in fresh and fresh + Met groups of oocytes. Scale bar: 20 μm. b Quantification analysis of the recovery of TMRM fluorescence intensity in fresh (n = 11) and fresh + Met (n = 11) groups of oocytes. c ATP levels analysis of oocytes in fresh (n = 40) and fresh + Met (n = 40) groups. d Oxygen consumption rate (OCR) was assessed by a Seahorse analyzer (XFe96) in fresh (n = 200) and fresh + Met (n = 200) groups. e ATP production rate was assessed by a Seahorse analyzer (XFe96) in fresh (n = 290) and fresh + Met (n = 245) groups. f Basal oxygen consumption rate was assessed by a Seahorse analyzer (XFe96) in fresh (n = 290) and fresh + Met (n = 245) groups. g Maximal oxygen consumption rate was assessed by a Seahorse analyzer (XFe96) in fresh (n = 290) and fresh + Met (n = 245) groups. Student’s t-test and One-way ANOVA was utilized for statistical analyses. “n” represented the oocyte number or mitochondria used in this test. *P < 0.05; **P < 0.01. Error bars indicated SEM.
Decreased membrane fluidity was found in metformin-pretreated oocytes
Next, we determined the effect of metformin on oocyte membrane fluidity during vitrification. We found that metformin pretreatment significantly decreased cell membrane fluidity in oocytes treated with cryoprotectants (P = 0.0061) (Fig. 5a–c). In addition, we found that after warming, compared with the vitrified counterparts, oocyte membrane fluidity significantly declined in the metformin pretreatment group (P = 0.0126) and recovered to that of the fresh control group (Fig. 5a–c).
Oocytes were divided into five groups: fresh oocytes, fresh oocytes exposed to cryoprotectants (fresh + toxicity), fresh oocytes pretreated with metformin exposed to cryoprotectants (fresh + toxicity + Met), vitrified oocytes (V), vitrified oocytes pretreated with metformin (oocytes were treated with metformin for 1 h, then oocytes were washed and transferred into solution for vitrification) (Met + V). After thawing and recovering for 2 h, the membrane fluidity was determined by FRAP analysis. a Representative images of oocyte membrane FRAP in fresh, fresh + toxicity, fresh + toxicity + Met, V, and Met + V groups. Scale bar: 20 μm. b Recovery of Dil fluorescence intensity in fresh (n = 21), fresh + toxicity (n = 7), fresh + toxicity + Met (n = 12), V (n = 14), and Met + V (n = 14) groups of oocytes. c Quantification analysis of the recovery of Dil fluorescence intensity in fresh (n = 21), fresh + toxicity (n = 7), fresh + toxicity + Met (n = 12), V (n = 14), and Met + V (n = 14) groups of oocytes within 3 min after bleaching. One-way ANOVA and student’s t-test were utilized for statistical analyses. “n” represented the oocyte number used in this test. *P < 0.05; **P < 0.01. Error bars indicated SEM.
Gene expression profiles in metformin-pretreated oocytes during vitrification
To further explore how metformin affects mitochondrial respiration and regulates cell membrane fluidity, fresh (F), vitrified (V), and metformin-treated vitrified (M) oocytes were collected for transcriptome sequencing. As shown in Fig. 6a, b and Supplementary Data 1, a total of 929, 1447, and 196 differentially expressed genes were found in pairwise comparison between the groups. When compared with the F group, 324 genes were upregulated and 605 genes were downregulated in the V group, while 480 genes were upregulated and 927 genes were downregulated in the M group, 108 genes were upregulated and 88 genes were downregulated in the M group compared to that of the V group.
Fresh oocytes (F), vitrified oocytes (V), and vitrified oocytes pretreated with metformin (M) were collected for transcriptome sequencing analysis. a Differentially expressed genes in F, V, and M groups. b Cluster plots of differentially expressed genes in F, V, and M groups. c GSEA pathway enrichment analysis in V group vs M group. d GSEA lattice diagram in V group vs M group. e Heatmap analysis of mRNA expression levels of fatty acid elongation-related mRNA in F, V, and M groups. f GO enrichment analysis of core genes in fatty acid elongation pathway.
As shown in Fig. 6c, the top 20 pathways with upregulation tendency were enriched between V and M groups by GSEA enrichment analysis (Fig. 6c and Supplementary Data 2). We found that the expression levels of genes involved in the fatty acid elongation pathway exhibited an upregulated tendency in the M group. When compared with V group, the levels of core genes in this pathway, including 3-hydroxyacyl-CoA dehydratase 1/3/4 (HACD1/3/4), enoyl-CoA hydratase short chain 1 (ECHS1), elongation of very long-chain fatty acids protein 6/3 (ELOVL 6/3), hydroxyacyl-CoA dehydrogenase (HADH), hydroxyacyl-CoA dehydrogenase trifunctional multienzyme complex subunit alpha (HADHA), hydroxysteroid 17-beta dehydrogenase 12 (HSD17B12), thioesterase superfamily member 5 (THEM5), acyl-CoA thioesterase 4 (ACOT4), were increased in the M group (Fig. 6d and Supplementary Data 3). Heatmap analysis showed that the expression of these core genes which decreased in the V group recovered to the normal level in the M group (Fig. 6e). Moreover, GO enrichment analysis indicated that the above key genes played a regulatory role in the fatty acid elongation process were enriched in mitochondrial matrix and endoplasmic reticulum terms according to the cellular component category (Fig. 6f). Taken together, these results show that metformin pretreatment may reduce membrane fluidity by upregulating fatty acids elongation after freezing and thawing.
Lipid contents were altered in metformin-pretreated vitrified oocytes
To verify the transcriptomic results, UPLC–MS/MS analysis was used to detect lipid content changes in fresh (F), vitrified (V), and metformin-treated vitrified (M) oocytes. The differential abundance of 89, 76, and 14 lipids was found in pairwise comparison between the three groups. In comparison with the F group, 75 lipids were upregulated and 14 lipids were downregulated in the V group, while 59 lipids were upregulated and 17 lipids were downregulated in the M group. Moreover, the accumulated level of 14 lipids was much higher in the M group than that of the V group (Fig. 7a and Supplementary Data 4). Among them, 12, 12, and 1 kinds of free fatty acid (FFA) contents were increased in V vs F, M vs F, and M vs V, respectively.
Fresh oocytes (F), vitrified oocytes (V), and vitrified oocytes pretreated with metformin (M) were collected for lipidome analysis. a Differentially expressed lipids in F, V, and M groups. b Heatmap analysis of relative expression abundance of lipids in F, V, and M groups. c K-means cluster analysis of differentially expressed lipids in F, V, and M groups. d Pie chart analysis of FFA expression trends in V, and M groups compared with F group, respectively.
Then the expression abundance of all the detected FFA was analyzed by heatmap (Fig. 7b). The result showed that most of the long-chain unsaturated fatty acids (LCUFA) were upregulated during the freezing process compared with the F group, while there was no difference between V and M groups (Fig. 7b and Supplementary Data 4). Interestingly, long-chain saturated fatty acid (LCSFA) myricinic acid (C31:0) showed significant elevations in the M group compared with the V group (Fig. 7b and Supplementary Data 4). Then lipids were clustered based on the overall relative variation trend by K-means cluster analysis. Results showed that eight sub-classes of lipid profiles were constructed (Fig. 7c and Supplementary Data 5). All FFAs showed three variation trends (class 3, class 5, class 8) in the three groups. We also found that LCUFA showed an upward trend in the V group compared with the F group. Meantime, much more LCSFAs showed an upward trend in the M group compared with F and V groups (Fig. 7d).
Discussion
Studies have shown that vitrification induced severe membrane damage, which was coincided with mitochondrial dysfunction in oocytes38. It is reported that cell evolves to stimuli adaptation by fine-tuning mitochondrial activity39. Up to now, it is still unknown the interrelationship between mitochondrial activity and membrane stability. Mitochondrial temperature is an indicator of mitochondrial activity, which is vital for maintaining normal physiological function in cells40. We suppose that modifying mitochondrial activity by temperature regulation would alleviate cell damage. Therefore, the purpose of this study was to investigate the effect of reducing intracellular mitochondrial temperature before vitrification and uncover the underlying mechanism in protecting oocytes from cryoinjuries. We found that mitochondrial inhibitor metformin could reduce mitochondrial temperature in advance and significantly improve oocyte survival after vitrification. Subsequent analysis showed that metformin could decrease membrane fluidity via fatty acid elongation.
Mitochondria, served as the cellular energy center, generate bioenergy and thermogenesis to meet the needs of normal physiological activities in the cell41. Thermogenesis regulation plays a pivotal role in oocyte development42. The mitochondrial temperature is mainly generated by the energy released from the mitochondrial electron transport chain and oxidative phosphorylation process28. In the present study, we found three mitochondrial inhibitors (Met, OA, and UK5099) could decrease mitochondrial temperature, and metformin as well as UK5099 had no negative effect on oocyte development ability. Further studies showed that only metformin significantly improved oocyte survival after warming. Metformin, as a hypoglycemic drug, played an important role in diabetes treatment43. Recent research confirmed that biguanides interacted with mitochondrial complex I, and the initial inhibitory binding site of drugs was located in the amphibian region of the quinone binding channel33,34. Research showed that inhibition of mitochondrial respiratory complex I mitigated mitochondrial dysfunction and cell damage induced by proteasome inhibition44. In oocytes, reduced mitochondrial respiration could effectively reduce oocyte oxidative damage45. Thus, we concluded that metformin could improve the survival rate of vitrified oocytes by inhibiting mitochondrial metabolic activity.
We further conduct experiments to verify the effect of metformin on mitochondrial activity. Results showed that metformin reduced oocyte ATP levels and mitochondrial temperature before vitrification, which was similar to the previous study that metformin slowed mitochondrial respiration and reduced cellular ATP production by inhibiting mitochondrial complex I46. This state was similar to mitochondrial respiration quiescence42,45,47, which was manifested by decreased ATP production and reduced mitochondrial activity. Studies showed that mitochondrial quiescence could resist various environmental stresses, such as sub-optimal temperature48, aging49, and oxidative stress45. In addition, we found that metformin could slow down intracellular mitochondrial respiration and movement, which suggested that metformin inhibited mitochondrial ATP production and thus failed to provide energy for mitochondrial movement in the microtubule network50. In brief, our findings also confirmed that mitochondrial quiescence would protect oocytes from vitrification stress.
Studies have shown that vitrification seriously damaged the mitochondrial structure and function in oocytes21,22,51. We found that vitrification caused ATP reduction in porcine oocytes, which was consistent with other literature reports23. Interestingly, we observed that vitrification caused decreased mitochondrial temperature in porcine oocytes, which seemed to contradict previous findings in mouse oocytes26,27. As we know, the intracellular lipid content in pig oocytes was extremely higher than that of mouse oocytes52. Studies have demonstrated that there was a negative correlation between lipid droplet contents and freezing tolerance because of the lipid phase transition (LPT) during cryopreservation53,54. Since lipid droplets closely aligned with mitochondria55, we supposed that the differed severity and extent of LPT accounted for the discrepant mitochondrial temperature observed in porcine and mouse vitrified oocytes. And in porcine oocytes, LPT and lipid droplet redistribution would cause serious damage to their interconnected mitochondrial membrane structure, resulting in decreased mitochondrial thermogenesis. Transmission electron microscopy results further confirmed the above hypothesis, for that swollen mitochondria, disrupted membrane structure, and reduced mitochondrial electron density were observed in vitrified oocytes. Similarly, the electron density is reduced and the mitochondrial membrane is damaged in human vitrified oocytes, affecting mitochondrial function and oocyte quality56. These results suggest that damage in mitochondrial membrane would result in mitochondrial contents outflow and mitochondrial dysfunction eventually. Study has shown that metformin could protect mitochondrial membrane structure from hyperglycemia-induced damage57. In agreement with the previous findings, our results also confirmed that metformin could protect mitochondrial membrane structure, and further restore mitochondrial thermogenesis function during cryopreservation.
Membrane fluidity, one of the key physical parameters of membrane integrity, was involved in cell function regulation58. It was reported that variations in membrane fluidity accounted for the divergent survival rates in different cell types under stress20. Accordingly, we further explored the effect of metformin on membrane fluidity. The result showed that metformin pretreatment could also reduce cellular membrane fluidity augment induced by vitrification. Studies indicated that cryoprotectants and ice crystals could also cause cell membrane damage during vitrification, resulting in membrane rupture and cell death11. When the membrane was damaged, protective mechanism would be initiated to prevent further injury by reducing membrane fluidity19. Herein, the above findings indicated that metformin played a crucial role in slowing down oocyte membrane fluidity to prevent membrane damage.
Factors, including temperature, osmotic pressure, length and saturation of fatty acids would affect membrane fluidity17. To further explore the mechanism underlying metformin-modulated membrane fluidity, we performed transcriptome analysis. GSEA analysis showed that the expression levels of fatty acid elongation pathway-related genes such as HACD4, HACD1, ECHS1, ELOVL6, HADHA, HADH, HACD3, ELOVL3, and HSD17B12 had an increasing tendency in metformin pretreatment group. Fatty acid elongation process was under the regulation of mitochondrial and endoplasmic reticulum pathways59. HADHA, ECHS1, and HADH were involved in fatty acid synthesis in mitochondrial pathway60,61,62. In the endoplasmic reticulum pathway, long-chain fatty acid elongase ELOVL6 and ELOVL3 were involved in the first and rate-limiting step of long-chain fatty acid elongation, respectively63, while HSD17B12, HACD4, HACD1, and HACD3 participated in the second and third steps of long-chain fatty acid extension reaction, respectively64,65. It was demonstrated that the increment of fatty acid carbon chain length would promote the density of membrane lipid accumulation and thus decrease membrane fluidity66. Study also showed that fatty acid chain lengthening reduced cell membrane fluidity67. In addition, in vitro research indicated that metformin drove lipid accumulation by inhibiting mitochondrial complex I68. Therefore, our result implied that inhibition of mitochondria by metformin would activate lipid anabolism in oocytes, and result in reduced membrane fluidity by upregulating fatty acids elongation after freezing and thawing. Similarly, the lipidomics also found that vitrification process led to an increase in LCUFAs in porcine oocytes. High content of unsaturated fatty acids could increase membrane fluidity69. Therefore, this may be the reason for the high membrane fluidity observed in frozen porcine oocytes. What’s more, metformin pretreatment increased the intracellular content of the LCSFA myricinic acid (C31:0), which further confirmed that metformin affected the membrane fluidity by altering the fatty acid elongation process.
In summary, the pretreatment of metformin before oocyte vitrification can decrease mitochondrial activity, and improve the survival rate after thawing by modulating the fatty acid elongation process to change membrane fluidity, and ultimately achieve effective alleviation of oocyte cryoinjuries. This study provides insights into deciphering the regulatory role of mitochondrial temperature in oocyte development and lays the foundation for understanding the pivotal role of mitochondria in regulating membrane stability, which would be beneficial to improve oocyte quality during cryopreservation.
Methods
Animals and chemicals
Animals used in the experiments were binary crosses of landrace and large white. The mean age of animal was 6 months. All animal study was reviewed and approved by the Animal Care and Use Committee of China Agricultural University (AW01302202-1-1). All chemicals in this study were purchased from Sigma (St. Louis, MO, USA) unless otherwise indicated.
Oocyte collection and in vitro maturation (IVM)
Porcine ovaries were collected in the 37 °C, 0.9% physiological saline containing 75 mg/L penicillin G potassium and 50 mg/L streptomycin sulfate at the local slaughterhouse (Hebei, China) and then transported to our laboratory in 2 h. Ovaries were washed three times with sterile physiological saline. Cumulus–oocyte complexes (COCs) were aspirated from follicles in 3–8 mm diameter using 18-gauge needles. COCs with more than three layers of cumulus cells were selected for IVM. COCs were washed by prewarmed Tyrode’s lactate (TL)–HEPES–polyvinyl alcohol (0.1% PVA). Then 50 COCs were transferred into IVM medium at 38 °C, 5% CO2 in a humidified atmosphere for 42–44 h.
The IVM medium was TCM-199 supplemented with 2.78 mM d-glucose, 0.91 mM sodium pyruvate, 0.57 mM cysteine, 50 mg/mL streptomycin, 75 mg/mL penicillin, 0.01 U/mL FSH (Sioux Biochemical, Sioux Center, IA), 0.01 U/mL LH (Sioux Biochemical, Sioux Center, IA), 10 ng/mL epidermal growth factor (EGF), and 10% porcine follicular fluid.
Parthenogenetic activation for maturated oocytes
After maturation culture, 0.1% (w/v) hyaluronidase was used to denude COCs. Oocytes with the first polar body extrusion were selected. The selected oocytes were washed three times with activation solution (0.3 M mannitol, 0.05 mM CaCl2, 0.1 mM MgCl2, and 0.4% (w/v) bovine serum albumin dissolved in water). Then oocytes were transferred into an electro cell manipulation pulse generator and activated with a single direct current pulse of 65 V/mm for 80 μs. The activated oocytes were washed three times and placed in porcine zygote medium-3 (PZM-3) containing 5 μg/mL cytochalasin B and 10 μg/mL cycloheximide for 4 h at 38.5 °C, 5% CO2, 100% humidity in the incubator. Then the oocytes were washed and cultured in PZM-3 (day 0). The number of cleavage embryos was counted on day 2 and blastocyst formation was recorded on day 7.
Mitochondrial inhibitor treatment
Metformin was dissolved in DPBS to 40 mM. The stock solution was diluted to different concentrations (0, 100, 200, 400 μM) in HEPES-buffered Tyrode’s lactate (TL–HEPES). Other mitochondrial inhibitors, rotenone, oligomycin, and UK5099, were dissolved in DMSO to 10, 10, and 200 mM, respectively. Then rotenone stock solution was diluted to 0, 0.3, 0.6, and 1 μM with TL–HEPES. The oligomycin stock solution was diluted to 0, 0.5, 1.5, and 2.5 μM with TL–HEPES. The UK5099 stock solution was diluted to 0, 0.5, 1, and 2 μM with TL–HEPES. Then the denuded MII oocytes were treated with metformin, rotenone, oligomycin, and UK5099 for 1 h, respectively.
Oocyte vitrification and thawing
Mature porcine oocytes were vitrified using cryotop method. In brief, oocytes were washed three times by base medium (BM, DPBS with 20% fetal bovine serum (FBS)). Then oocytes were pre-equilibrated in E20 solution (BM with 20% ethylene glycol (EG)) for 3 min. After that, oocytes were equilibrated in EFS40 (DPBS with 12% FBS, 0.3 mol/L sucrose, 18% Ficoll, 40% EG) for 20–30 s, and then placed on the cryotop device in droplets. The volume of each droplet was no more than 1 μL. Then the cryotop device was immediately inserted into liquid nitrogen obliquely, and the time period from the exposure of oocytes in EFS40 to plunging into liquid nitrogen was within 1 min.
Oocytes thawing was performed by gradient thawing. First, the end of the cryotop containing oocytes was quickly immersed into a droplet containing 1 M sucrose. Oocytes were equilibrated on a warm table at 37 °C for 1 min. Then oocytes were sequentially transferred to BM containing 0.5, 0.25, and 0 M sucrose solution for 3, 3, and 5 min, respectively. Finally, oocytes were recovered for 2 h in IVM medium.
Fluorescein diacetate (FDA) staining
Only living cells could convert the nonfluorescent FDA into the green fluorescent compound fluorescein following intracellular lipase activity, thus, FDA was used for cell viability detection. First, the FDA stock solution (5 mg/mL) was diluted to 2.5 μg/mL with TL solution, and then the oocytes were incubated in the FDA solution for 1 min. Subsequently, the oocytes were washed three times with 0.1% PVA–PBS, placed in the microdroplets, and photographed using a fluorescence microscope (IX73, Olympus, Tokyo, Japan). Oocytes that exhibited green fluorescence were considered to be alive. Oocyte survival rate was calculated as the number of oocytes with green fluorescence divided by the corresponding total examined oocytes.
Mitochondrial temperature detection
Thermo-sensitive mitochondrial-targeted fluorescent dye Mito-Thermo-Yellow (MTY) was used to detect oocyte mitochondrial temperature. The MTY fluorescence intensity was negatively correlated with temperature70. MTY was diluted to 0.5 μmol/L with TL and prewarmed at 37 °C, 5% CO2 for 15 min. Then oocytes were incubated in MTY solution at 37 °C, 5% CO2 for 15 min. After that, oocytes were washed three times with TL and transferred into the laser confocal cell culture dish. Oocytes were observed with a laser confocal microscope (A1 Cell Imaging System; Nikon, Tokyo, Japan). The fluorescence intensity was evaluated by NISElements AR software (Nikon Instruments, Tokyo, Japan).
ATP detection
An enhanced ATP detection kit (Beyotime, Shanghai, China) was used to evaluate the oocyte’s ATP. Briefly, oocytes were lysed with lysis buffer on ice followed by vortex oscillation and centrifugation. Then ATP assay solution was added to a 96-well plate and equilibrated for 3–5 min at room temperature. ATP standards with different concentration gradients were prepared (0, 2, 4, 8, 16, 32 pmol). ATP standard solution and samples were added to each well respectively. Then a luminometer (Infinite F200; Tecan, Austria, G.m.b.H) was used to detect luminescence activity. ATP level was calculated by the standard curve method.
Transmission electron microscopy (TEM) analysis
Oocytes collected were washed three times with DPBS, fixed in 4% paraformaldehyde for 1 h at room temperature, and then fixed in 2.5% glutaraldehyde fixative for 14 h. The samples were then fixed with special osmic acid (1% osmic acid and 1.5% potassium ferrocyanide) on ice for 60 min and washed three times with ddH2O. After that, 1% uranyl acetate was added and samples were stained at room temperature for 1 h. Then samples were dehydrated with different concentrations of ethanol (50%, 70%, 80%, 90%, 100%). Samples were soaked twice with propylene oxide and permeated with 812 resin and propylene oxide overnight at room temperature. After embedding, the samples were serially sectioned with an automated microtome at a thickness of 70 nm, and the oocyte sections with the largest diameter were fixed on a copper mesh. The sections were stained with uranium dioxyacetate. Then images were taken under TEM with selected magnifications of 7000×, 20,000×. The number of mitochondria, mitochondrial electron density, and mitochondrial area were evaluated for each image in the fixed area of oocytes.
Fluorescence recovery after photobleaching (FRAP)
TMRM (Invitrogen, Carlsbad, CA, USA) fluorescent dye was used in the mitochondrial FRAP experiment. Briefly, TMRM was diluted to 100 nM with H199. Then oocytes were transferred to TMRM solution at 37 °C, 5% CO2 for 30 min. Oocytes were washed three times and transferred into a glass bottom dish. FRAP was performed on the oocytes using Nikon A1 confocal microscope (A1 Cell Imaging System; Nikon, Tokyo, Japan). First, a bleached area was selected, and three fluorescence images were collected before bleaching with an interval of 5 s. The bleaching laser intensity was 100%, and the bleaching time was 7.93 s. Recovery time was 3 min after bleaching, and fluorescence images were collected at 5 s intervals. The fluorescence intensity of the bleaching area was statistically analyzed by NISElements AR software (Nikon Instruments, Tokyo, Japan). Fi represented the fluorescence intensity before bleaching. F0 represented the fluorescence intensity after bleaching, and F1 represented the recovery fluorescence intensity after bleaching. The data were normalized, and the fluorescence recovery rate was calculated by the formula R = (F1 − F0)/(Fi − F0).
Membrane FRAP was conducted with Dil (Beyotime, Shanghai, China) fluorescent dye. Briefly, Dil was diluted to 10 μM with TL. Oocytes were placed in Dil solution at 37 °C, 5% CO2 for 10 min. Then oocytes were washed three times and transferred into a glass bottom dish. FRAP was performed on the oocyte membrane using Nikon A1 confocal microscope (A1 Cell Imaging System; Nikon, Tokyo, Japan). The laser bleaching time was 15.82 s, and the manipulating steps were in accordance with that of mitochondrial FRAP method as mentioned above.
Mitochondrial respiration test
Oocyte mitochondrial respiration was measured by Seahorse metabolic flux analyzer (XFe96). In short, the XFe96 sensor cartridge was first hydrated overnight with sterile water in a CO2-free incubator at 37 °C. After that, Cell Tak was added to the cell culture plate. 15–50 porcine oocytes (one biological duplicate) were put into 50 μL test solution (supplementing XF Base medium with 1 mM sodium pyruvate, 2 mM glutamine, 5 mM glucose, and the pH was adjusted to 7.4 ± 0.05). Then the test solution containing porcine oocytes was added to the test hole. 125 μL test solution was added to it, and then placed back to culture for another 20 min. The final concentrations (per well) of oligomycin, FCCP (carbonyl cyanide p-trifluoromethoxyphenylhydrazone) and Rot/AA (rotenone/antamycin A) of the Mitochondrial Stress Test Kit reagent were 1, 2.5, and 1 μM, respectively. Several different mitochondrial electron transport chain inhibitors were injected sequentially. Then oocyte oxygen consumption was recorded. The Wave software (Agilent Technologies, Santa Clara, CA, USA) was used to analyze mitochondrial basal respiration, maximum respiration, and ATP-coupled respiration. The data were normalized by calculating the number of oocytes per sample.
Transcriptome analysis
The experiment consisted of three groups: fresh group (F), vitrified group (V), and metformin pretreatment vitrified group (M). Oocytes were collected from each group and placed in centrifuge tubes, and each group had three biological replicates. TRIzol method (Invitrogen, CA, USA) and RNase-free DNase I (Takara, Kusatsu, Japan) were used to extract total RNA. 1.5 μg RNA/sample was used as input materials. Sequencing libraries were generated using NEBNext® Ultra™ RNA Library Prep Kit for Illumina® (NEB, USA) following the manufacturer’s recommendations. The library preparations were sequenced on an Illumina Novaseq 6000 platform by the Beijing Allwegene Technology Company Limited (Beijing, China) and paired-end 150 bp reads were generated. Clean data (clean reads) were obtained by removing low-quality reads and those containing adapter and ploy-N sequences from raw data of fastq format. Further analysis and annotation were performed by alignment with the reference genome (ftp://ftp.ensembl.org:21/pub/release-100/fasta/sus_scrofa/).
HTSeq software and FPKM (Fragments Per Kilobase of exon model per Million mapped reads) were used to analyze the gene expression levels of each sample, and DESeq was used to analyze transcripts expressions. The criteria for screening differentially expressed mRNA was |log2 (FoldChange)| > 1, q value < 0.05. Logarithm base two was taken for each mRNA fold change, and the absolute value of logarithm base 10 was taken for p value, and a histogram was drawn. The relative expression level value log2(ratios) of differential expressed genes were clustered using H-cluster, K-means, and SOM.
Gene Set Enrichment Analysis (GSEA) and Gene Ontology (GO) were performed to identify biological functions and heatmap was used for gene cluster analysis. Gene functional annotations were conducted by String (https://cn.string-db.org/). Columnar classification map was drawn by the Lc-Bio Technologies online platform (Hangzhou, China).
Lipidomic analysis
Fresh, vitrified, and metformin-pretreated vitrified MII oocytes were collected and stored at −80 °C, respectively. Each group had three biological replicates. Oocytes were thawed on ice and the reagent (MTBE:MeOH = 3:1, v/v) containing an internal standard mixture was used for lipids extraction. Briefly, the mixture was rotated for 15 min, and 200 μL water was added to the mixture. Followed by vortex oscillation for 1 min, the mixture was centrifuged at 12,000 rpm for 10 min. Then the upper organic layer was evaporated by the vacuum concentrator, and the dry extract was dissolved in a reconstituted solution (ACN:IPA = 1:1, v/v) for LC–MS/MS analysis.
LC–ESI–MS/MS system (UPLC, ExionLC AD, MS, QTRAP® System) was used to quantify the lipid contents in the extracted samples. Briefly, lipids were performed on the UPLC column, Thermo Accucore™ C30 (2.6 μm, 2.1 mm*100 mm i.d.). The solvent system contained A, acetonitrile/water (60/40, V/V, 0.1% formic acid, 10 mmol/L ammonium formate) and B, acetonitrile/isopropanol (10/90 V/V, 0.1% formic acid, 10 mmol/L ammonium formate). The injection volume was 2 μL, and the gradient program was A/B (80:20, V/V) at 0 min, 70:30 V/V at 2.0 min, 40:60 V/V at 4 min, 15:85 V/V at 9 min, 10:90 V/V at 14 min, 5:95 V/V at 15.5 min, 5:95 V/V at 17.3 min, 80:20 V/V at 17.3 min, 80:20 V/V at 20 min with the flow rate of 0.35 mL/min under 45 °C. The effluent was alternatively connected to an ESI-triple quadrupole-linear ion trap (QTRAP)-MS. LIT and triple quadrupole (QQQ) scans were acquired on a triple quadrupole-linear ion trap mass spectrometer (QTRAP), QTRAP® LC–MS/MS System, equipped with an ESI Turbo Ion-Spray interface, operating in positive and negative ion mode and controlled by Analyst 1.6.3 software (Sciex).
After UPLC–MS/MS analysis, raw data were processed using the software Analyst 1.6.3. Based on the metabolic database of the Beijing Allwegene Technology Company Limited (Beijing, China), the lipids of the samples were qualitatively and quantitatively analyzed by mass spectrometry. The quality control sample was prepared by mixing sample extracts, which was used to perform sample quality control analysis. For two-group analysis, differential lipids were determined by VIP (VIP > 1) and P value (P < 0.05, Student’s t-test). Hierarchical clustering heatmap was drawn using the R (Version 1.0.12) software. Columnar classification map was drawn by origin 2022 (Microcal Software Inc., Northampton, MA, USA). The trend of relative lipid content variation in all different lipids was analyzed by K-means cluster analysis using the R (Version 3.5.1). Unit variance scaling was used for K-means cluster analysis, and the calculation formula was as follows: x′ = (x − μ)/σ, where µ is the mean and σ is the standard deviation.
Statistics and reproducibility
Statistical analysis was performed using GraphPad Prism 9 (GraphPad Software Inc., La Jolla, CA, USA). Oocyte survival rate was analyzed by Chi-square test. Student’s t-test was used for comparison between two independent samples and one-way ANOVA test for more than two groups. Independent biological replication ensures reproducibility of experimental results. At least three replicates were performed for each experiment in this study. All data were expressed as means ± standard error of the mean (SEM). Statistical significance was set at P < 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data generated or analyzed during this study are included in this published article, and available from the corresponding author upon reasonable request. The source data for the main figure are provided in Supplementary Data 6. RNA-seq data are deposited in the NCBI Sequence Read Archive (Accession Number PRJNA1129607).
References
Poli, M. & Capalbo, A. Oocyte cryopreservation at a young age provides an effective strategy for expanding fertile lifespan. Front. Reprod. Health 3, 704283 (2021).
Giannopapa, M., Sakellaridi, A., Pana, A. & Velonaki, V. S. Women electing oocyte cryopreservation: characteristics, information sources, and oocyte disposition: a systematic review. J. Midwifery Womens Health 67, 178–201 (2022).
Mara, L., Casu, S., Carta, A. & Dattena, M. Cryobanking of farm animal gametes and embryos as a means of conserving livestock genetics. Anim. Reprod. Sci. 138, 25–38 (2013).
Tharasanit, T. & Thuwanut, P. Oocyte cryopreservation in domestic animals and humans: principles, techniques and updated outcomes. Animals 11, 17 (2021).
Imesch, P. et al. Developmental potential of human oocytes matured in vitro followed by vitrification and activation. J. Ovarian Res. 6, 5 (2013).
Song, W. Y. et al. Effects of vitrification on outcomes of in vivo-mature, in vitro-mature and immature human oocytes. Cell. Physiol. Biochem. 38, 2053–2062 (2016).
Molina, I. et al. Osmotic-shock produced by vitrification solutions improves immature human oocytes in vitro maturation. Reprod. Biol. Endocrinol. 14, 9 (2016).
Cao, Y. X. et al. Cryopreservation of immature and in-vitro matured human oocytes by vitrification. Reprod. Biomed. Online 19, 369–373 (2009).
Cohen, Y. et al. Decreased pregnancy and live birth rates after vitrification of in vitro matured oocytes. J. Assist. Reprod. Genet. 35, 1683–1689 (2018).
Iussig, B. et al. A brief history of oocyte cryopreservation: arguments and facts. Acta Obstet. Gynecol. Scand. 98, 550–558 (2019).
Ma, Y. M. et al. Advanced biomaterials in cell preservation: hypothermic preservation and cryopreservation. Acta Biomater. 131, 97–116 (2021).
Edmond, V. et al. Downregulation of ceramide synthase-6 during epithelial-to-mesenchymal transition reduces plasma membrane fluidity and cancer cell motility. Oncogene 34, 996–1005 (2015).
Matsuzaki, T. et al. Defining lineage-specific membrane fluidity signatures that regulate adhesion kinetics. Stem Cell Rep. 11, 852–860 (2018).
Ben-Dov, N. & Korenstein, R. Proton-induced endocytosis is dependent on cell membrane fluidity, lipid-phase order and the membrane resting potential. Biochim. Biophys. Acta 1828, 2672–2681 (2013).
Gasser, K. W., Goldsmith, A. & Hopfer, U. Regulation of chloride transport in parotid secretory granules by membrane fluidity. Biochemistry 29, 7282–7288 (1990).
Yasumiba, S. et al. Cyclosporin A reduces canalicular membrane fluidity and regulates transporter function in rats. Biochem. J. 354, 591–596, (2001).
Los, D. A. & Murata, N. Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys. Acta 1666, 142–157 (2004).
Pedrera, L. et al. Sticholysin I-membrane interaction: an interplay between the presence of sphingomyelin and membrane fluidity. Biochim. Biophys. Acta 1838, 1752–1759 (2014).
Lin, Y. C., Chipot, C. & Scheuring, S. Annexin-V stabilizes membrane defects by inducing lipid phase transition. Nat. Commun. 11, 13 (2020).
Mizuno, M. et al. Cell membrane fluidity and ROS resistance define DMSO tolerance of cryopreserved synovial MSCs and HUVECs. Stem Cell Res. Ther. 13, 16 (2022).
Cao, B. J. et al. Oxidative stress and oocyte cryopreservation: recent advances in mitigation strategies involving antioxidants. Cells 11, 18 (2022).
Chang, C. C., Shapiro, D. B. & Nagy, Z. P. The effects of vitrification on oocyte quality. Biol. Reprod. 106, 316–327 (2022).
Dai, J. J. et al. Changes in mitochondrial function in porcine vitrified MII-stage oocytes and their impacts on apoptosis and developmental ability. Cryobiology 71, 291–298 (2015).
Xu, J. H. et al. Mitophagy is involved in the mitochondrial dysfunction of vitrified porcine oocytes. Mol. Reprod. Dev. 88, 427–436 (2021).
Jia, B. Y. et al. Proteomic changes of porcine oocytes after vitrification and subsequent in vitro maturation: a tandem mass tag-based quantitative analysis. Front. Cell Dev. Biol. 8, 11 (2020).
Zhuan, Q. R. et al. Antioxidant procyanidin B2 protects oocytes against cryoinjuries via mitochondria regulated cortical tension. J. Anim. Sci. Biotechnol. 13, 22 (2022).
Gao, L. et al. Melatonin rescues the aneuploidy in mice vitrified oocytes by regulating mitochondrial heat product. Cryobiology 89, 68–75 (2019).
Chretien, D. et al. Mitochondria are physiologically maintained at close to 50 °C. PLoS Biol. 16, e2003992 (2018).
El-Gammal, Z. et al. Regulation of mitochondrial temperature in health and disease. Pflugers Arch. 474, 1043–1051 (2022).
Wei-Dong, L. et al. Metformin protects against mouse oocyte apoptosis defects induced by arecoline. Cell Prolif. 53 https://doi.org/10.1111/cpr.12809 (2020).
Yongzhi, C. et al. Metformin promotes in vitro maturation of oocytes from aged mice by attenuating mitochondrial oxidative stress via SIRT3-dependent SOD2ac. Front. Cell Dev. Biol. 10 https://doi.org/10.3389/fcell.2022.1028510 (2022).
Melanie, F. et al. Metformin in reproductive biology. Front. Endocrinol. 9 https://doi.org/10.3389/fendo.2018.00675 (2018).
Bridges, H. R. et al. Structural basis of mammalian respiratory complex I inhibition by medicinal biguanides. Science 379, 351–357 (2023).
Vial, G., Detaille, D. & Guigas, B. Role of mitochondria in the mechanism(s) of action of metformin. Front. Endocrinol. 10, 8 (2019).
Niu, Y. J. et al. Melatonin enhances mitochondrial biogenesis and protects against rotenone-induced mitochondrial deficiency in early porcine embryos. J. Pineal Res. 68, 16 (2020).
Kansaku, K. et al. Differential effects of mitochondrial inhibitors on porcine granulosa cells and oocytes. Theriogenology 103, 98–103 (2017).
Tanaka, K. et al. Abnormal early folliculogenesis due to impeded pyruvate metabolism in mouse oocytes. Biol. Reprod. 105, 64–75 (2021).
Gualtieri, R. et al. Mitochondrial dysfunction and oxidative stress caused by cryopreservation in reproductive cells. Antioxidants 10, 24 (2021).
Bennett, C. F., Latorre-Muro, P. & Puigserver, P. Mechanisms of mitochondrial respiratory adaptation. Nat. Rev. Mol. Cell Biol. 23, 817–835 (2022).
Bertholet, A. M. & Kirichok, Y. Mitochondrial H+ leak and thermogenesis. Annu. Rev. Physiol. 84, 381–407 (2022).
Okabe, K. & Uchiyama, S. Intracellular thermometry uncovers spontaneous thermogenesis and associated thermal signaling. Commun. Biol. 4, 1377 (2021).
Zhou, D. et al. Mito-Q promotes porcine oocytes maturation by maintaining mitochondrial thermogenesis via UCP2 downregulation. Theriogenology 187, 205–214 (2022).
Maruthur, N. M. et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann. Intern. Med. 164, 740 (2016).
Lee, S. J., Youn, Y. C., Han, E. S. & Lee, C. S. Depressant effect of mitochondrial respiratory complex inhibitors on proteasome inhibitor-induced mitochondrial dysfunction and cell death in PC12 cells. Neurochem. Res. 30, 1191–1200 (2005).
He, B. et al. Melatonin-induced increase of lipid droplets accumulation and in vitro maturation in porcine oocytes is mediated by mitochondrial quiescence. J. Cell. Physiol. 233, 302–312 (2018).
Wang, Y. et al. Metformin improves mitochondrial respiratory activity through activation of AMPK. Cell Rep. 29, 1511 (2019).
Hocaoglu, H. & Sieber, M. Mitochondrial respiratory quiescence: a new model for examining the role of mitochondrial metabolism in development. Semin. Cell Dev. Biol. 138, 94–103 (2023).
Alekseenko, L. L. et al. Quiescent human mesenchymal stem cells are more resistant to heat stress than cycling cells. Stem Cells Int. 2018, 15 (2018).
Unal, E., Kinde, B. & Amon, A. Gametogenesis eliminates age-induced cellular damage and resets life span in yeast. Science 332, 1554–1557 (2011).
McCarron, J. et al. From structure to function: mitochondrial morphology, motion and shaping in vascular smooth muscle. J. Vasc. Res. 50, 357–371 (2013).
Mateo-Otero, Y., Yeste, M., Damato, A. & Giaretta, E. Cryopreservation and oxidative stress in porcine oocytes. Res. Vet. Sci. 135, 20–26 (2021).
Baenziger, J., Morris, M., Darsaut, T. & Ryan, S. Effect of membrane lipid composition on the conformational equilibria of the nicotinic acetylcholine receptor. J. Biol. Chem. 275, 777–784 (2000).
Amstislavsky, S. et al. Influence of cellular lipids on cryopreservation of mammalian oocytes and preimplantation embryos: a review. Biopreserv. Biobank. 17, 76–83 (2019).
Abe, H., Yamashita, S., Satoh, T. & Hoshi, H. Accumulation of cytoplasmic lipid droplets in bovine embryos and cryotolerance of embryos developed in different culture systems using serum-free or serum-containing media. Mol. Reprod. Dev. 61, 57–66 (2002).
Sturmey, R. G., O’Toole, P. J. & Leese, H. J. Fluorescence resonance energy transfer analysis of mitochondrial: lipid association in the porcine oocyte. Reproduction 132, 829–837 (2006).
Gualtieri, R. et al. Slow cooling of human oocytes: ultrastructural injuries and apoptotic status. Fertil. Steril. 91, 1023–1034 (2009).
Waisundara, V. Y., Hsu, A., Tan, B. K. H. & Huang, D. J. Baicalin reduces mitochondrial damage in streptozotocin-induced diabetic Wistar rats. Diabetes Metab. Res. Rev. 25, 671–677 (2009).
Los, D., Mironov, K. & Allakhverdiev, S. Regulatory role of membrane fluidity in gene expression and physiological functions. Photosynth. Res. 116, 489–509 (2013).
Tehlivets, O., Scheuringer, K. & Kohlwein, S. D. Fatty acid synthesis and elongation in yeast. Biochim. Biophys. Acta 1771, 255–270 (2007).
Kamijo, T., Aoyama, T., Komiyama, A. & Hashimoto, T. Structural analysis of cDNAs for subunits of human mitochondrial fatty acid beta-oxidation trifunctional protein. Biochem. Biophys. Res. Commun. 199, 818–825 (1994).
Yamada, K. et al. Clinical, biochemical and metabolic characterisation of a mild form of human short-chain enoyl-CoA hydratase deficiency: significance of increased N-acetyl-S-(2-carboxypropyl) cysteine excretion. J. Med. Genet. 52, 691–698 (2015).
Clayton, P. T. et al. Hyperinsulinism in short-chain L-3-hydroxyacyl-CoA dehydrogenase deficiency reveals the importance of beta-oxidation in insulin secretion. J. Clin. Investig. 108, 457–465 (2001).
Guillou, H., Zadravec, D., Martin, P. G. P. & Jacobsson, A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Prog. Lipid Res. 49, 186–199 (2010).
Kihara, A. Very long-chain fatty acids: elongation, physiology and related disorders. J. Biochem. 152, 387–395 (2012).
Ikeda, M. et al. Characterization of four mammalian 3-hydroxyacyl-CoA dehydratases involved in very long-chain fatty acid synthesis. FEBS Lett. 582, 2435–2440 (2008).
Hazel, J. R. & Williams, E. E. The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog. Lipid Res. 29, 167–227 (1990).
Kingston, A. W., Subramanian, C., Rock, C. O. & Helmann, J. D. A σW-dependent stress response in Bacillus subtilis that reduces membrane fluidity. Mol. Microbiol. 81, 69–79 (2011).
Lord, S. R. et al. Transcriptomic analysis of human primary breast cancer identifies fatty acid oxidation as a target for metformin. Br. J. Cancer 122, 258–265 (2020).
Brenner, R. R. Effect of unsaturated acids on membrane structure and enzyme kinetics. Prog. Lipid Res. 23, 69–96 (1984).
Arai, S. et al. Mitochondria-targeted fluorescent thermometer monitors intracellular temperature gradient. Chem. Commun. 51, 8044–8047 (2015).
Acknowledgements
We thank Professor Yong-Tae Chang from POSTECH for providing us with the mitochondrial temperature dye MTY. This work was supported by National Key Research and Development Program Topics, Grant/Award Number: 2021YFD1200402; National Natural Science Foundation of China, Grant/Award Number: 32072736; Natural Science Foundation of Hebei province, Grant/Award Number: H2023206029; Central Guidance on Local Science and Technology Development Fund of Hebei province, Grant/Award Number: 226Z7713G; Specific Project of Hebei Province for Outstanding Talents in Clinical Medicine (LS202206); Innovative Project for Clinical Medicine of Hebei Medical University (2022LCTD-B1); Xinghuo Program of the First Hospital of Hebei Medical University, Grant/Award Number: XH202005.
Author information
Authors and Affiliations
Contributions
D.Z. and H.L. designed and performed major experiments and analyzed data. A.L. contributed to the analysis of transcriptomics and lipidomics. L.Z. conducted the part of the experiments. Q.Z., Y.L., G.Z., and L.M. contributed to the samples collection, performed partial experiment, and analyzed data. J.L. and X.F. initiated, organized, and designed the study. D.Z., J.L., and X.F. wrote the manuscript. Y.H. and G.W. revised the manuscript. All authors commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Pascal Froment and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Christopher Hine and Dario Ummarino. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, D., Liu, H., Zheng, L. et al. Metformin alleviates cryoinjuries in porcine oocytes by reducing membrane fluidity through the suppression of mitochondrial activity. Commun Biol 7, 925 (2024). https://doi.org/10.1038/s42003-024-06631-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-024-06631-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.