Abstract
Coral polyps are composed of four tissues; however, their characteristics are largely unexplored. Here we report biological characteristics of tentacles (Te), mesenterial filaments (Me), body wall (Bo), and mouth with pharynx (MP), using comparative genomic, morpho-histological, and transcriptomic analyses of the large-polyp coral, Fimbriaphyllia ancora. A draft F. ancora genome assembly of 434 Mbp was created. Morpho-histological and transcriptomic characterization of the four tissues showed that they have distinct differences in structure, primary cellular composition, and transcriptional profiles. Tissue-specific, highly expressed genes (HEGs) of Te are related to biological defense, predation, and coral-algal symbiosis. Me expresses multiple digestive enzymes, whereas Bo expresses innate immunity and biomineralization-related molecules. Many receptors for neuropeptides and neurotransmitters are expressed in MP. This dataset and new insights into tissue functions will facilitate a deeper understanding of symbiotic biology, immunology, biomineralization, digestive biology, and neurobiology in corals.
Similar content being viewed by others
Introduction
Scleractinian corals are the primary builders of coral reef ecosystems, which nurture a wide variety of marine organisms1,2. Coral reefs support tropical and sub-tropical fisheries and tourism3. Despite their ecological and economic importance, biological characteristics of corals are still largely unknown. A comprehensive understanding of coral biology will not only allow more accurate predictions of anthropogenic impacts on corals4. but may help to establish methods for preservation and propagation of extant corals.
Corals belong to the phylum Cnidaria, a group of animals possessing cnidocytes, also known as stinging cells5. Corals and other cnidarians, e.g., sea anemones, hydras, and jellyfish, are classically regarded as diploblastic animals5. However, data suggesting a triploblastic nature have been reported6. They have simple body structures, and tissues, organs, and organ systems characteristic of vertebrates are not present7. Yet, individual coral polyps are composed largely of several functionally defined tissues: tentacle, mouth with pharynx, mesenterial filament, and body wall5. Tentacles, located atop each polyp, are responsible for predation, attack, and defense. The mouth with the associated pharynx is located in the upper part of the polyp not only to engulf prey, but also to release excreta, gametes, and larvae. Mesenterial filaments are located in the body cavity and constitute the primary tissue responsible for digestion and defense8. The body wall separates the interior of a coral polyp from the environment. Although differences and functions can be inferred from tissue locations and behavioral observations9,10, molecular and cellular characteristics and detailed functions of those tissues remain largely unexplored. Fine characterization of each tissue comprising coral polyps should promote better understanding of biological characteristics of corals.
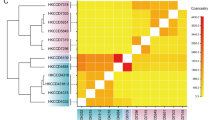
Tissue-specific analysis is a powerful approach to characterize structure, cellular composition, and function. Detailed cellular and molecular analyses of isolated target tissues, rather than of whole individuals, allow us to explore differences in organization and function among tissues, as well as intrinsic mechanisms underlying specific functions11,12. However, tissue-specific analyses have rarely been conducted in corals. This is because the coral species often used for cellular and/or molecular studies, e.g., Acropora millepora13, Pocillopora damicornis14, Stylophora pistillata15, have very small polyps (about 1 mm in diameter), making tissue isolation difficult. Fimbriaphyllia ancora (family Euphyllidae), formerly called Euphyllia ancora (Fig. 1a), is distributed widely in reefs of the Indo-Pacific Ocean16,17, and is one of the most popular coral species in the aquarium industry18. Morphologically, it has tentacles with swollen, anchor-like tips and a flabello-meandroid skeleton (Fig. 1b, c)16. The most notable morphological characteristic is its polyp size (3–5 cm in diameter), and techniques for isolating tissues from these polyps have been established19.
a External appearance of an F. ancora colony. b, c Top and side views of an F. ancora skeleton. The skeleton was photographed after removal of polyp tissue. Hammer or anchor-like tentacles and the flabello-meandroid skeleton typify F. ancora. d Molecular phylogeny of F. ancora based on 4208 single-copy Orthogroups (OGs) identified from published scleractinian genomes. All nodes are supported with 100% bootstrap values. e A heatmap showing numbers of shared OGs among scleractinian genomes.
Using F. ancora, we sought to determine cellular and molecular characteristics of the four major tissues (tentacle, mesenterial filament, body wall, and mouth with pharynx) constituting coral polyps. Ultimately, such fundamental data will enhance our understanding of biological characteristics of corals. To this end, we generated a draft genome of F. ancora and performed morpho-histological and transcriptomic analyses of all four polyp tissues.
Results
The Fimbriaphyllia ancora draft genome
In total, 14.6 Gbp of PacBio HiFi reads (QV > 20, 1.8 M reads, average length: 8145 bp) were obtained. Then, we assembled those reads into an F. ancora draft genome assembly of 434 Mbp, comprising 205 scaffold sequences with an N50 size of 5.18 Mbp (Table 1). The HiFi raw sequences had a single GC peak at around 40% (Supplementary Fig. S1a, b), and only a few reads (0.25%) mapped to reported Symbiodiniaceae genomes (BLASTN, <e−10). This indicated that there were very few contaminations of zooxanthellae sequences. A total of 27,537 protein-coding genes were predicted, and the number of genes was comparable to those of other coral genomes (Table 120,21,22,23,24,25,26). Benchmarking Universal Single-Copy Orthologs (BUSCO) analyses27,28, which assess whether universal single-copy orthologous genes observed in more than 90% of metazoan species (from the OrthoDB database of orthologs) (www.orthodb.org; version 9) are recovered in a genome/transcriptome assembly, yielded completeness scores for the F. ancora genome assembly and gene models of about 95.6% and 96.1% (Complete BUSCO %), respectively. This indicated that the genome assembly and gene models are of comparable quality to those of previously reported coral genomes.
Next, orthologous relationships with other scleractinian corals were investigated using genome sequences of 4 acroporid species (Acropora millepora, Acropora tenuis, Montipora cactus, and Astreopora myriophthalma), Porites australiensis, Stylophora pistillata, Pocillopora damicornis, and Orbicella faveolata. We identified 23,701 orthologous gene families (OGs) from scleractinian genomes and obtained 17,128 OGs for F. ancora. Phylogenomic analysis of these anthozoan genomes using concatenated amino acid sequences of 4208 single-copy orthologous group genes (1,817,638 AAs) yielded robust phylogenetic relationships, with all clades supported by 100% bootstrap values (Fig. 1d), clearly indicating that F. ancora belongs to the Complexa coral clade, as reported in previous molecular phylogenic analyses (Fukami et al.29; Kitahara et al.30). Among the OGs in F. ancora, 10,016 are shared by all scleractinians, 16,511 OGs are shared with the Complexa group, and 371 gene families appear unique to F. ancora (Fig. 1e). The latter gene families comprise 1176 genes, of which 267 showed similarities to genes in the SwissProt database, whereas 277 resembled those in Pfam. These genes may have originated by gene duplication in the Fimbriaphyllia lineage.
Morphological and histological characteristics of the four tissues comprising F. ancora polyps
Four major tissues: tentacle, mesenterial filament, body wall, and mouth with pharynx (Fig. 2a–c) were isolated, and their morpho-histological characteristics were examined (Table 2).
a A F. ancora polyp showing the 4 tissues analyzed in this study. b Top view of an F. ancora colony in an aquarium. The mouth is at the center of the polyp, and tentacles surround the mouth. c Representative picture of a dissected F. ancora polyp with tentacles, part of the mouth with the pharynx, mesenterial filaments, and part of the body wall. d–o Appearances of isolated polyp tissues and their histological micrographs. d–f A tentacle. g–i A mesenterial filament. j–l A piece of the body wall. m–o A piece of the mouth with the pharynx. d, g, j, m Bright views of isolated polyp tissues. e, h, k, n U‐MWIB 2 (GFP) filter view of the same field as in d, g, j, and m. Dashed lines in h, k, and n show outlines of each tissue. f, i, l, o Histological sections of isolated tentacle, mesenterial filament, body wall, and mouth and pharynx, respectively. epi epidermis, gas gastrodermis, zoo zooxanthellae, nem nematocyte, muc mucocytes.
Tentacle
Tentacles were basically tubular, and their tips were hammer- or anchor-like (Fig. 2a–c). They were hollow and expanded by retaining seawater inside. Each tentacle was 3–5 cm long and 1–2 cm wide when fully expanded. Tips were white to light green, and columns were brown, due to zooxanthellae (Fig. 2b). White areas at tentacle tips showed strong green fluorescence under a fluorescence microscope (Fig. 2d, e). Histologically, two distinct cell layers, epidermis, and gastrodermis, were observed. Epidermis was characterized by a high density of nematocytes and mucocytes, whereas gastrodermis cells and zooxanthellae were observed in the gastrodermis (Fig. 2f).
Mesenterial filament
Mesenterial filaments, located in the aboral part of the polyp, are formed by gastrodermal extensions toward the body cavity (Fig. 2a, c). Isolated mesenterial filaments were white to pale yellow with an undulating structure (Fig. 2g). Individual mesenterial filaments (when contracted) were 2–3 mm in diameter. Slight green fluorescence was observed (Fig. 2h). Histologically, the margins were distinctive and club-shaped, and only a few zooxanthellae were observed. A number of nematocytes and mucocytes were present in the marginal area (Fig. 2i).
Body wall
The isolated part of the body wall was white (Fig. 2j) and buoyant in seawater. Slight green fluorescence was observed under fluorescence microscopy (Fig. 2k). Histologically, the body wall consisted of a distinct two-cell layer, a thin epidermis containing a calicodermis layer, and a relatively thick, porous gastrodermis (Fig. 2l). Only a few nematocytes, mucocytes, and zooxanthellae were observed in the body wall.
Mouth with pharynx
Isolated pieces of mouth and pharynx were white to gray with a furrowed structure (Fig. 2m). Under the fluorescence microscope, spatial localization of green fluorescence was observed in the mouth, but not in the pharynx (Fig. 2n). Histologically, resembling tentacles, the mouth and pharynx consisted of two distinct layers, epidermis with many nematocytes and mucocytes and gastrodermis with many zooxanthellae (Fig. 2o).
Transcriptome analysis of the four tissues
Numbers of genes expressed included 19,057 in tentacles, 19,582 in mesenterial filaments, 18,777 in the body wall, and 19,587 in the mouth with pharynx (Fig. 3a). Hierarchical clustering and a correlation heatmap showed that tentacles have the most specialized expression profiles (Fig. 3b). NMDS analysis further showed that transcriptional profiles of the four tissues were significantly different (Fig. 3c). In order to identify genes that characterize each tissue, we examined tissue-specific, highly expressed genes (HEGs): tentacles (856) mesenterial filaments (326), body wall (479), and mouth and pharynx (173) (q-value < 0.05, Fig. 3d). Functional enrichment analysis of HEGs (Fig. 3e) revealed tentacle-specific HEGs related to neurotransmitter transport, amino-acid transport, chloride channels, toxins, and nematocysts (>6-fold enrichment). Mesenterial filament-specific HEGs were related to polysaccharide degradation, serine proteases, and protease inhibitors, whereas body wall-specific HEGs were associated with basement membrane and extracellular matrix. In mouth and pharynx, genes associated with the Wnt signaling pathway were significantly enriched (Fig. 3e).
a Total numbers of genes with detected transcripts in each tissue type using five abundance levels for transcripts per million (TPM) values; 1–5 TPM (brown), 5–20 TPM (dark yellow), 20–100 TPM (light orange), 100–500 TPM (dark orange), more than 500 TPM (dark red). In tentacle, 19,057 genes were detected, with 19,582 in mesenterial filaments, 18,777 in body wall, and 19,587 in mouth and pharynx. b Pearson correlation of tissue-specific transcriptomes of the four tissues using 16,703 expressed genes in all samples (TPM, >1). c Nonmetric multidimensional scaling (NMDS) plot based on Bray-Curtis distances of gene expression profiles from tentacle, mesenterial filament, body wall, and mouth and pharynx. 12,302 genes TPM > 1 in 16 samples) were used for comparisons. Colored lines surrounding each sample type represent covariance ellipsoids. d Relative gene expression levels of specifically highly expressed genes (total 1834 genes) in the four polyp tissues. TPM values were scaled to row Z-scores for each gene. e Significantly upregulated UniProt keywords detected from tissue-specific HEGs. Significantly (>4-fold change, P < 0.05) enriched UniProt keywords in biological processes (red bar), molecular function (blue bar), and cellular component (green bar). The X-axis represents the magnitude of fold enrichment. The Y-axis represents the functional category.
Biological characteristics of the four tissues revealed by transcriptomic analysis
Based on results of morpho-histological observation and functional enrichment analysis of HEGs described above, we explored and further selected expressed genes characterizing each tissue.
Tentacles
Tentacles highly expressed genes associated with biological defense and predation (Fig. 4). This included cnidocytes (Nematocyst expressed proteins), venom (DELTA-thalatoxin, DELTA-alicitoxin, and U-actitoxin), mucus (Mucin-like protein), innate immunity (Techylectin-5B, Antimicrobial peptide, Toll-like receptor 2, and Scavenger receptor), and defense against oxidative stress (Cell surface Cu-only superoxide, Glutathione peroxidase 5, Catalase, GFP-like fluorescent chromoprotein). Tentacles also highly expressed genes encoding extracellular matrix (Collagen alpha-1,2,4, and 5, Fibronectin) and a cell adhesion molecule (Protocadherin Fat 4), which may support the three-dimensional structure of tentacles. Genes associated with coral-algal symbiosis/metabolic interactions (Amino acid transporter AVT1A, Major facilitator superfamily domain-containing protein 10, NPC intracellular cholesterol transporter 2 homolog, and Ammonium transporter Rh type B-B) were also detected. Moreover, genes for the light-sensing molecule, melanopsin-B, and the precursor of Antho-RF amide neuropeptide, which is involved in tentacle contraction, were also highly expressed. Two genes encoding a steroidogenesis-related enzyme, Steroid 17-alpha-hydroxylase/17,20 lyase, were also highly expressed (Fig. 4).
Mesenterial filaments
Mesenterial filaments highly expressed a number of genes encoding proteases such as chymotrypsinogens, chymotrypsins, zinc metalloproteinases, carbopeptidases, aminopeptidase, meprin A subunit beta, and acidic mammalian chitinase. Mesenterial filaments also highly expressed genes encoding light-sensing molecules such as violet-sensitive opsin, melanopsin A, and melanopsin B (Fig. 4).
Body wall
Body wall highly expressed a number of genes encoding skeletal organic matrix proteins (SOMPs), e.g., acidic SOMPs, an uncharacterized protein, extracellular matrix, cell adhesion molecules, and enzymes (Fig. 4). Genes encoding molecules related to innate immunity (Toll-like receptor 1, MAPK 2/5/6, Complement C3, integumentary mucin) were also highly expressed (Fig. 4).
Mouth and pharynx
In mouth and pharynx, genes encoding a transcription factor, Brachyury, essential for pharynx formation during embryogenesis in corals, were highly expressed. Molecules related to the Wnt signaling pathway, innate immunity, and the precursor of GLWamide neuropeptides, involved in polyp contraction, were also detected (Fig. 4). Mouth and pharynx also highly expressed a number of receptors for neuropeptides, e.g., neuropeptide SIFamide, tachykinin-like peptides, and neurotransmitters, e.g., 5-hydroxytryptamine, octopamine, dopamine, GABA (Fig. 4).
Tissue-specific expression patterns of specific gene families
Coral color is primarily attributed to pigment proteins, including fluorescent proteins (FPs), non-fluorescent chromoproteins, and brown-pigmented zooxanthellae. In the F. ancora genome, we identified 11 candidate genes encoding FPs. Genes encoding non-fluorescent chromoproteins were not identified. Based on molecular phylogenetic analysis of two known F. ancora FP genes (one GreenFP and one RedFP)31,32 and FP-like genes from diverse anthozoan taxa, we estimated that 3 of the identified FP candidate genes encode GreenFPs (GFPs), while 1 encodes a RedFP (RFP) (Fig. 5a). Our molecular phylogenetic analysis also showed that 7 of 11 FP candidate genes in F. ancora were expanded by tandem duplication, independently of those in Acropora (Fig. 5a). These 7 FP genes are also arranged in tandem in the genome (Fig. 5b). Tissue-specific transcriptome analyses revealed that 6 of 11 FP candidate genes, including 1 GFP gene, were highly and significantly expressed in tentacles (Fig. 5c), in agreement with fluorescent microscopy. One FP-like gene, s020 g104, is also highly, but not significantly, expressed in the mouth and pharynx (Fig. 5c). Immunohistochemical analysis with anti-GFP antibody showed that GFP was expressed in epidermis of tentacles (Fig. 5d).
a Molecular phylogenetic analysis of possible F. ancora FP-like genes with two known F. ancora FP genes (one GreenFP and one RedFP), FP genes, and non-fluorescent chromoprotein genes of diverse anthozoan taxa, including Acropora, Montipora, and Astreopora corals. Red arrows indicate tentacle-specific HEGs. Circles on branches indicate bootstrap values higher than 80%. b Arrangement and orientation of seven FP-like genes (shown as green arrows) in the F. ancora genome. Numbers in arrows indicate gene IDs in scaffolds. c A heatmap showing transcript levels of 11 FP-like genes in tentacle, mesenterial filament, body wall, and mouth and pharynx. Asterisks indicate tissue-specific HEGs. d Epidermal expression of GFP in tentacles as assessed by immunohistochemical analysis with anti-GFP antibody.
Homeobox genes function in developmental patterning in animals33. In the F. ancora genome, the Antennapedia (ANTP) class of homeobox genes, including Hox-like, ParaHox-like (Supplementary Fig. S2), and NK cluster genes, were identified. Tissue distribution analysis revealed that 8 of 10 NK genes cluster in scaffold 19 and all 4 in scaffold 12 showed tissue-specific expression patterns (Fig. 6a): 3 MSX genes in mouth and pharynx, two NKX2 genes and one NKX3 gene in body wall, HLX, LBX, NKX3 and two NKX2 genes in mesenterial filaments, and one MSX gene in tentacle (Fig. 6a, b).
a Organization of NK homeobox gene clusters in the F. ancora genome. Gene IDs and their relative positions and orientations in scaffolds 19 and 12 are indicated with arrows. Arrow directions indicate directions of transcription. Arrow colors indicate the tissue in which genes are highly expressed. Relative expression levels in the four polyp tissues are shown with heat maps under the arrows. Te tentacle (green), MP mouth and pharynx (blue), Bo body wall (yellow), Me mesenterial filament (red). b Schematic diagram of a coral polyp showing locations of the four tissues analyzed in this study. c Organization of Wnt genes in the F. ancora genome. Gene IDs and their relative positions and orientations in scaffolds are indicated by arrows. As in a above, colors of arrows indicate tissues in which genes are highly expressed. Relative expression levels in the four tissues are shown with heat maps under the arrows.
The Wnt gene family encodes secreted signaling molecules that control cell fate in animal development and disease. In the F. ancora genome, 20 possible Wnt genes with conserved wnt family domains (PF00110) were identified (Fig. 6c, Supplementary Fig. S3). Tissue distribution analysis found that 10 of 20 Wnt genes were assigned as tissue-specific HEGs (Fig. 6c): 5 genes (Wnt1,-2,-7,-9, and -11) in mouth and pharynx, 4 genes (Wnt5,-8,-8 and -11) in body wall, and 1 gene (Wnt6) in mesenterial filaments (Fig. 6c).
Discussion
Establishment of an F. ancora draft genome and the tissue-specific gene expression profiles of coral polyps
The present study established a draft genome and tissue-specific transcriptome assemblies of F. ancora. We previously created an F. ancora gonadal transcriptome assembly that allowed us to identify sex- and gonadal, phase-specific genes associated with germ cell development34. Altogether, tissue-specific transcriptomes of 6 tissues, i.e., tentacles, mesenterial filaments, body wall, mouth with pharynx, testes, and ovary, are now available for F. ancora. Although genomes of more than 25 coral species have been reported so far4,20,21,22,23,24,25,26,35, tissue-specific spatial expression patterns have not been undertaken in those species. These transcriptomic datasets of F. ancora not only shed light on unexplored functions of coral tissues, but provide a useful foundation for better understanding of gene functions.
Tentacles: multifunctional tissues involved in biological defense, predation, external-factor sensing, symbiosis, and steroid synthesis
Our morpho-histological observations demonstrated that some nematocytes and mucocytes are located in the epidermis, whereas zooxanthellae are present in the gastrodermis of tentacles. Consistent with these observations, genes associated with defense and predation36,37,38,39, innate immunity40,41,42,43,44,45, and symbiosis (antioxidant defense, immune regulation, and metabolite exchange46 are highly expressed. Light-sensing molecule are also highly expressed47,48. These findings indicate that tentacles are multifunctional tissues that not only serve in biological defense, predation, and light-sensing, but are also central to symbiosis.
FPs create vivid displays of coral color49,50. To date, diverse functions of FPs have been shown. Since FPs absorb ultraviolet A and emit light of lower energy, the functions include photoprotection from high UVA/blue irradiation and photosynthetic enhancement of zooxanthellae51,52. In addition, FPs also serve antioxidant functions32,53,54, and participate in innate immunity55,56, stress response57, establishment of symbiosis with free-living dinoflagellates (family Symbiodiniaceae)58, and prey attraction57. Previously, we reported identification of RedFP (RFP) gene, specifically expressed in oocytes of F. ancora, and suggested its involvement in coral oogenesis32. The present study identified 6 FP genes that are highly upregulated in tentacles. The FPs may have acquired functions specific to tentacles such as protection of coral cells and zooxanthellae from intense UV light51, or in attracting free-living dinoflagellate 58and prey59.
Of particular interest is the significantly higher expression of the gene encoding steroid 17α-hydroxylase/17,20-lyase (Cyp17a), a key enzyme in production of sex steroids and cortisol60, in tentacles. Steroids are biologically active compounds derived from cyclopenta[a]phenanthrene61. They function as important components of cell membranes and are involved in a wide range of physiological processes, such as stress response, immune response, behavior, reproduction, etc62.. Steroid biosynthesis is catalyzed by activities of various steroidogenic enzymes63. To date, steroidogenic enzyme activities, plus estrogens and testosterone have been demonstrated in tissue extracts of some scleractinians64,65,66,67. Some genes encoding steroidogenic enzymes were also demonstrated68,69. Although further research is required to clarify transcript localization and steroidal activity, our findings imply that steroid biosynthesis occurs in tentacles, and that produced steroids/cortisol could be associated with some functions in tentacles, such as maintenance of cell membrane integrity, biological defense, or symbiosis.
Digestion and light-sensing in mesenterial filaments
Our histological analysis demonstrated that many cnidocytes and mucocytes are present in the margins of mesenterial filaments. Further, we identified a variety of digestive enzymes that are highly expressed in mesenterial filaments. Enzymes similar to those expressed in digestive tissues of other cnidarians, such as the sea anemone, Nematostella vectensis, and the jellyfish, Aurelia aurita, were also identified6. Few studies have reported on the presence and expression of digestive enzymes in corals70,71. Therefore, this study may provide important insights into understanding the digestive system of corals. Since corals feed on various types of prey72,73, it is possible that expression levels of the identified digestive enzymes may also vary depending on prey.
Some coral behaviors such as tentacle expansion/contraction are strongly influenced by light74,75,76,77,78. Additionally, it usually releases gametes at night76. However, it is still largely unknown how corals, which have no special light-sensing organs, perceive light. In the jellyfish Clytia hemisphaerica, spawning timing is controlled by light, and that process involves the photosensing molecule, opsin, expressed in gonads79. In Acropora millepora, multiple opsins have been identified47, including one that responds to UV light80. In the F. ancora genome, there are 32 genes encoding opsins/melanopsins and some exhibit tissue-specific expression. Melanopsin B is highly expressed in tentacles and mesenterial filaments, whereas Violet-sensitive opsin, Melanopsin A, is highly expressed in mesenterial filaments. This suggests that corals may enhance sensitivity to light by highly expressing photo-sensing molecules not only in tentacles, but also in mesenterial filaments.
Body wall: skeleton formation and a chemical-physical barrier
Nematocytes and zooxanthellae are nearly absent in the body wall of F. ancora, suggesting that body wall is little involved in predation or symbiosis. Surprisingly, body wall highly expresses a variety of molecules involved in biomineralization (acidic SOMPs, uncharacterized proteins, extracellular matrix, cell adhesion molecules, and enzymes)81,82,83. This is possibly because F. ancora forms its skeleton entirely outside the polyp body wall. The epidermis of the body wall basically comprises a layer of calicodermis, in contact with the skeleton. Further, the body wall also expresses some molecules involved in innate immunity, such as Toll-like receptor 1, Complement C3, integumentary mucin40,44,84. These results indicate that the body wall is not only the primary tissue for skeleton formation, but also functions as a chemical-physical barrier against parasitic and biofouling organisms.
Mouth and pharynx: key tissues for the coral nervous system?
A notable finding was the high expression of many neuropeptides and biogenic amine receptors in the mouth and pharynx. Both peptidergic and non-peptidergic neurotransmission/neuromodulation are fundamental to cnidarian physiology85,86,87. In corals, GLWamide-positive and RFamide-positive neurons have been found in the mouth and pharynx, and are involved in polyp contraction13,88,89. Some neuroactive compounds such as dopamine induce larval settlement in the coral, Leptastrea purpurea90. Increases levels of neuroactive compounds were detected during a synchronous spawning event in Acropora intermedia91. Evidence for neuropeptides and neurotransmitters in coral physiology is slowly accumulating; however, many coral neuropeptides, neurotransmitters, and their receptors have not yet been identified in corals. Their future identification will lead to a better understanding of coral physiology, targeting especially the mouth and pharynx.
Tissue-specific expression of NK homeobox clusters and Wnt genes
Homeobox genes were identified in the F. ancora genome, as reported in some other corals92,93. Notably, 11 of 14 genes in NK homeobox clusters showed tissue-specific expression patterns in F. ancora. In a sea anemone, Nematostella vectensis (Anthozoa), NK homeobox genes also show tissue-specific expression patterns. Gbx is expressed in pharyngeal endoderm94, Hlx and Nk6 in pharyngeal ectoderm, and Nk3 in nutrient-storing somatic gonads in mesentery6. Further detailed expression analysis in corals is likely to reveal other similarities and differences with N. vectensis.
Of particular interest is the discovery of significantly higher expression of genes encoding various Wnt proteins in F. ancora mouth and pharynx. In Hydra, various types of Wnt mRNA (HyWnt1, -3, -7, -9/10a, -9/10c, -11, and -16) are expressed in hypostomes of both adult polyps and new buds, and their involvement in head formation was demonstrated95,96. In Nematostella, induction of Wnt signaling with alsterpaullone results in formation of ectopic oral tissue during regeneration and embryogenesis97. Accordingly, Wnt may also be involved in formation of oral tissue in corals.
Future perspectives
Given the serious plight of coral reefs, promoting coral conservation and increasing our understanding of coral biology is essential. Genomic information from F. ancora will facilitate coral conservation activities. Genome-wide SNP markers will reveal detailed population structures in nature98,99,100, and will be applicable to F. ancora aquaculture to monitor genetic diversity in captivity101,102,103,104.
Although morphohistological and transcriptome characteristics of coral polyp tissues are presented here, numbers of cell types and compositional ratios of cells constituting each tissue are still unknown. Single-cell transcriptome analysis105 of each coral tissue will clarify these issues in the future.
In conclusion, we have established a draft genome and tissue-specific transcriptome assemblies of Fimbriaphyllia ancora. The established dataset for each polyp tissue will not only shed light on unexplored functions of coral tissues, but provide a useful foundation for better understanding of gene functions.
Materials and methods
Sampling, DNA and RNA extraction, transcriptome and genome sequencing
Four colonies of F. ancora were collected on reef slopes in the nearshore of Onna Village, Okinawa, Japan, under Okinawa prefectural permit #30-8 in 2018. Four polyp tissues, tentacle, mouth, mesenterial filament, and body wall, were isolated and photographed under a stereomicroscope19. Collected tissues were snap-frozen in liquid nitrogen and stored at −80 °C until use. Total RNA was isolated from each tissue with an RNeasy Plant Kit (QIAGEN Inc., Valencia, CA). For transcriptome sequencing, a TruSeq Stranded mRNA Library Kit (Illumina, San Diego, CA) was used for mRNA sequencing and library preparation, and each library was sequenced from 150-bp paired-end reads using a HiSeq 4000 (Illumina). To prepare genomic DNA for sequencing, isolated mesenterial filaments from one colony were maintained in Petri dishes for 1 week with 50 mL of filtered seawater containing 0.2 mM menthol106 to eliminate symbiotic algae. Bleached mesenterial filaments were snap-frozen in liquid nitrogen and stored at −80 °C until use. Genomic DNA was isolated using the phenol-chloroform method, and sequenced on a PacBio platform to obtain highly accurate long-read sequencing data. Genome shotgun sequencing (150-bp paired-end) of the same DNA sample was also performed on an Illumina HiSeq4000.
Genome assembly and gene prediction
PacBio HiFi reads with quality values > 20 were assembled with Hifiasm version 0.14-r312 using default settings107. Possible diploid scaffolds were first removed with Purge_haplotigs ver. 1.1.1108, and then merged with HaploMerger2109. Further scaffolding was performed with LINKS (ver. 1.8.7) with a kmer size of 21110. Possible errors in genome assembly were corrected with Hypo ver. 1.0.3111 using Illumina shotgun data with default settings. For gene prediction from assembled genome sequences, we used the above-prepared RNA-Seq data from the four tissues and RNA-Seq data from F. ancora male and female gonads in different developmental stages34. Low-quality reads (quality score <20 and length <20 bp) and sequence adapters in RNA-Seq data were trimmed using CUTADAPT v1.18112. Repetitive elements in scaffolds were identified de novo with RepeatScout v1.0.6113 and RepeatMasker v4.1.0 (http://www.repeatmasker.org). Repetitive elements were filtered out by length (>50 bp) and occurrence. Gene prediction was first performed with the BRAKER pipeline v2.1.2114, with AUGUSTUS v3.3.3. RNA-seq reads were aligned to genome sequences with HISAT v2.1.0115,116. Then, alignment information was used for BRAKER gene prediction with options “UTR=on”, “soft- masking”, and “AUGUSTUS_ab_initio.” To improve gene prediction, we further executed genome-guided transcriptome assembly using StringTie114 with option “-m 500.” Genome-based transcript structure was predicted with TransDecoder (https://github.com/TransDecoder/TransDecoder/wiki). During read alignment, we used soft-masked repeats for genome-guided transcriptome assembly and hard-masked repeats for BRAKER gene prediction. Finally, genes present in genome-guided assembly or ab initio prediction, but absent in predictions from the hint file were added to the predicted file using GffCompare117. We assessed completeness of the genome assembly and gene prediction with Benchmarking Universal Single-Copy Orthologs (BUSCO) ver. 5.2.227,28 using the Metazoa set (978 genes).
Gene annotation, clustering of orthologous genes, and molecular phylogenetic analysis
Predicted gene models were BLASTed against the Uniprot/Swissprot (UniProt Consortium 2018) database and were analyzed with InterProScan 5 with a cutoff of e−5118. To cluster orthologous genes in scleractinian genomes, we used four acroporid species (A. millepora, A. tenuis, Montipora cactus, and Astreopora myriophthalma4,21,22,23,35, Porites australiensis20, Stylophora pistillata24, Pocillopora damicornis26 and Orbicella faveolata25. For the A. millepora, S. pistillata, O. faveolata, and P. damicornis genomes, we downloaded data from the NCBI RefSeq database. Then, using OrthoFinder version 2.5.4119, we performed clustering of possible orthologs, and Orthogroups (OGs) were used for subsequent analyses.
For phylogenomic analysis of scleractinian genomes, we used 4208 genes that were identified by OrthoFinder as single-copy genes in all of the above scleractinian genomes. All amino acid sequences belonging to same OG were aligned with MAFFT (ver. 7.310. with –auto option)120 and all gaps in the alignment were removed with TrimAL121 with the –nogaps option. Then all sequences from the same species were concatenated. Finally, a maximum likelihood analysis was performed using concatenated sequences (1,817,638 amino acids in length) from RAxML (maximum likelihood method) with 100 bootstraps replicates and the “protgammaauto” option122. For phylogenetic analysis of each gene, amino acid sequences were aligned using MAFFT (ver. 7.310. with –auto option)120, and gaps in aligned sequences were trimmed using TrimAL121 with the –gappyout option. After that, poorly aligned sequences were removed (-resoverlap 0.75 -seqoverlap 80). Then we performed molecular phylogenetic analysis of the selected alignments using RAxML (maximum likelihood method) with 100 bootstrap replicates and “protgammaauto” option.
Tissue-specific gene expression analysis
RNA-Seq data obtained from the 16 samples (4 tissue types × 4 colonies) were used. Low-quality reads (quality score <20 and length <20 bp) and Illumina sequence adapters were trimmed with CUTADAPT v1.16112, and then mapped to F. ancora gene models (mRNA) using SALMON v1.8.0. Mapping counts were normalized with the trimmed mean of M values (TMM) method, and then converted to counts per million (CPM) using EdgeR v3.32.1 in R v4.0.3. Gene expression levels (numbers of mapped reads) of each tissue were compared pairwise with the other three tissues. p-values were adjusted using the Benjamini–Hochberg method in EdgeR. When the gene expression level was significantly higher (False discovery rate < 0.05) in one tissue than the other three, genes were considered tissue-specific HEGs.
Histology and immunohistochemistry
Histological and immunohistological analysis was performed according to methodology described previously123. Briefly, isolated tissues were fixed in filtered seawater containing 20% Zinc Formal Fixx (Thermo Scientific Shandon, Cheshire, UK) for 16 h and preserved in 70% ethanol until use. Dehydrated samples were embedded in paraplast plus (Sherwood Medical, St. Louis, MO), sliced into 4-mm serial sections, and stained with haematoxylin and eosin Y (H & E staining, Thermo Shandon). For immunohistochemical staining, hydrated sections were incubated for 30 min with HistoVT ONE (Nacalai Tesque, Inc, Kyoto, Japan) for antigen retrieval. After washing with phosphate-buffered saline containing 0.1% Tween 20 (PBT), sections were incubated for 10 min in 3% H2O2, and for 1 h in in 5% skim milk for blocking. Sections were then incubated for 16 h in anti-monomeric Azami-Green 1 pAb antibody (a polyclonal antibody against GFP of the stony coral G. fascicularis; item no. PM052M, Medical & Biological Laboratories, Nagoya, Japan) (1:4,000 in PBT with 2% skim milk) at 4 °C. For the secondary antibody reaction, sections were incubated with a biotinylated goat anti-rat IgG antibody (Vector Laboratories, Burlingame, USA; diluted 1: 2000 in PBT with 2% skim milk) for 30 min. Immunoreactive signals were visualized with avidin–biotin–peroxidase complex (ABC) solution (Vector Laboratories), and 3,30-diaminobenzidine (DAB; Sigma-Aldrich). Sections were counterstained with haematoxylin. Stained sections were observed and photographed under a BX51 microscope (Olympus, Tokyo, Japan).
Data availability
Raw RNA-sequencing data and raw genomic sequencing data have been deposited in the DDBJ/EMBL/GenBank databases under accession numbers DRR397929–DRR397944 and DRR397945–DRR397946 (BioProject ID: PRJDB14104), respectively. The genome assembly and mitochondrial genome assembly of F. ancora have been deposited in the DDBJ/EMBL/GenBank under accession numbers BRZB01000001-BRZB01000205 and LC811399, respectively. Source data of TPM values for the heatmaps shown in Figs. 3, 5, and 6 can be found in the Supplementary Data Sheet 1–5.
References
Odum, H. T. & Odum, E. P. Trophic structure and productivity of a windward coral reef community on eniwetok atoll. Ecol. Monog. 25, 291–320 (1955).
Fisher, R. et al. Species richness on coral reefs and the pursuit of convergent global estimates. Curr. Biol. 25, 500–505 (2015).
De Groot, R. et al. Global estimates of the value of ecosystems and their services in monetary units. Ecosyst. Serv. 1, 50–61 (2012).
Shinzato, C. et al. Using the Acropora digitifera genome to understand coral responses to environmental change. Nature 476, 320–323 (2011).
Ruppert, E. E., Fox R. S. & Barnes, R. D. In Invertebrate zoology: A functional evolutionary approach, 7th ed. 111–180 (Thomson-Brooks/Cole, 2003).
Steinmetz, P. R. H., Aman, A., Kraus, J. E. M. & Technau, U. Gut-like ectodermal tissue in a sea anemone challenges germ layer homology. Nat. Ecol. Evol. 1, 1535–1542 (2017).
Technau, U. & Steele, R. E. Evolutionary crossroads in developmental biology: Cnidaria. Development 138, 1447–1458 (2011).
Roff, G., Dove, S. & Dunn, S. R. Mesenterial filaments make a clean sweep of substrates for coral growth. Coral Reefs 28, 79 (2008).
Nugues, M. M., Delvoye, L. & Bak, R. P. M. Coral defense against macroalgae: differential effects of mesenterial filaments on the green alga Halimeda opuntia. Mar. Ecol. Prog. Ser. 278, 103–114 (2004).
Shikina, S. et al. Culturing reef-building corals on a laboratory dish: a simple experimental platform for stony corals. Front. Mar. Sci. 10, 1149495 (2023).
Su, A. I. et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl Acad. Sci. USA 101, 6062–6067 (2004).
Fagerberg, L. et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 13, 397–406 (2014).
Attenborough, R. et al. Expression of the neuropeptides RFamide and LWamide during development of the coral Acropora millepora in relation to settlement and metamorphosis. Dev. Biol. 446, 56–67 (2019).
Domart‐Coulon, I., Elbert, D. C., Scully, E. P., Calimlim, P. S. & Ostrander, G. K. Aragonite crystallization in primary cell cultures of multicellular isolates from a hard coral, Pocillopora damicornis. Proc. Natl Acad. Sci. USA 98, 11885–11890 (2001).
Muscatine, L., Tambutté, É. & Allemand, D. Morphology of coral desmocytes, cells that anchor the calicoblastic epithelium to the skeleton. Coral Reefs 16, 205–213 (1997).
Luzon, K. S., Lin, M., Ablan-Lagman, M. C., Licuanan, W. R. Y. & Chen, C. A. Resurrecting a subgenus to genus: molecular phylogeny of Euphyllia and Fimbriaphyllia (order Scleractinia; family Euphyllidae; clade V). PeerJ 5, e4074 (2017).
Hoeksema, B. W. & Cairns, S. World List of Scleractinia. https://www.marinespecies.org/scleractinia. Accessed 2023-10-24. (2023).
Bruckner, A. W. Tracking the trade in ornamental coral reef organisms: The importance of CITES and its limitations. Aquar. Sci. Conserv. 3, 79–94 (2001).
Shikina, S. et al. Yolk formation in a stony coral Euphyllia ancora (Cnidaria, Anthozoa): insight into the evolution of vitellogenesis in nonbilaterian animals. Endocrinology 154, 3447–3459 (2013).
Shinzato, C. et al. Whole-genome sequencing highlights conservative genomic strategies of a stress-tolerant, long-lived scleractinian coral, Porites australiensis Vaughan, 1918. Genome Biol. Evol. 13, evab270 (2021b).
Yoshioka, Y., Suzuki, G., Zayasu, Y., Yamashita, H. & Shinzato, C. Comparative genomics highlight the importance of lineage-specific gene families in evolutionary divergence of the coral genus, Montipora. BMC Ecol. Evol. 22, 71 (2022).
Shinzato, C. et al. Eighteen coral genomes reveal the evolutionary origin of Acropora strategies to accommodate environmental changes. Mol. Biol. Evol. 38, 16–30 (2021a).
Fuller, Z. L. et al. Population genetics of the coral Acropora millepora: Toward genomic prediction of bleaching. Science 369, eaba4674 (2020).
Voolstra, C. C. et al. Comparative analysis of the genomes of Stylophora pistillata and Acropora digitifera provides evidence for extensive differences between species of corals. Sci. Rep. 7, 17583 (2017).
Prada, C. et al. Empty niches after extinctions increase population sizes of modern corals. Curr. Biol. 26, 3190–3194 (2016).
Cunning, R., Bay, R. A., Gillette, P., Baker, A. C. & Traylor-Knowles, N. Comparative analysis of the Pocillopora damicornis genome highlights role of immune system in coral evolution. Sci. Rep. 8, 16134 (2018).
Simão, F. A., Waterhouse, R. M., Ioannidis, P., Kriventseva, E. V. & Zdobnov, E. M. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31, 3210–3212 (2015).
Waterhouse, R. M. et al. BUSCO applications from quality assessments to gene prediction and phylogenomics. Mol. Biol. Evol. 35, 543–548 (2018).
Fukami, H. et al. Mitochondrial and nuclear genes suggest that stony corals are monophyletic but most families of stony corals are not (Order Scleractinia, Class Anthozoa, Phylum Cnidaria). PLoS One 3, e3222 (2008).
Kitahara, M. V., Cairns, S. D., Stolarski, J., Blair, D. & Miller, D. J. A comprehensive phylogenetic analysis of the scleractinia (Cnidaria, anthozoa) based on mitochondrial CO1 sequence data. PLoS One 5, e11490 (2010).
Chiu, Y., Shikina, S. & Chang, C. Testicular somatic cells in the stony coral Euphyllia ancora express an endogenous green fluorescent protein. Mol. Reprod. Dev. 86, 798–811 (2019).
Shikina, S. et al. Oocytes express an endogenous red fluorescent protein in a stony coral, Euphyllia ancora: a potential involvement in coral oogenesis. Sci. Rep. 6, 25868 (2016).
Hubert, K. A. & Wellik, D. M. Hox genes in development and beyond. Development 150, dev192476 (2023).
Chiu, Y., Shikina, S., Yoshioka, Y., Shinzato, C. & Chang, C. De novo transcriptome assembly from the gonads of a scleractinian coral, Euphyllia ancora: molecular mechanisms underlying scleractinian gametogenesis. BMC Genom. 21, 732 (2020).
Ying, H. et al. The whole-genome sequence of the coral Acropora millepora. Genome Biol. Evol. 11, 1374–1379 (2019).
Moran, Y. et al. Analysis of soluble protein contents from the nematocysts of a model sea anemone sheds light on venom evolution. Mar. Biotechnol. 15, 329–339 (2013).
Shiomi, K. et al. Novel polypeptide toxins with crab lethality from the sea anemone Anemonia erythraea. Biochim. Biophys. Acta 1335, 191–198 (1997).
Klompen, A. M. L., Macrander, J., Reitzel, A. M. & Stampar, S. N. Transcriptomic analysis of four cerianthid (Cnidaria, Ceriantharia) venoms. Mar. Drugs 18, 413 (2020).
Trevisan-Silva, D. et al. Astacin-like metalloproteases are a gene family of toxins present in the venom of different species of the brown spider (genus Loxosceles). Biochimie 92, 21–32 (2010).
Sheng, Y. & Hasnain, S. Z. Mucus and mucins: the underappreciated host defence system. Front. Cell. Infect. Microbiol. 12, 856962 (2022).
Angthong, P., Roytrakul, S., Jarayabhand, P. & Jiravanichpaisal, P. Characterization and function of a tachylectin 5-like immune molecule in Penaeus monodon. Dev. Comp. Immunol. 76, 120–131 (2017).
Mason, B. M. et al. AmAMP1 from Acropora millepora and damicornin define a family of coral-specific antimicrobial peptides related to the Shk toxins of sea anemones. Dev. Comp. Immunol. 114, 103866 (2021).
Areschoug, T. & Gordon, S. Scavenger receptors: role in innate immunity and microbial pathogenesis. Cell. Microbiol. 11, 1160–1169 (2009).
Duan, T., Du, Y., Xing, C., Wang, H. Y. & Wang, R. Toll-like receptor signaling and its role in cell-mediated immunity. Front. Immunol. 13, 812774 (2022).
Yoshioka, Y. et al. Whole-genome transcriptome analyses of native symbionts reveal host coral genomic novelties for establishing coral–algae symbioses. Genome Biol. Evol. 13, evaa240 (2021).
Yoshioka, Y. et al. Genes possibly related to symbiosis in early life stages of Acropora tenuis inoculated with Symbiodinium microadriaticum. Commun. Biol. 6, 1027 (2023).
Mason, B. M. et al. Evidence for multiple phototransduction pathways in a reef-building coral. PLoS ONE 7, e50371 (2012).
Emanuel, A. J. & Do, H., M. T. The multistable melanopsins of mammals. Front. Ophthalmol. 3, https://doi.org/10.3389/fopht.2023.1174255 (2023).
Dove, S., Hoegh‐Guldberg, O. & Ranganathan, S. Major colour patterns of reef-building corals are due to a family of GFP-like proteins. Coral Reefs 19, 197–204 (2001).
Alieva, N. O. et al. Diversity and evolution of Coral fluorescent proteins. PLoS ONE 3, e2680 (2008).
Salih, A., Larkum, A., Cox, G., Kühl, M. & Hoegh-Guldberg, O. Fluorescent pigments in corals are photoprotective. Nature 408, 850–853 (2000).
Roth, M. S., Latz, M. I., Goericke, R. & Dehyn, D. D. Green florescent protein regulation in the coral Acropora yongei during photoacclimation. J. Exp. Biol. 213, 3644–3655 (2010).
Bou‐Abdallah, F., Chasteen, N. D. & Lesser, M. P. Quenching of superoxide radicals by green fluorescent protein. Biochim. Biophys. Acta 1760, 1690–1695 (2006).
Palmer, C. V., Modi, C. K. & Mydlarz, L. D. Coral fluorescent proteins as antioxidants. PLoS One 4, e7298 (2009a).
Palmer, C. V., Roth, M. & Gates, R. D. Red fluorescent protein responsible for pigmentation in trematode-infected Porites compressa tissues. Biol. Bull. 216, 68–74 (2009b).
D’Angelo, C. J. et al. Locally accelerated growth is part of the innate immune response and repair mechanisms in reef-building corals as detected by green fluorescent protein (GFP)-like pigments. Coral Reefs 31, 1045–1056 (2012).
Seneca, F. O. et al. Patterns of gene expression in a scleractinian coral undergoing natural bleaching. Mar. Biotechnol. 12, 594–604 (2010).
Aihara, Y. et al. Green fluorescence from cnidarian hosts attracts symbiotic algae. Proc. Natl Acad. Sci. USA 116, 2118–2123 (2019).
Ben-Zvi, O., Lindemann, Y., Eyal, G. & Loya, Y. Coral fluorescence: a prey-lure in deep habitats. Commun. Biol. 5, 537 (2022).
Guengerich, F. P., McCarty, K. D., Tateishi, Y. & Liu, L. Steroid 17α-hydroxylase/17, 20-lyase (cytochrome P450 17A1). Methods Enzymol. 689, 39–63 (2023).
Sultan, A. & Raza, A. R. Steroids: a diverse class of secondary metabolites. Med. Chem. 5, 310–317 (2015).
Rasheed, A. & Qasim, M. D. A review of natural steroids and their applications. Int. J. Pharm. Sci. Res. 4, 520–531 (2013).
Miller, W. L. & Auchus, R. J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 32, 81–151 (2011).
Atkinson, S. & Atkinson, M. J. Detection of estradiol-17-beta during a mass coral spawn. Coral Reefs 11, 33–35 (1999).
Tarrant, A. M., Atkinson, S. & Atkinson, M. J. Estrone and estradiol-17 beta concentration in tissue of the scleractinian coral, Montipora verrucosa. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 122, 85–92 (1999).
Slattery, M., Hines, G. A., Starmer, J. & Paul, V. J. Chemical signals in gametogenesis, spawning, and larval settlement and defense of the soft coral Sinularia polydactyla. Coral Reefs 18, 75–84 (1999).
Twan, W., Hwang, J. & Chang, C. Sex steroids in scleractinian coral, Euphyllia ancora: implication in mass spawning. Biol. Reprod. 68, 2255–2260 (2003).
Shikina, S. et al. Molecular cloning and characterization of a steroidogenic enzyme, 17β-hydroxysteroid dehydrogenase type 14, from the stony coral Euphyllia ancora (Cnidaria, Anthozoa). Gen. Comp. Endocrinol. 228, 95–104 (2016b).
Tan, E. S. et al. Does estrogen regulate vitellogenin synthesis in corals? Comp. Biochem. Physiol. A Mol. Integr. Physiol. 255, 110910 (2021).
Yoshioka, Y., Tanabe, T. & Iguchi, A. The presence of genes encoding enzymes that digest carbohydrates in coral genomes and analysis of their activities. PeerJ 5, e4087 (2017).
Raz-Bahat, M., Douek, J., Моисеева, ЕГ, Peters, E. C. & Rinkevich, B. The digestive system of the stony coral Stylophora pistillata. Cell Tissue Res. 368, 311–323 (2017).
Poter, J. W. Zooplankton feeding by the Caribbean reef-building coral Montastrea cavernosa. Proc. 2nd int Coral reef. Sym 21, 111–125 (1974).
Sebens, K. P., Vandersall, K. S., Savina, L. A. & Graham, K. R. Zooplankton capture by two scleractinian corals, Madracis mirabilis and Montastrea cavernosa, in a field enclosure. Mar. Biol. 127, 303–317 (1996).
Sweeney, B. M. Circadian rhythms in corals, particularly fungiidae. Biol. Bull. 151, 236–246 (1976).
Sebens, K. P. & DeRiemer, K. Diel cycles of expansion and contraction in coral reef anthozoans. Mar. Biol. 43, 247–256 (1977).
Lasker, H. R. Light dependent activity patterns among reef corals: Montastrea cavernosa. Biol. Bull. 156, 196–211 (1979).
Hoadley, K. D., Szmant, A. M. & Pyott, S. J. Circadian clock gene expression in the coral Favia fragum over diel and lunar reproductive cycles. PLoS One 6, e19755 (2011).
Shikina, S. et al. Cellular and biochemical changes in early embryonic development of a scleractinian coral, Fimbriaphyllia (Euphyllia) ancora. Zool. Stud. 62, 38 (2023).
Quiroga Artigas, G. et al. A gonad-expressed opsin mediates light-induced spawning in the jellyfish Clytia. eLife 7, e29555 (2018).
Mason, B. M. et al. Multiple opsins in a reef-building coral, Acropora millepora. Sci. Rep. 13, 1628 (2023).
Ramos-Silva, P. et al. The skeletal proteome of the coral Acropora millepora: the evolution of calcification by co-option and domain shuffling. Mol. Biol. Evol. 30, 2099–2112 (2013).
Drake, J. L. et al. Proteomic analysis of skeletal organic matrix from the stony coral Stylophora pistillata. Proc. Natl Acad. Sci. USA 110, 3788–3793 (2013).
Takeuchi, T., Yamada, L., Shinzato, C., Sawada, H. & Satoh, N. Stepwise evolution of coral biomineralization revealed with genome-wide proteomics and transcriptomics. PLoS One 11, e0156424 (2016).
Dunkelberger, J. & Song, W. Complement and its role in innate and adaptive immune responses. Cell Res. 20, 34–50 (2010).
Kass-Simon, G. & Pierobon, P. Cnidarian chemical neurotransmission, an updated overview. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 146, 9–25 (2007).
Takahashi, T. & Takeda, N. Insight into the molecular and functional diversity of cnidarian neuropeptides. Int. J. Mol. Sci. 16, 2610–2625 (2015).
Takahashi, T. Comparative aspects of structure and function of cnidarian neuropeptides. Front. Endocrinol. 11, 339 (2020).
Shikina, S. et al. Involvement of GLWamide neuropeptides in polyp contraction of the adult stony coral Euphyllia ancora. Sci. Rep. 10, 9427 (2020).
Zhang, Y. et al. Involvement of RFamide neuropeptides in polyp contraction of the adult scleractinian corals Euphyllia ancora and Stylophora pistillata. Gen. Comp. Endocrinol. 314, 113905 (2021).
Moeller, M., Nietzer, S. & Schupp, P. J. Neuroactive compounds induce larval settlement in the scleractinian coral Leptastrea purpurea. Sci. Rep. 9, 2291 (2019).
Taira, J., Higa, I., Tsuchida, E., Isomura, N. & Iguchi, A. Neurotransmitters in hermatypic coral, Acropora spp., and its contribution to synchronous spawning during reproductive event. Biochem. Biophys. Res. Commun. 501, 80–84 (2018).
DuBuc, T. Q., Ryan, J. F., Shinzato, C., Satoh, N. & Martindale, M. Q. Coral comparative genomics reveal expanded hox cluster in the Cnidarian-Bilaterian ancestor. Integr. Comp. Biol. 52, 835–841 (2012).
Ying, H. et al. Comparative genomics reveals the distinct evolutionary trajectories of the robust and complex coral lineages. Genome Biol. 19, 175 (2018).
Matus, D. et al. Molecular evidence for deep evolutionary roots of bilaterality in animal development. Proc. Natl Acad. Sci. USA 103, 11195–11200 (2006).
Hobmayer, B. et al. WNT signalling molecules act in axis formation in the diploblastic metazoan Hydra. Nature 407, 186–189 (2000).
Lengfeld, T. et al. Multiple Wnts are involved in Hydra organizer formation and regeneration. Dev. Biol. 330, 186–199 (2009).
Trevino, M. A., Stefanik, D., Rodríguez, R., Harmon, S. & Burton, P. M. Induction of canonical Wnt signaling by alsterpaullone is sufficient for oral tissue fate during regeneration and embryogenesis in Nematostella vectensis. Dev. Dyn. 240, 2673–2679 (2011).
Shinzato, C., Mungpakdee, S., Arakaki, N. & Satoh, N. Genome-wide SNP analysis explains coral diversity and recovery in the Ryukyu Archipelago. Sci. Rep. 5, 18211 (2015).
Tsuchiya, K. et al. Genomic analysis of a reef‐building coral, Acropora digitifera, reveals complex population structure and a migration network in the Nansei Islands, Japan. Mol. Ecol. 31, 5270–5284 (2022).
Zhang, J. et al. Evolutionary responses of a reef-building coral to climate change at the end of the last glacial maximum. Mol. Biol. Evol. 39, msac201 (2022).
Shinzato, C. et al. Development of novel, cross-species microsatellite markers for Acropora corals using next-generation sequencing technology. Front. Mar. Sci. 1, fmars.2014.00011 (2014).
Zayasu, Y. et al. Unexpectedly complex gradation of coral population structure in the Nansei Islands, Japan. Ecol. Evol. 6, 5491–5505 (2016).
Zayasu, Y., Satoh, N. & Shinzato, C. Genetic diversity of farmed and wild populations of the reef-building coral, Acropora tenuis. Restor. Ecol. 26, 1195–1202 (2018).
Zayasu, Y. & Suzuki, G. Comparisons of population density and genetic diversity in artificial and wild populations of an arborescent coral, Acropora yongei: implications for the efficacy of “artificial spawning hotspots.”. Restor. Ecol. 27, 440–446 (2019).
Levy, S. et al. A stony coral cell atlas illuminates the molecular and cellular basis of coral symbiosis, calcification, and immunity. Cell 184, 2973–2987 (2021).
Wang, J., Chen, Y., Tew, K. S., Meng, P. & Chen, C. A. Physiological and biochemical performances of Menthol-Induced aposymbiotic corals. PLoS One 7, e46406 (2012).
Cheng, H., Concepcion, G. T., Feng, X., Zhang, H. & Li, H. Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nat. Methods 18, 170–175 (2021).
Roach, M. J., Schmidt, S. & Borneman, A. R. Purge Haplotigs: allelic contig reassignment for third-gen diploid genome assemblies. BMC Bioinform 19, 460 (2018).
Huang, S., Kang, M. & Xu, A. HaploMerger2: rebuilding both haploid sub-assemblies from high-heterozygosity diploid genome assembly. Bioinformatics 33, 2577–2579 (2017).
Warren, R. L. et al. LINKS: Scalable, alignment-free scaffolding of draft genomes with long reads. GigaScience 4, 35 (2015).
Kundu, R., Casey, J. & Sung, W. HyPo: Super Fast & amp; Accurate polisher for long read genome assemblies. bioRxiv https://doi.org/10.1101/2019.12.19.882506 (2019).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Price, A. L., Jones, N. & Pevzner, P. A. De novo identification of repeat families in large genomes. Bioinformatics 21, i351–i358 (2005).
Brůna, T., Hoff, K. J., Lomsadze, A., Stanke, M. & Borodovsky, M. BRAKER2: automatic eukaryotic genome annotation with GeneMark-EP+ and AUGUSTUS supported by a protein database. NAR Genom. Bioinform. 3, qaa108 (2021).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Pertea, M. et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 33, 290–295 (2015).
Pertea, G. & Pertea, M. GFF Utilities: GffRead and GffCompare. F1000Res. 9, https://doi.org/10.12688/f1000research.23297.2 (2020).
Jones, P. et al. InterProScan 5: genome-scale protein function classification. Bioinformatics 30, 1236–1240 (2014).
Emms, D. & Kelly, S. OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genom. Biol. 16, 157 (2015).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Capella-Gutíerrez, S., Silla-Martínez, J. M. & Gabaldón, T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973 (2009).
Stamatakis, A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014).
Shikina, S. et al. Germ cell development in the scleractinian coral Euphyllia ancora (Cnidaria, Anthozoa). PLoS One 7, e41569 (2012).
Acknowledgements
We thank all members of the DNA Sequencing Section at OIST for their enormous help in the project. We also thank Dr. Steven Aird for editing the manuscript.
Funding
This research was funded by grants from the Ministry of Science and Technology, Taiwan (MOST 108-2628-B-019-003 to S.S.), and was supported by JSPS KAKENHI Grants (20H03235, 20K21860, and 24K01847 for C.S.) and Grant-in-Aid for JSPS Fellows to Y.Y. (20J21301). Computations were partially performed on the NIG supercomputer at ROIS National Institute of Genetics.
Author information
Authors and Affiliations
Contributions
S.S. and C.S. conceptualized and designed the project. Y.Z. collected the specimens. S.S. and T.-C.L. dissected tissues and collected samples. C.S. and N.S. supervised and organized whole genome sequencing and RNA-Seq. MiK., MaK. and M.F. prepared sequencing libraries and produced sequencing data. C.S. assembled genomes and Y.Y. performed gene predictions. S.S., Y.Y., Yi-L.C., T.U., E.C., Y.-C.C., Yu-L.C., T.T., and C.S. performed data analyses. S.S. and C.S. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
Experiments were conducted in accordance with principles and procedures approved by the Institutional Animal Care and Use Committee of National Taiwan Ocean University.
Peer review
Peer review information
Communications Biology thanks Zuhong Lu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Tobias Goris.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shikina, S., Yoshioka, Y., Chiu, YL. et al. Genome and tissue-specific transcriptomes of the large-polyp coral, Fimbriaphyllia (Euphyllia) ancora: a recipe for a coral polyp. Commun Biol 7, 899 (2024). https://doi.org/10.1038/s42003-024-06544-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-024-06544-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.