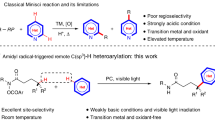
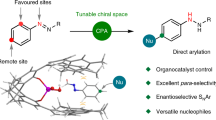
Abstract
Catalytic site-selective functionalization of distal C–H bonds represents a formidable challenge in organic synthesis. Particularly, the precise functionalization of distal aromatic C(sp2)–H bonds remains largely unexplored. Here we present a highly para-selective acylation strategy to target ultraremote aryl C(sp2)–H bonds, eight chemical bonds away from an activated functionality, through radical N-heterocyclic carbene organocatalysis. This method is developed on the basis of a unique single-electron pathway involving the site-selective activation of aryl C–H bonds by a nitrogen-centred radical generated in situ. Importantly, this organocatalytic approach shows potential for the functionalization of drugs, amino acids and peptides, thus highlighting its importance for medicinal chemistry. Our investigation encompassed meticulous mechanistic studies, including control experiments and density functional theory calculations, to unravel the intricacies behind the observed site selectivity and shed light on the mechanism of radical N-heterocyclic carbene organocatalysis.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within this Article and its Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2245194 (3a), 2355142 (3bu) and 2245196 (3ch). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Data are available from the corresponding authors upon request.
References
Lam, N. Y. S., Wu, K. & Yu, J.-Q. Advancing the logic of chemical synthesis: C–H activation as strategic and tactical disconnections for C–C bond construction. Angew. Chem. Int. Ed. 60, 15767–15790 (2021).
Leow, D., Li, G., Mei, T.-S. & Yu, J.-Q. Activation of remote meta-C–H bonds assisted by an end-on template. Nature 486, 518–522 (2012).
Zhang, Z., Tanaka, K. & Yu, J.-Q. Remote site-selective C–H activation directed by a catalytic bifunctional template. Nature 543, 538–542 (2017).
Roy, S., Panja, S., Sahoo, S. R., Chatterjee, S. & Maiti, D. Enroute sustainability: metal free C–H bond functionalisation. Chem. Soc. Rev. 52, 2391–2479 (2023).
Rogge, T. et al. C–H activation. Nat. Rev. Methods Prim. 1, 43 (2021).
Maiti, D. Handbook of CH-Functionalization (Wiley, 2022).
Lyons, T. W. & Sanford, M. S. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 110, 1147–1169 (2010).
Gensch, T., Hopkinson, M. N., Glorius, F. & Wencel-Delord, J. Mild metal-catalyzed C–H activation: examples and concepts. Chem. Soc. Rev. 45, 2900–2936 (2016).
Lucas, E. L. et al. Palladium-catalyzed enantioselective β-C(sp3)–H activation reactions of aliphatic acids: a retrosynthetic surrogate for enolate alkylation and conjugate addition. Acc. Chem. Res. 55, 537–550 (2022).
Sinha, S. K. et al. Toolbox for distal C–H bond functionalizations in organic molecules. Chem. Rev. 122, 5682–5841 (2022).
Maiti, D. & Guin, S. Remote C–H Bond Functionalizations: Methods and Strategies in Organic Synthesis. (Wiley, 2021).
Fan, Z. et al. Molecular editing of aza-arene C–H bonds by distance, geometry and chirality. Nature 610, 87–93 (2022).
Lam, N. Y. S. et al. Empirical guidelines for the development of remote directing templates through quantitative and experimental analyses. J. Am. Chem. Soc. 144, 2793–2803 (2022).
Li, J.-J. et al. Atroposelective remote meta-C–H activation. Chem 9, 1452–1463 (2023).
Leitch, J. A. & Frost, C. G. Ruthenium-catalysed σ-activation for remote meta-selective C–H functionalisation. Chem. Soc. Rev. 46, 7145–7153 (2017).
Hofmann, N. & Ackermann, L. meta-Selective C–H bond alkylation with secondary alkyl halides. J. Am. Chem. Soc. 135, 5877–5884 (2013).
Davies, H. M. L. & Manning, J. R. Catalytic C–H functionalization by metal carbenoid and nitrenoid insertion. Nature 451, 417–424 (2008).
Nobile, E., Castanheiro, T. & Besset, T. Radical-promoted distal C–H functionalization of C(sp3) centers with fluorinated moieties. Angew. Chem. Int. Ed. 60, 12170–12191 (2021).
Nechab, M., Mondal, S. & Bertrand, M. P. 1,n-Hydrogen-atom transfer (HAT) reactions in which n ≠ 5: an updated inventory. Chem. Eur. J. 20, 16034–16059 (2014).
Guo, W., Wang, Q. & Zhu, J. Visible light photoredox-catalysed remote C–H functionalisation enabled by 1,5-hydrogen atom transfer (1,5-HAT). Chem. Soc. Rev. 50, 7359–7377 (2021).
Choi, G. J., Zhu, Q., Miller, D. C., Gu, C. J. & Knowles, R. R. Catalytic alkylation of remote C–H bonds enabled by proton-coupled electron transfer. Nature 539, 268–271 (2016).
Chu, J. C. K. & Rovis, T. Amide-directed photoredox-catalysed C–C bond formation at unactivated sp3 C–H bonds. Nature 539, 272–275 (2016).
Proctor, R. S. J. & Phipps, R. J. Recent advances in Minisci-type reactions. Angew. Chem. Int. Ed. 58, 13666–13699 (2019).
Allen, A. R., Noten, E. A. & Stephenson, C. R. J. Aryl transfer strategies mediated by photoinduced electron transfer. Chem. Rev. 122, 2695–2751 (2022).
Li, W., Xu, W., Xie, J., Yu, S. & Zhu, C. Distal radical migration strategy: an emerging synthetic means. Chem. Soc. Rev. 47, 654–667 (2018).
Leifert, D. & Studer, A. The persistent radical effect in organic synthesis. Angew. Chem. Int. Ed. 59, 74–108 (2020).
Smith B. M. & March J. Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (Wiley-Interscience, 2007).
Hartwig, J. F. & Larsen, M. A. Undirected, homogeneous C–H bond functionalization: challenges and opportunities. ACS Cent. Sci. 2, 281–292 (2016).
Meng, G. et al. Achieving site-selectivity for C–H activation processes based on distance and geometry: a carpenter’s approach. J. Am. Chem. Soc. 142, 10571–10591 (2020).
Tang, R.-Y., Li, G. & Yu, J.-Q. Conformation-induced remote meta-C–H activation of amines. Nature 507, 215–220 (2014).
Wan, L., Dastbaravardeh, N., Li, G. & Yu, J.-Q. Cross-coupling of remote meta-C–H bonds directed by a U-shaped template. J. Am. Chem. Soc. 135, 18056–18059 (2013).
Yang, G. et al. Pd(II)-catalyzed meta-C–H olefination, arylation, and acetoxylation of indolines using a U-shaped template. J. Am. Chem. Soc. 136, 10807–10813 (2014).
Li, M. et al. Remote para-C–H acetoxylation of electron-deficient arenes. Org. Lett. 21, 540–544 (2019).
Chang, W. et al. Computationally designed ligands enable tunable borylation of remote C–H bonds in arenes. Chem 8, 1775–1788 (2022).
Hopkinson, M. N., Richter, C., Schedler, M. & Glorius, F. An overview of N-heterocyclic carbenes. Nature 510, 485–496 (2014).
Liu, K., Schwenzer, M. & Studer, A. Radical NHC catalysis. ACS Catal. 12, 11984–11999 (2022).
Dai, L. & Ye, S. Recent advances in N-heterocyclic carbene-catalyzed radical reactions. Chin. Chem. Lett. 32, 660–667 (2021).
Ishii, T., Nagao, K. & Ohmiya, H. Recent advances in N-heterocyclic carbene-based radical catalysis. Chem. Sci. 11, 5630–5636 (2020).
Li, Q.-Z., Zeng, R., Han, B. & Li, J.-L. Single-electron transfer reactions enabled by N-heterocyclic carbene organocatalysis. Chem. Eur. J. 27, 3238–3250 (2021).
Song, R. & Chi, Y. R. N-heterocyclic carbene catalyzed radical coupling of aldehydes with redox-active esters. Angew. Chem. Int. Ed. 58, 8628–8630 (2019).
Ishii, T., Kakeno, Y., Nagao, K. & Ohmiya, H. N-heterocyclic carbene-catalyzed decarboxylative alkylation of aldehydes. J. Am. Chem. Soc. 141, 3854–3858 (2019).
Ishii, T., Ota, K., Nagao, K. & Ohmiya, H. N-heterocyclic carbene-catalyzed radical relay enabling vicinal alkylacylation of alkenes. J. Am. Chem. Soc. 141, 14073–14077 (2019).
Meng, Q.-Y., Lezius, L. & Studer, A. Benzylic C–H acylation by cooperative NHC and photoredox catalysis. Nat. Commun. 12, 2068 (2021).
Matsuki, Y. et al. Aryl radical-mediated N-heterocyclic carbene catalysis. Nat. Commun. 12, 3848 (2021).
Bay, A. V., Fitzpatrick, K. P., Betori, R. C. & Scheidt, K. A. Combined photoredox and carbene catalysis for the synthesis of ketones from carboxylic acids. Angew. Chem. Int. Ed. 59, 9143–9148 (2020).
Li, J. L. et al. Radical acylfluoroalkylation of olefins through N-heterocyclic carbene organocatalysis. Angew. Chem. Int. Ed. 59, 1863–1870 (2020).
Goto, Y., Sano, M., Sumida, Y. & Ohmiya, H. N-heterocyclic carbene- and organic photoredox-catalysed meta-selective acylation of electron-rich arenes. Nat. Synth. 2, 1037–1045 (2023).
Pitzer, L., Schäfers, F. & Glorius, F. Rapid assessment of the reaction-condition-based sensitivity of chemical transformations. Angew. Chem. Int. Ed. 58, 8572–8576 (2019).
Guillemard, L., Kaplaneris, N., Ackermann, L. & Johansson, M. J. Late-stage C–H functionalization offers new opportunities in drug discovery. Nat. Rev. Chem. 5, 522–545 (2021).
Börgel, J. & Ritter, T. Late-stage functionalization. Chem 6, 1877–1887 (2020).
Wang, W., Lorion, M. M., Shah, J., Kapdi, A. R. & Ackermann, L. Late-stage peptide diversification by position-selective C–H activation. Angew. Chem. Int. Ed. 57, 14700–14717 (2018).
Yuan, Z., Zhu, C., Zhang, Y. & Rao, Y. Post-modification of amino acids and peptides for the rapid synthesis of C-glycoamino acids and C-glycopeptides. Eur. J. Org. Chem. 2022, e202201036 (2022).
Zard, S. Z. Recent progress in the generation and use of nitrogen-centred radicals. Chem. Soc. Rev. 37, 1603–1618 (2008).
Jiang, H. & Studer, A. Intermolecular radical carboamination of alkenes. Chem. Soc. Rev. 49, 1790–1811 (2020).
Gentry, E. C. & Knowles, R. R. Synthetic applications of proton-coupled electron transfer. Acc. Chem. Res. 49, 1546–1556 (2016).
Jiang, H. & Studer, A. Chemistry with N-centered radicals generated by single-electron transfer-oxidation using photoredox catalysis. CCS Chem. 1, 38–49 (2019).
Pratley, C., Fenner, S. & Murphy, J. A. Nitrogen-centered radicals in functionalization of sp2 systems: generation, reactivity, and applications in synthesis. Chem. Rev. 122, 8181–8260 (2022).
Zhang, Y. et al. N-heterocyclic carbene-catalyzed radical reactions for highly enantioselective β-hydroxylation of enals. J. Am. Chem. Soc. 137, 2416–2419 (2015).
Li, Q.-Z. et al. Remote C(sp3)–H acylation of amides and cascade cyclization via N-heterocyclic carbene organocatalysis. Angew. Chem. Int. Ed. 61, e202116629 (2022).
Zhang, X.-M., Tu, Y.-Q., Zhang, F.-M., Chen, Z.-H. & Wang, S.-H. Recent applications of the 1,2-carbon atom migration strategy in complex natural product total synthesis. Chem. Soc. Rev. 46, 2272–2305 (2017).
Regnier, V. et al. What are the radical intermediates in oxidative N-heterocyclic carbene organocatalysis? J. Am. Chem. Soc. 141, 1109–1117 (2019).
Bay, A. V. et al. Light-driven carbene catalysis for the synthesis of aliphatic and α-amino ketones. Angew. Chem. Int. Ed. 60, 17925–17931 (2021).
Li, Q.-Z. et al. Oxidative radical NHC catalysis: divergent difunctionalization of olefins through intermolecular hydrogen atom transfer. Angew. Chem. Int. Ed. 61, e202207824 (2022).
Ess, D. H. & Houk, K. N. Distortion/interaction energy control of 1,3-dipolar cycloaddition reactivity. J. Am. Chem. Soc. 129, 10646–10647 (2007).
Hayden, A. E. & Houk, K. N. Transition state distortion energies correlate with activation energies of 1,4-dihydrogenations and Diels–Alder cycloadditions of aromatic molecules. J. Am. Chem. Soc. 131, 4084–4089 (2009).
Acknowledgements
We are grateful for the financial support from the NSFC (no. 22271028 to J.-L.L., 22071011 to Q.-Z.L. and 22203010 to Z.-Y.Y.), the Science & Technology Department of Sichuan Province (no. 2023NSFSC2001 to J.-L.L.) and Longquan Talents Program, Key-Area Research and Development Program of Guangdong Province (no. 2022B1111050003 to J.-L.L.). We express our profound gratitude for the insightful discussions with Y. Lan and X.-T. Qi regarding the DFT calculations in this study.
Author information
Authors and Affiliations
Contributions
J.-L.L. supervised this study. Q.-Z.L. and W.-L.Z. conducted the main experiments and prepared the supplementary information of the experimental section. X.-X.K. and Y.-Q.L. prepared some substrates and performed the synthetic and mechanistic experiments. Y.H. and X.Z. helped with characterizing some compounds. Z.-Y.Y. performed the DFT calculations. Z.-Y.Y. and X.Z. prepared the supplementary information of the calculation section. J.-L.L. and Q.-Z.L. designed the project and wrote the manuscript. Q.-Z.L. and W.-L.Z. contributed equally to this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Jan Philipp Götze, Liliana Dobrzańska, Xiangyang Liu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, discussion, note and references.
Supplementary Data 1
The atomic coordinates of the optimized computational models studied in this paper.
Supplementary Data 2
CIF file of 3a.
Supplementary Data 3
CIF file of 3bu.
Supplementary Data 4
CIF file of 3ch.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, QZ., Zou, WL., Yu, ZY. et al. Remote site-selective arene C–H functionalization enabled by N-heterocyclic carbene organocatalysis. Nat Catal (2024). https://doi.org/10.1038/s41929-024-01194-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41929-024-01194-5