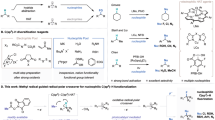
Abstract
Nickel photoredox catalysis has resulted in a rich development of transition-metal-catalysed transformations for carbon–heteroatom bond formation. By harnessing light energy, the transition metal can attain oxidation states that are difficult to achieve through thermal chemistry in a catalytic manifold. For example, nickel photoredox reactions have been reported for both the synthesis of anilines and aryl ethers from aryl(pseudo)halides. However, oxidative addition to simple nickel systems is often sluggish in the absence of special, electron-rich ligands, leading to catalyst decomposition. Electron-rich aryl electrophiles therefore currently fall outside the scope of many transformations in the field. Here we provide a conceptual solution to this problem and demonstrate nickel-catalysed C–heteroatom bond-forming reactions of arylthianthrenium salts, including amination, oxygenation, sulfuration and halogenation. Because the redox properties of arylthianthrenium salts are primarily dictated by the thianthrenium, oxidative addition of highly electron-rich aryl donors can be unlocked using simple NiCl2 under light irradiation to form the desired C‒heteroatom bonds.

Similar content being viewed by others
Main
The canonical transition-metal-catalysed C–N and C–O cross-coupling reactions proceed through well-established M(0)/M(II) redox cycles (Fig. 1a), largely dominated by palladium catalysts1,2,3. Building on these often well-understood transformations, and seminal work with nickel4,5 and copper catalysis6,7, recent efforts to improve the reactivity with more sustainable elements have appeared8,9,10,11. An increasing number of reports in this area have targeted mimicking the low-valent two-electron redox cycle12,13,14. To this end, a variety of elegant ligand classes based on phosphorous13,15,16 or N-heterocyclic carbenes17,18 have been designed to successfully achieve productive catalysis. This general low-valent redox paradigm was challenged by a report from MacMillan and Buchwald, who reported a nickel-catalysed C–N bond formation without ancillary ligands between aryl halides and amines, using a photocatalyst and light irradiation19. The protocol is characterized by facile access to high-valent Ni(III) species, which are responsible for a fast and energetically downhill C–N reductive elimination (Fig. 1a)20,21. A related nickel/photochemical approach has also been reported by the MacMillan group for C–O bond formation with aryl halides and alcohols22; in this case, a N-based ligand was necessary to form the C–O bond. The absence of complex exogenous ligands for a historically challenging C–N bond formation rapidly attracted the interest of the community, leading to many reports contributing to the expansion of the scope and understanding of its mechanistic intricacies22,23,24. Based on this redox manifold25, C–N and C–O bond-forming reactions have also been achieved using nickel in combination with electrochemistry26,27 or energy transfer to an excited Ni(II) complex28. More recently, thermally sustained Ni(I)/Ni(III) coupling processes have been postulated29. Regardless of the subtle mechanistic differences between them, the use of electron-rich aryl (pseudo)halides as coupling partners remains a challenge (Fig. 1b). It is speculated that the slower rate of oxidative addition of low-valent nickel complexes to electron-rich aryl halides when no supporting ligands are present results in accumulation and aggregation of nickel species, resulting in their deactivation. Only specific, isolated examples of electron-rich aryl halides have been shown to be suitable for C–N bond formation with current approaches, and these require up to a week of reaction time for primary alkyl amines30, and no general solution to the problem has been reported.
a, Canonical low-valent two-electron cross-coupling versus the high-valent NiI/III redox cycle. b, Electron-rich aryl halide: a recurrent limitation. c, This work: a general ligand-free nickel-catalysis platform for the C–N, C–O and C–S coupling of electron-rich and neutral arenes. EDG, electron donating group.
Intrigued by this persistent fundamental challenge in catalysis, we focused our attention on providing a general solution to include electron-rich aryl pseudohalides. Several pathways have been invoked for the oxidative addition of L–Ni(I) complexes into the C–X bond31, namely nucleophilic aromatic substitution (SNAr)32, single-electron transfer (SET)33, concerted oxidative addition34 and halogen atom abstraction35. Regardless of the mechanism, the success of the oxidative addition is largely dependent on the electronic structure of the aryl group (Fig. 1b). Although the redox properties of aryl halides are solely a function of the substituents on the arene, the redox properties of arylthianthrenium (ArTT) salts are predominantly determined by the thianthrenium unit itself, which acts as a redox antenna for SET events36. Upon single-electron reduction by a low-valent catalyst such as a Ni(I) salt, rapid mesolytic cleavage of the C–S bond can provide a synthetically useful aryl radical. This process depends on the stereo-electronic alignment of the σ* orbital of the exocyclic C–S bond with the π-system of the thianthrenium scaffold37, and hence is favourable even for arenes with electron-releasing substituents. Based on the demonstrated reducing ability of Ni(I) under light irradiation, we intended to utilize such species for the initial single-electron reduction of ArTT salts, followed by oxidative ligation of the aryl radical to form the required Ni(III) complex for C–N reductive elimination (Fig. 1c). Based on this approach, we show, here, a straightforward nickel-catalysed C–heteroatom bond cross-coupling with electron-neutral and electron-rich ArTT salts, thus providing a solution to the long-standing challenge in nickel photochemistry19,22,25,30. The protocol operates at room temperature, does not require the addition of ancillary ligands, and is suitable for the coupling of large and densely functionalized nucleophiles and thianthrenium salt fragments, thus rapidly generating complexity in late-stage contexts. The combination of selective C−H functionalization with a practical and broadly applicable cross-coupling protocol for electron-rich and electron-neutral arenes renders this approach complementary to known nickel-catalysed C–heteroatom bond-forming reactions.
Results
C–N bond formation
The reaction between ArTT salt 1 and piperidine 2 under irradiation with blue light-emitting-diode (LED) light, catalysed by 2 mol% NiCl2·6H2O, results in near-quantitative yield of 3 in N,N-dimethylacetamide (DMA) as solvent at 25 °C within 16 h (Table 1a, entry 1). Control experiments confirmed the necessity for light (entry 2). NiBr2·diglyme is also a competent catalyst, although NiCl2·6H2O provides a cheaper alternative (entry 3). Previously, we reported a conceptually different nickel-catalysed halogenation in the absence of light38, in which zinc was used as a reducing reagent. However, these conditions, even with 1.0 equiv. zinc, only resulted in less than 10% of product 3 (entry 4). The reaction system reported here is distinct from the previous approach. It proceeds through nickel photocatalysis, unlike our previous work, allowing for various C–heteroatom bond-formation reactions via the same approach, whereas the previous approach failed beyond halogenation. Initially, 2 equiv. amine was required to achieve a high product yield, consistent with its role as the substrate, the reductant for light-induced Ni(II) reduction and the base to neutralize the HBF4 formed during the reaction (vide infra). Although acceptable for simple, inexpensive amines, the two equivalents are not favourable for late-stage functionalization of complex small-molecule amines. We thus investigated the use of exogenous tertiary amines (for example, 3.0 equiv. DABCO), with the reagent amine as the limiting reagent, which afforded 3 in 84% yield (entry 5). The use of UV-A light (390 nm) did not lead to product formation, but resulted in decomposition of the starting material (entry 6). Adding 5.0 equiv. water to the reaction resulted in only slightly lower yield (85%, entry 7). An inert atmosphere is particularly important for this reaction; only trace amounts of product were generated when the reaction was executed in air (Supplementary Table 3 provides details).
Electron-rich and electron-neutral ArTT salts can be converted to products in 50–93% yields, as shown in Table 1b. Complementary to other Ni-catalysed amination protocols19,22,28,39,40, electron-deficient substrates react less efficiently (for example, 18). Oxidative addition of electron-poor arenes proceeds faster (vide infra), but the yields are lower due to the observed competing side reaction of hydrodefunctionalization. The detailed reasons for this side reaction were not established, in large part because analysis of the arylnickel species in the absence of ancillary ligands is challenging, but it may be a consequence of hydrogen atom transfer (HAT) from the α-C−H position of the amine or the solvent41. Functional-group compatibility is high, accommodating sulfonamides, amides, cyclopropyls, ethers, biaryls, halides, nitriles, esters, heterocycles, carbamates and amines. Complex, functionalized small molecules such as flurbiprofen (10), nefiracetam (14), benzyloxazolidinone (15), boscalid (19), strychnine (20), fenbufen (24) and pyriproxyfen (25) can be converted into the corresponding aminated products, providing a useful protocol for late-stage modification. Substrates with para- and meta-substitution can participate in the reaction as well. The ortho-substituted ArTT salts fall beyond the scope of the protocol, and result in hydrodefunctionalized by-products as well as unreacted starting materials.
A variety of N-nucleophiles, including primary alkyl amines, secondary alkyl amines, anilines, sulfonamides and amides, performed well under the reaction conditions, as demonstrated in Table 2. Cyclic secondary amines with different ring sizes, α-methyl substituted pyrrolidine, N-Boc protected piperazine, morpholine, and fused or spirocyclic-, pyrimidine- and benzoisothiazole-amines are all compatible in the reaction. Dimethylamine could efficiently yield the target product 37 with 80% yield. However, when using a linear secondary amine with larger steric hindrance compared to dimethylamine, the yield decreased, as shown for product 51. The lower yield may be due to steric hindrance slowing the reaction and causing β-H elimination upon coordination with the nickel catalyst19. However, with the addition of 3 equiv. 2-tert-butyl-1,1,3,3-tetramethylguanidine (BTMG), switching the nickel source to NiCl2·glyme with a loading of 10 mol%, and irradiation of the reaction mixture, primary amines could also engage in the reaction to furnish the desired products. Although blue LEDs were also effective, white LEDs provided slightly higher yields in the case of primary amines (Supplementary Fig. 11). Cyclopropylamine, propargylamine, α-CF3-substituted and primary alkyl amines containing furan rings can all participate in the reaction to furnish the desired products in 40–89% yields. Substrates with functional groups that are susceptible to side reactions in Pd-catalysed amination reactions, such as olefins (Heck) and boronic esters (Suzuki), are tolerated under the reaction conditions reported here. In addition, acidic functional groups are also compatible (for example, 38), and are problematic in our previously reported Pd-catalysed amination protocol42.
Extension to other C−X bond formation
The developed catalyst system was extended to medicinally relevant methoxylation using MeOH as nucleophile. Both simple and highly functionalized ArTT salts bearing alkyls, halogens, amides, heterocycles and esters can be engaged in this process, as shown in Table 3. Other primary and secondary alcohols, as well as phenols, can also participate in the reaction, with hydrodefunctionalized compounds as the main by-products. Although the construction of C−O bonds with ArTT salts has been reported previously43, those transformations were not catalytic, and stoichiometric amounts of copper were required. Additionally, for halogenation, the use of heterogeneous zinc powder was required for the formation of the Ni(I) catalyst, which could pose challenges on a larger scale. Furthermore, the formation of C−S bonds was limited to aromatic thiols, leaving alkyl thiols beyond the scope44,45. In this context, our current protocol overcomes these previous limitations and provides a unified approach across nitrogen, oxygen and sulfur nucleophiles. Moreover, sodium iodide, sodium bromide and tetrabutylammonium chloride (TBACl) can be used as nucleophiles in the same catalytic manifold, which provides a straightforward and easy method for the halogenation of drug-like molecules in a late-stage functionalization, although the transformation has thus far not been extendable to fluorination (Table 3).
To validate the scalability of our protocol, we performed a gram-scale fragment coupling of two drug molecules, as highlighted in Fig. 2a. Compound 79 was previously46 synthesized in a seven-step sequence, starting from aryl bromide 80. With our protocol, we can begin with thianthrenation of 78, followed by amination and a nucleophilic substitution (SN2) reaction, and are able to streamline the synthesis to three steps starting from a cheaper starting material (Fig. 2b).
a, Gram-scale process for fragment coupling. TT, thianthrene. b, Alternative retro-synthetic strategy. See Supplementary Methods for the detailed experimental procedure. The price of compounds 78 and 80 was retrieved from Sigma-Aldrich on 5 July 2023.
A working hypothesis for the Ni-catalysed C–X bond formation is depicted in Fig. 3a. Based on previous examples of photoredox nickel-catalysed reactions23,40, it is speculated that Ni(I) species may be generated from the simple Ni(II) salt through a ligand-to-metal charge transfer (LMCT) process through the ligated amine under light irradiation40. When mixing NiBr2·diglyme with amine 2 for 5 min under blue LED irradiation, signals consistent with a paramagnetic Ni(I) species were observed by electron paramagnetic resonance (EPR) spectroscopy (Supplementary Fig. 4 provides details). Additional evidence for the involvement of Ni(I) was provided by experiments performed in the absence of light. When a mixture of 1 and 2 was combined with Ni(COD)2 (100 mol%) and one-electron oxidant FeCp2BArF (100 mol%) in the dark, a 90% yield of C–N bond product 3 was obtained (Fig. 3b, entry 1). However, the sole presence of Ni(0) [Ni(COD)2] or Ni(II) (NiBr2·diglyme) in the absence of irradiation did not produce any target product, and 1 remained unreacted (Fig. 3b, entries 2 and 3). Oxidative addition could proceed by initial SET to the ArTT cation. A measured positive but relatively small Hammett rho value of ρ = 1.1 is consistent with the hypothesis that the substituents on the arene play less of a role for the rate of oxidative addition, and the process is primarily governed by the electronic structure of the thianthrenium substituent itself. Although different ρ values have been observed previously depending on the mechanism of the nickel-based oxidative addition reactions, a larger ρ value of 4 was observed for the oxidative addition of nickel by SET to aryl iodides31,47,48,49. We also confirmed the requirement for continuous visible-light irradiation by performing light on/off experiments, excluding the possibility of a self-sustained Ni(I)/Ni(III) cycle; both the amination reaction and the oxygenation reaction proceeded only in the presence of light (Supplementary Figs. 5 and 6). The requirement of light for reactivation to a catalytically active Ni(I) complex after reductive elimination is in agreement with previous research regarding related nickel photoredox C–N bond formation23,24,40. Although specific information about the mechanism could be obtained here—such as the relevance and importance of the involvement of Ni(I) species—details on the structure of the nickel complex after reductive elimination remain elusive due to the simple catalytic system deprived of any ancillary ligands, which could otherwise support isolation and characterization of the intermediate metal complexes. Similarly, different pathways prior to reductive elimination are conceivable, with the subtle differences not being discernible with our current set of data. For example, reductive elimination could be preceded by oxidative addition via oxidative ligation to a Ni(III) intermediate as suggested, but an energy transfer from a Ni(II) aryl intermediate cannot be excluded at this stage.
Conclusion
The Ni(I)-catalysed C–heteroatom bond formation presented here represents a general approach to include electron-rich aryl electrophiles in nickel photoredox catalysis based on simple nickel salts. Through a fundamentally distinct SET oxidative addition process, thianthrenium salts provide a solution to the long-standing challenge of coupling electron-rich aryl (pseudo)halides. The combination of a Ni(I)/Ni(III) redox cycle appears well suited to the electronic structure of ArTT salts and provides a fundamental advance over previous reaction developments. We demonstrate that, together with site-selective C−H thianthrenation, our contribution offers a distinct approach for late-stage diversification.
Methods
General procedure for amination of ArTT salts (secondary alkyl amines)
A culture tube with a Teflon screw-cap equipped with a Teflon-coated stir bar was used. ArTT salt (0.20 mmol, 1.0 equiv.) and amine (if solid, 0.40 mmol, 2.0 equiv.) were introduced into the culture tube, then, inside a glovebox, a stock solution of NiCl2·6H2O in dry DMA (2 ml, 2–10 mol%) was added into the tube via syringe at 25 °C. The reaction tube was taken out of the glovebox, and amine (if liquid, 0.40 mmol, 2.0 equiv.) was added via a microsyringe at 25 °C. The reaction mixture was stirred at 25 °C with irradiation by blue LEDs (456 nm, 34 W × 2; the culture tube containing the reaction mixture was put in the centre of the two light sources, and the distance to each light source was ~5 cm). After 24 h, the mixture was diluted with ethyl acetate (~4 ml), washed with brine (~3 ml) and dried over Na2SO4. On filtration, the organic layer was concentrated and purified by flash column chromatography on silica gel or preparative thin layer chromatography (pTLC) to afford the desired product.
General procedure for amination of ArTT salts (primary alkyl amines, anilines, amides and sulfonamides)
A culture tube with a Teflon screw-cap equipped with a Teflon-coated stir bar was used. ArTT salt (0.20 mmol, 1.0 equiv.) and nitrogen nucleophile (0.60 mmol, 3.0 equiv., if solid) were introduced into the culture tube. The culture tube was introduced into a glovebox. Inside the glovebox, NiCl2·glyme (4.4 mg, 20 µmol, 10 mol%) was introduced into the tube, and dry DMA (2.0 ml, c = 0.10 M) was added using a syringe. The reaction tube was taken out of the glovebox, and nitrogen nucleophile (0.60 mmol, 3.0 equiv., if liquid) and BTMG (122 µl, 0.600 mmol, 3.00 equiv.) were added using microsyringes. The mixture was stirred at 25 °C with white LED irradiation (light source ~5 cm away). After 24 h, the mixture was diluted with ethyl acetate (~4 ml), washed with brine (~3 ml) and dried over Na2SO4. On filtration, the organic layer was concentrated and purified by flash column chromatography on silica gel or pTLC to afford the desired product.
General procedure for catalytic C−heteroatom bond formation of ArTT salts (alcohols, thiols, phenols and halogens)
A culture tube with a Teflon screw-cap equipped with a Teflon-coated stir bar was used. ArTT salt (0.10 mmol, 1.0 equiv.), quinuclidine (33.4 mg, 300 µmol, 3.00 equiv.) and nucleophile (if solid, 0.20–1.0 mmol, 2.0–10 equiv.) were introduced into the culture tube. The culture tube was introduced into a glovebox. Inside the glovebox, NiCl2·glyme (2.2 mg, 10 µmol, 10 mol%) was introduced into the tube, and dry DMA (1.0 ml, 0.10 M) was added using a syringe. The reaction tube was taken out of the glovebox, then nucleophile (if liquid, 0.20–1.0 mmol, 2.0–10 equiv.) was added using a microsyringe. The reaction mixture was stirred at 25 °C with irradiation by 465-nm blue LEDs (the culture tube containing the reaction mixture was put in the centre of the light source, and the distance to the light source was ~5 cm). After 24 h, the mixture was diluted with ethyl acetate (~4 ml), washed with brine (~3 ml), and dried over Na2SO4. On filtration, the organic layer was concentrated and purified by flash column chromatography on silica gel or pTLC to afford the desired product.
Data availability
All data relating to the materials and methods, experimental procedures, mechanistic studies and NMR spectra are available in the Supplementary Information or from the authors upon reasonable request.
References
Hartwig, J. F. Evolution of a fourth generation catalyst for the amination and thioetherification of aryl halides. Acc. Chem. Res. 41, 1534–1544 (2008).
Ruiz-Castillo, P. & Buchwald, S. L. Applications of palladium-catalyzed C–N cross-coupling reactions. Chem. Rev. 116, 12564–12649 (2016).
Dorel, R., Grugel, C. P. & Haydl, A. M. The Buchwald-Hartwig amination after 25 years. Angew. Chem. Int. Ed. 58, 17118–17129 (2019).
Hughes, E. C., Veatch, F. & Elersich, V. N-methylaniline from chlorobenzene and methylamine. Ind. Eng. Chem. 42, 787–790 (1950).
Cramer, R. & Coulson, D. R. Nickel-catalyzed displacement reactions of aryl halides. J. Org. Chem. 40, 2267–2273 (1975).
Ullmann, F. Ueber eine neue Bildungsweise von Diphenylaminderivaten. Chem. Ber. 36, 2382–2384 (1903).
Cristau, H.-J. & Desmurs, J.-R. in Industrial Chemistry Library Vol. 7 (eds Desmurs, J.-R. et al.) 240–263 (Elsevier, 1995).
Marín, M., Rama, R. J. & Nicasio, M. C. Ni-catalyzed amination reactions: an overview. Chem. Rec. 16, 1819–1832 (2016).
Ma, D. & Cai, Q. Copper/amino acid catalyzed cross-couplings of aryl and vinyl halides with nucleophiles. Acc. Chem. Res. 41, 1450–1460 (2008).
Creutz, S. E., Lotito, K. J., Fu, G. C. & Peters, J. C. Photoinduced Ullmann C-N coupling: demonstrating the viability of a radical pathway. Science 338, 647–651 (2012).
Ziegler, D. T. et al. A versatile approach to Ullmann C-N couplings at room temperature: new families of nucleophiles and electrophiles for photoinduced, copper-catalyzed processes. J. Am. Chem. Soc. 135, 13107–13112 (2013).
Morrison, K. M., Yeung, C. S. & Stradiotto, M. Nickel-catalyzed chemoselective arylation of amino alcohols. Angew. Chem. Int. Ed. 62, e202300686 (2023).
Reichert, E. C., Feng, K., Sather, A. C. & Buchwald, S. L. Pd-catalyzed amination of base-sensitive five-membered heteroaryl halides with aliphatic amines. J. Am. Chem. Soc. 145, 3323–3329 (2023).
Gowrisankar, S. et al. A general and efficient catalyst for palladium-catalyzed C-O coupling reactions of aryl halides with primary alcohols. J. Am. Chem. Soc. 132, 11592–11598 (2010).
Tassone, J. P., England, E. V., MacQueen, P. M., Ferguson, M. J. & Stradiotto, M. PhPAd-DalPhos: ligand-enabled, nickel-catalyzed cross-coupling of (hetero)aryl electrophiles with bulky primary alkylamines. Angew. Chem. Int. Ed. 58, 2485–2489 (2019).
Newman-Stonebraker, S. H., Wang, J. Y., Jeffrey, P. D. & Doyle, A. G. Structure-reactivity relationships of Buchwald-type phosphines in nickel-catalyzed cross-couplings. J. Am. Chem. Soc. 144, 19635–19648 (2022).
Marion, N. et al. Modified (NHC)Pd(allyl)Cl (NHC = N-heterocyclic carbene) complexes for room-temperature Suzuki-Miyaura and Buchwald-Hartwig reactions. J. Am. Chem. Soc. 128, 4101–4111 (2006).
Rull, S. G. et al. Elucidating the mechanism of aryl aminations mediated by NHC-supported nickel complexes: evidence for a nonradical Ni(0)/Ni(II) pathway. ACS Catal. 8, 3733–3742 (2018).
Corcoran, E. B. et al. Aryl amination using ligand-free Ni(II) salts and photoredox catalysis. Science 353, 279–283 (2016).
Barker, T. J. & Jarvo, E. R. Umpolung amination: nickel-catalyzed coupling reactions of N,N-dialkyl-N-chloroamines with diorganozinc reagents. J. Am. Chem. Soc. 131, 15598–15599 (2009).
Koo, K. & Hillhouse, G. L. Carbon-nitrogen bond formation by reductive elimination from nickel(II) amido alkyl complexes. Organometallics 14, 4421–4423 (1995).
Terrett, J. A., Cuthbertson, J. D., Shurtleff, V. W. & MacMillan, D. W. C. Switching on elusive organometallic mechanisms with photoredox catalysis. Nature 524, 330–334 (2015).
Till, N. A., Tian, L., Dong, Z., Scholes, G. D. & MacMillan, D. W. C. Mechanistic analysis of metallaphotoredox C-N coupling: photocatalysis initiates and perpetuates Ni(I)/Ni(III) coupling activity. J. Am. Chem. Soc. 142, 15830–15841 (2020).
Bradley, R. D., McManus, B. D., Yam, J. G., Carta, V. & Bahamonde, A. Mechanistic evidence of a Ni(0/II/III) cycle for nickel photoredox amide arylation. Angew. Chem. Int. Ed. 62, e202310753 (2023).
Chan, A. Y. et al. Metallaphotoredox: the merger of photoredox and transition metal catalysis. Chem. Rev. 122, 1485–1542 (2022).
Li, C. et al. Electrochemically enabled, nickel-catalyzed amination. Angew. Chem. Int. Ed. 56, 13088–13093 (2017).
Kawamata, Y. et al. Electrochemically driven, Ni-catalyzed aryl amination: scope, mechanism and applications. J. Am. Chem. Soc. 141, 6392–6402 (2019).
Kudisch, M., Lim, C.-H., Thordarson, P. & Miyake, G. M. Energy transfer to Ni-amine complexes in dual catalytic, light-driven C-N cross-coupling reactions. J. Am. Chem. Soc. 141, 19479–19486 (2019).
Sun, R., Qin, Y. & Nocera, D. G. General paradigm in photoredox nickel-catalyzed cross-coupling allows for light-free access to reactivity. Angew. Chem. Int. Ed. 59, 9527–9533 (2020).
Gisbertz, S., Reischauer, S. & Pieber, B. Overcoming limitations in dual photoredox/nickel-catalysed C–N cross-couplings due to catalyst deactivation. Nat. Catal. 3, 611–620 (2020).
Tang, T. et al. Interrogating the mechanistic features of Ni(I)-mediated aryl iodide oxidative addition using electroanalytical and statistical modeling techniques. J. Am. Chem. Soc. 145, 8689–8699 (2023).
Portnoy, M. & Milstein, D. Mechanism of aryl chloride oxidative addition to chelated palladium(0) complexes. Organometallics 12, 1665–1673 (1993).
Tsou, T. T. & Kochi, J. K. Mechanism of oxidative addition. Reaction of nickel(0) complexes with aromatic halides. J. Am. Chem. Soc. 101, 6319–6332 (1979).
Amatore, C. & Pfluger, F. Mechanism of oxidative addition of palladium(0) with aromatic iodides in toluene, monitored at ultramicroelectrodes. Organometallics 9, 2276–2282 (1990).
Biscoe, M. R., Fors, B. P. & Buchwald, S. L. A new class of easily activated palladium precatalysts for facile C-N cross-coupling reactions and the low temperature oxidative addition of aryl chlorides. J. Am. Chem. Soc. 130, 6686–6687 (2008).
Berger, F. et al. Site-selective and versatile aromatic C−H functionalization by thianthrenation. Nature 567, 223–228 (2019).
Li, J. et al. Photoredox catalysis with aryl sulfonium salts enables site-selective late-stage fluorination. Nat. Chem. 12, 56–62 (2020).
Ni, S. et al. Nickel meets aryl thianthrenium salts: Ni(I)-catalyzed halogenation of arenes. J. Am. Chem. Soc. 145, 9988–9993 (2023).
Ghosh, I. et al. General cross-coupling reactions with adaptive dynamic homogeneous catalysis. Nature 619, 87–93 (2023).
Lim, C.-H., Kudisch, M., Liu, B. & Miyake, G. M. C-N cross-coupling via photoexcitation of nickel-amine complexes. J. Am. Chem. Soc. 140, 7667–7673 (2018).
Shields, B. J., Kudisch, B., Scholes, G. D. & Doyle, A. G. Long-lived charge-transfer states of nickel(II) aryl halide complexes facilitate bimolecular photoinduced electron transfer. J. Am. Chem. Soc. 140, 3035–3039 (2018).
Engl, P. S. et al. C-N cross-couplings for site-selective late-stage diversification via aryl sulfonium salts. J. Am. Chem. Soc. 141, 13346–13351 (2019).
Sang, R. et al. Site-selective C-H oxygenation via aryl sulfonium salts. Angew. Chem. Int. Ed. 58, 16161–16166 (2019).
Cabrera-Afonso, M. J., Granados, A. & Molander, G. A. Sustainable thioetherification via electron donor-acceptor photoactivation using thianthrenium salts. Angew. Chem. Int. Ed. 61, e202202706 (2022).
Zhu, J., Ye, Y., Yan, Y., Sun, J. & Huang, Y. Highly regioselective dichalcogenation of alkenyl sulfonium salts to access 1,1-dichalcogenalkenes. Org. Lett. 25, 5324–5328 (2023).
Urade, Y. et al. Preparation of 4-((pyrrol-2-yl)carbonyl)-N-(piperidin-4-yl)-1-piperazinecarboxamide compounds having a prostaglandin D synthase (PGDS) inhibitory effect. PCT patent WO2011090062 (2011).
Till, N. A., Oh, S., MacMillan, D. W. C. & Bird, M. J. The application of pulse radiolysis to the study of Ni(I) intermediates in Ni-catalyzed cross-coupling reactions. J. Am. Chem. Soc. 143, 9332–9337 (2021).
Ting, S. I., Williams, W. L. & Doyle, A. G. Oxidative addition of aryl halides to a Ni(I)-bipyridine complex. J. Am. Chem. Soc. 144, 5575–5582 (2022).
Pierson, C. N. & Hartwig, J. F. Mapping the mechanisms of oxidative addition in cross-coupling reactions catalysed by phosphine-ligated Ni(0). Nat. Chem. https://doi.org/10.1038/s41557-024-01451-x (2024).
Acknowledgements
Financial support for this work was provided by the Max-Planck-Gesellschaft, Max-Planck-Institut für Kohlenforschung, and the Fonds der Chemischen Industrie (VCI-FCI). We thank the analytical departments (NMR spectroscopy, mass spectrometry and HPLC) at the MPI für Kohlenforschung for support in the characterization of compounds.
Funding
Open access funding provided by Max Planck Society.
Author information
Authors and Affiliations
Contributions
S.N., J.C. and T.R. conceived this project. S.N. developed the C–heteroatom bond formation. S.N. and R.H. explored the scope of C−N bond formation. S.N. and D.A. explored the scope of C–O, S and halogen bond formation. E.J.R. performed EPR experiments. S.N., J.C. and T.R. wrote the paper. J.C. and T.R. directed the project.
Corresponding authors
Ethics declarations
Competing interests
T.R. may benefit from sales of thianthrene-related compounds. The other authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Yinhua Huang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Tables 1–11, Figs. 1–11, spectroscopic data and references.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ni, S., Halder, R., Ahmadli, D. et al. C–heteroatom coupling with electron-rich aryls enabled by nickel catalysis and light. Nat Catal (2024). https://doi.org/10.1038/s41929-024-01160-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41929-024-01160-1