Abstract
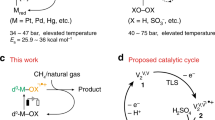
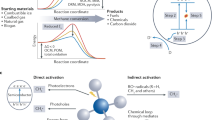
Reforming of methane with H2S is a promising path to directly utilize sour natural gas reserves, although some aspects of the mechanism and structure–function relations remain elusive. Here we show that metal oxides of group 4–6 elements, which are inert for steam and dry methane reforming reactions, are active and stable (pre)catalysts for the H2S reforming of methane. The key active sites are sulfur species (S*) that are dynamically bound to metal cations during catalysis. Similar H/D isotope exchange patterns and universal rate inhibition by H2 on representative catalysts indicate that H2S decomposition and recombination of surface hydrogen atoms are quasi-equilibrated, whereas CH4 dissociation steps are reversible, yet not quasi-equilibrated. An in-depth analysis of the kinetic data and isotopic substitution effects identifies S*-mediated C–H bond cleavage as the most plausible rate-limiting step common for all catalysts, with subtle yet essential differences between 3d and 4d/5d catalysts in the thermodynamic stability of S*.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data supporting this work are available in the main text and Supplementary Information. Source data are provided with this paper.
References
York, A. P. E., Xiao, T. C., Green, M. L. H. & Claridge, J. B. Methane oxyforming for synthesis gas production. Catal. Rev. 49, 511–560 (2007).
Choudhary, T. V. & Choudhary, V. R. Energy-efficient syngas production through catalytic oxy-methane reforming reactions. Angew. Chem. Int. Ed. 47, 1828–1847 (2008).
Taifan, W. & Baltrusaitis, J. Minireview: direct catalytic conversion of sour natural gas (CH4 + H2S + CO2) components to high-value chemicals and fuels. Catal. Sci. Technol. 7, 2919–2929 (2017).
Gutiérrez, O. Y., Kaufmann, C. & Lercher, J. A. Synthesis of methanethiol from carbonyl sulfide and carbon disulfide on (Co)K-promoted sulfide Mo/SiO2 catalysts. ACS Catal. 1, 1595–1603 (2011).
Gutiérrez, O. Y., Zhong, L., Zhu, Y. & Lercher, J. A. Synthesis of methanethiol from CS2 on Ni-, Co-, and K-doped MoS2/SiO2 catalysts. ChemCatChem 5, 3249–3259 (2013).
Olah, G. A. et al. Onium ylide chemistry. 1. Bifunctional acid-base-catalyzed conversion of heterosubstituted methanes into ethylene and derived hydrocarbons. The onium ylide mechanism of the C → C2 conversion. J. Am. Chem. Soc. 106, 2143–2149 (1984).
Huguet, E. et al. A highly efficient process for transforming methyl mercaptan into hydrocarbons and H2S on solid acid catalysts. Appl. Catal. B 134–135, 344–348 (2013).
Hulea, V. et al. Conversion of methyl mercaptan and methanol to hydrocarbons over solid acid catalysts—a comparative study. Appl. Catal. B 144, 547–553 (2014).
Cammarano, C. et al. Selective transformation of methyl and ethyl mercaptans mixture to hydrocarbons and H2S on solid acid catalysts. Appl. Catal. B 156–157, 128–133 (2014).
Erekson, E. J. Gasoline from Natural Gas by Sulfur Processing Final Technical Report, June 1993–July 1996 (OSTI, 1996); https://doi.org/10.2172/303990
Miao, F. Q. & Erekson, E. J. Method for direct production of carbon disulfide and hydrogen from hydrocarbons and hydrogen sulfide feedstock. US patent 9018957 (1998).
Espinosa, G., Dominguez, J. M., Diaz, L. & Angeles, C. Catalytic behavior of CoMo/ZSM5 catalysts for CS2 conversion. Catal. Today 148, 153–159 (2009).
Martínez-Salazar, A. L. et al. Technoeconomic analysis of hydrogen production via hydrogen sulfide methane reformation. Int. J. Hydrogen Energy 44, 12296–12302 (2019).
Huang, C. & T-Raissi, A. Liquid hydrogen production via hydrogen sulfide methane reformation. J. Power Sources 175, 464–472 (2008).
Waterman, H. I. & Van Vlodrop, C. The preparation of carbon disulfide from methane and hydrogen sulfide. J. Soc. Chem. Ind. Lond. 58, 109–110 (1939).
Karan, K. & Behie, L. A. CS2 formation in the Claus reaction furnace: a kinetic study of methane−sulfur and methane−hydrogen sulfide reactions. Ind. Eng. Chem. Res. 43, 3304–3313 (2004).
El-Melih, A. M., Al Shoaibi, A. & Gupta, A. K. Hydrogen sulfide reformation in the presence of methane. Appl. Energy 178, 609–615 (2016).
El-Melih, A. M., Iovine, L., Al Shoaibi, A. & Gupta, A. K. Production of hydrogen from hydrogen sulfide in presence of methane. Int. J. Hydrogen Energy 42, 4764–4773 (2017).
Li, Y. et al. Kinetic study of decomposition of H2S and CH4 for H2 production using detailed mechanism. Energy Procedia 142, 1065–1070 (2017).
Megalofonos, S. K. & Papayannakos, N. G. Hydrogen production from natural gas and hydrogen sulphide. Int. J. Hydrogen Energy 16, 319–327 (1991).
Megalofonos, S. K. & Papayannakos, N. G. Kinetics of catalytic reaction of methane and hydrogen sulphide over MoS2. Appl. Catal. A 165, 249–258 (1997).
Megalofonos, S. K. & Papayannakos, N. G. Kinetics of the catalytic reaction of methane and hydrogen sulphide over a Pt–Al2O3 catalyst. Appl. Catal. A 138, 39–55 (1996).
Galindo-Hernández, F., Domínguez, J. M. & Portales, B. Structural and textural properties of Fe2O3/γ-Al2O3 catalysts and their importance in the catalytic reforming of CH4 with H2S for hydrogen production. J. Power Sources 287, 13–24 (2015).
Martínez-Salazar, A. L. et al. Hydrogen production by methane reforming with H2S using Mo,Cr/ZrO2–SBA15 and Mo,Cr/ZrO2–La2O3 catalysts. Int. J. Hydrogen Energy 40, 17272–17283 (2015).
Martínez-Salazar, A. L. et al. Hydrogen production by methane and hydrogen sulphide reaction: kinetics and modeling study over Mo/La2O3–ZrO2 catalyst. Int. J. Hydrogen Energy 40, 17354–17360 (2015).
Wang, H. et al. Sulfidation of MoO3/γ-Al2O3 towards a highly efficient catalyst for CH4 reforming with H2S. Catal. Sci. Technol. 11, 1125–1140 (2021).
Fremy, G., Barre, P. & Raymond, J.-M. Method for preparing methyl mercaptan. US patent 2017/0158631 (2017).
Kaloidas, V. & Papayannakos, N. Kinetics of thermal, non-catalytic decomposition of hydrogen sulphide. Chem. Eng. Sci. 44, 2493–2500 (1989).
Liu, S. et al. ‘Soft’ oxidative coupling of methane to ethylene: mechanistic insights from combined experiment and theory. Proc. Natl Acad. Sci. USA 118, e2012666118 (2021).
Peter, M. & Marks, T. J. Platinum metal-free catalysts for selective soft oxidative methane → ethylene coupling. Scope and mechanistic observations. J. Am. Chem. Soc. 137, 15234–15240 (2015).
Zhu, Q. et al. Sulfur as a selective ‘soft’ oxidant for catalytic methane conversion probed by experiment and theory. Nat. Chem. 5, 104–109 (2013).
Ashcroft, A. T., Cheetham, A. K., Green, M. L. H. & Vernon, P. D. F. Partial oxidation of methane to synthesis gas using carbon dioxide. Nature 352, 225–226 (1991).
Akri, M. et al. Atomically dispersed nickel as coke-resistant active sites for methane dry reforming. Nat. Commun. 10, 5181 (2019).
Yamaguchi, A. & Iglesia, E. Catalytic activation and reforming of methane on supported palladium clusters. J. Catal. 274, 52–63 (2010).
Wei, J. & Iglesia, E. Structural and mechanistic requirements for methane activation and chemical conversion on supported iridium clusters. Angew. Chem. Int. Ed. 43, 3685–3688 (2004).
Wei, J. & Iglesia, E. Isotopic and kinetic assessment of the mechanism of methane reforming and decomposition reactions on supported iridium catalysts. Phys. Chem. Chem. Phys. 6, 3754–3759 (2004).
Wei, J. & Iglesia, E. Isotopic and kinetic assessment of the mechanism of reactions of CH4 with CO2 or H2O to form synthesis gas and carbon on nickel catalysts. J. Catal. 224, 370–383 (2004).
Wei, J. & Iglesia, E. Mechanism and site requirements for activation and chemical conversion of methane on supported Pt clusters and turnover rate comparisons among noble metals. J. Phys. Chem. B 108, 4094–4103 (2004).
Wei, J. & Iglesia, E. Structural requirements and reaction pathways in methane activation and chemical conversion catalyzed by rhodium. J. Catal. 225, 116–127 (2004).
Wei, J. & Iglesia, E. Reaction pathways and site requirements for the activation and chemical conversion of methane on Ru-based catalysts. J. Phys. Chem. B 108, 7253–7262 (2004).
Toulhoat, H. & Raybaud, P. Kinetic interpretation of catalytic activity patterns based on theoretical chemical descriptors. J. Catal. 216, 63–72 (2003).
Shang, H., Wang, T. & Zhang, W. Sulfur vacancy formation at different MoS2 edges during hydrodesulfurization process: a DFT study. Chem. Eng. Sci. 195, 208–217 (2019).
Dibeler, V. H. & Mohler, F. L. Mass spectra of the deuteromethanes. J. Res. Natl Bur. Stand. 45, 441–444 (1950).
Dibeler, V. H. & Rosenstock, H. M. Mass spectra and metastable transitions of H2S, HDS, and D2S. J. Chem. Phys. 39, 3106–3111 (1963).
Acknowledgements
This work was financially supported by Evonik Industries. J.A.L. acknowledges support by the US Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences and Biosciences (Impact of catalytically active centers and their environment on rates and thermodynamic states along reaction paths, FWP 47319). We thank L. Wahl and X. Hecht for the construction of the reaction device, S. Ren and F. Chew for catalyst screening, R. Weindl, A. Wellmann (Technical University of Munich) and J. Hu (Xiamen University) for transmission electron microscopy and Raman characterizations.
Author information
Authors and Affiliations
Contributions
Y.W. and X.C. performed the experiments; Y.W. and H.S. conceived the work, designed the experiments and analysed the data; H.S. and J.A.L. supervised the project. All the authors discussed the results and were involved in the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Ping Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–36, Tables 1–10 and Notes 1−5.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Chen, X., Shi, H. et al. Catalytic reforming of methane with H2S via dynamically stabilized sulfur on transition metal oxides and sulfides. Nat Catal 6, 204–214 (2023). https://doi.org/10.1038/s41929-023-00922-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-00922-7
This article is cited by
-
Balancing elementary steps enables coke-free dry reforming of methane
Nature Communications (2023)