Abstract
Triple-negative breast cancer (TNBC) patients are more likely to have BRCA1/2 mutations, with a prevalence rate of about 10–20%. Although several studies have analyzed the oncologic outcomes between BRCA1/2 carriers and non-carriers, the impact on breast cancer patients is still unclear. A retrospective review was performed to determine the long-term outcomes of TNBC patients, focusing on the impact of BRCA1/2 mutations. A total of 953 TNBC patients who underwent primary breast cancer surgery from June 2008 to January 2016 were included. We examined long-term outcomes, including contralateral breast cancer (CBC) incidence, recurrence patterns, and survival rates over a median follow-up of 80.9 months (range 3–152 months). 122 patients (12.8%) had BRCA1/2 mutations. BRCA1/2 mutation carriers were significantly younger at diagnosis and more likely to have a family history of breast/ovarian cancer. CBC incidence at 60, 120, and 150 months was significantly higher in BRCA1/2 mutation carriers compared to non-carriers (P = 0.0250, 0.0063, and 0.0184, respectively). However, there were no significant differences in disease-free survival, overall survival, breast cancer-specific survival, or distant-metastasis-free survival between the two groups. BRCA1/2 mutation status was a significant risk factor for CBC (HR = 6.242, P < 0.0001). Interestingly, among 29 patients with CBC recurrence, 24 patients (82.8%) had recurring TNBC subtype and among the CBC recurrence patients, 19 patients (65.5%) resumed chemotherapy. In the TNBC subtype, appropriate genetic testing and counseling are pivotal for surgical decisions like risk-reducing mastectomy (RRM). Furthermore, long-term surveillance is warranted, especially in BRCA1/2 carriers who did not receive RRM.
Similar content being viewed by others
Introduction
Breast cancer comprises subtypes with distinct morphologies and clinical implications. Triple-negative breast cancer (TNBC) is defined by little or lack of expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER-2)1. The TNBC subtype has poor oncologic outcomes; a higher risk of early recurrence and a lower risk of late recurrence compared to luminal-type breast cancer2,3,4. Among all breast cancer patients, TNBC patients are more likely to have BRCA1/2 germline mutations, with a prevalence rate of approximately 10–20%5,6,7,8.
Several studies have analyzed prognosis and oncologic outcomes between BRCA1/2 carriers and non-carriers, but whether BRCA1/2 carriers have a worse prognosis is unclear. Some reports have shown that BRCA1/2 mutation carriers have poor overall survival (OS) relative to non-carriers9,10. Other studies have reported no difference in OS between BRCA1/2 carriers and non-carriers11,12,13,14, and some reports indicate that BRCA1/2 carriers showed better survival outcomes than non-carriers6. Recently, results from the OlympiA trial reported improved survival with the use of poly (adenosine diphosphate-ribose) polymerase inhibitor (PARPi), olaparib in BRCA1/2 mutation associated early breast cancer patients15. To date, only a few studies have focused on the effect of BRCA1/2 mutations on long-term oncologic prognosis in unselected TNBC patients.
Previously, we reported the prevalence and oncologic outcomes (median follow-up of 53.6 months) of germline BRCA1/2 mutations among unselected Korean patients with TNBC. Poor contralateral breast cancer (CBC)-free survival and a prevalence of 14.5% in TNBC patients aged ≤ 60 years were observed, contributing to the update in BRCA1/2 genetic testing guidelines in Korea16. Here, we present the follow-up results of our previous study, which assessed the long-term oncologic outcomes and recurrence rates after 5 years with a median follow-up of 80.9 months.
Results
Baseline characteristics
The baseline patient characteristics according to BRCA1/2 mutation are shown in Table 1. All patients were Korean. BRCA1/2 mutation carriers were significantly younger (mean age 45.5 ± 11.0 vs. 50.1 ± 10.8) and premenopausal (63.1% vs. 47.3%) at the time of diagnosis compared to non-carriers (P = 0.002 and P = 0.0011, respectively). Also, BRCA1/2 mutation carriers were more likely to have a family history of breast cancer and/or ovarian cancer (40.2% vs. 9.4%, P < 0.0001), personal history of ovarian cancer (9.0% vs. 0.5%, P < 0.0001), bilateral breast cancer (4.9% vs. 1.2%, P = 0.0105), and a higher nuclear grade (86.0% vs. 73.4%, P = 0.0140). Pathologic stage, lympho-vascular invasion, multiplicity, and proportion of mastectomy were not significantly different in those with or without BRCA1/2 mutation. Although differences in the proportion of adjuvant chemotherapy (81.2% vs. 70.2%, P = 0.0120) and neo-adjuvant chemotherapy (13.1% vs. 21.8%, P = 0.0273) were observed, the total proportion of chemotherapy treatment was comparable between the two groups (94.3% vs. 89.4%, P = 0.1314).
Long-term oncologic outcomes
With a median follow-up duration of 80.9 months (range, 3–152 months), we compared the long-term oncologic outcomes between BRCA1/2 mutation carriers and non-carriers including the cumulative risks on 60-, 120-, and 150-month period (Table 2 and Fig. 1). 60- and 120-month cumulative disease incidence rates were 18.5% and 31.2% for BRCA1/2 carriers vs. 19.3% and 22.7% for non-carriers (P = 0.8346 and 0.1359, respectively). Although not statistically significant, the cumulative disease incidence rate at 150 months was higher in BRCA1/2 mutation carriers compared to non-carriers (36.2% vs. 23.5%, P = 0.0800). 60-, 120-, and 150-month CBC incidence rate estimates were 6.7%, 18.7%, and 25.5% for BRCA1/2 mutation carriers vs. 1.1%, 2.7%, and 5.2% for non-carriers, which showed statistically significant differences (P = 0.0250, 0.0063, and 0.0184, respectively). There were no significant differences in cumulative mortality rates between the two groups in all three time periods. 60-, 120-, and 150-month breast cancer-specific survival (BCSS) had no significant difference, but poorer BCSS was observed in the non-carrier group (91.4%, 88.8%, and 88.8% for BRCA1/2 carriers vs. 87.4%, 82.4%, and 82.4% for non-carriers P = 0.1571, 0.0724, and 0.0724, respectively). There was no significant difference in distant-metastasis free survival (DMFS) between BRCA1/2 mutation carriers and non-carriers (P = 0.2254, 0.3302, and 0.3302, respectively).
Kaplan–Meier curves with corresponding log-rank tests for BCSS (a), DMFS (b), CBCFS and cumulative CBC incidence rate (c), DFS and cumulative disease incidence rate (d), and OS (e) according to BRCA1/2 mutation status. DFS disease-free survival, DMFS distant metastasis-free survival, CBCFS contralateral breast cancer-free survival, OS overall survival, BCSS breast cancer-specific survival.
Recurrence patterns according to BRCA1/2 mutation status
In BRCA1/2 mutation carriers, there were 6 locoregional recurrences, 12 distant metastases, and 7 CBC recurrences within five years. Late recurrence patterns in BRCA1/2 mutation carriers who had no recurrence within the first 5 years showed 1 locoregional recurrence, 1 distant metastasis, and 7 CBC recurrences (Tables 3 and 4). Early and late locoregional recurrence and distant metastasis showed no significant differences between BRCA1/2 mutation carriers and non-carriers (P = 0.1409, 0.4878, and 0.2754, 0.8327, respectively). Notably, the CBC recurrence rate showed a significant difference between BRCA1/2 mutation carriers and non-carriers in both early- and late recurrences (P = 0.0002 and 0.0001, respectively). A total of 15 ovarian cancer occurrences were observed in the total population. 11 cases were observed in the BRCA1/2 mutation carrier group and 4 cases were observed in the non-carrier group.
Among 29 patients with CBC recurrence, 24 patients (82.8%) had TNBC breast cancer again and among recurred patients, 19 patients (65.5%) resumed chemotherapy. Overall, 14 patients were BRCA1/2 mutation carriers, and 15 patients were non-carriers. Among 14 BRCA1/2 mutation carriers with CBC recurrence, 11 patients (78.6%) had recurred triple-negative type breast cancer and 10 patients (71.4%) resumed chemotherapy. Additionally, within the 14 BRCA1/2 mutation carriers with CBC recurrence, 12 patients had BRCA1 mutation, and 2 patients had BRCA2 mutation.
Risk factors of CBC occurrence
To investigate the risk factors of CBC, we performed univariate and multivariate analyses using the Cox regression model (supplementary Table S1). Age at diagnosis was classified into 2 groups with a cutoff value of 40 years. Although younger age had a higher risk of CBC events, there was no significance (HR = 2.011, P = 0.074 in the univariate model and HR = 1.663, P = 0.200 in the multivariate model). TNM stage was not a significant risk factor. Notably, BRCA1/2 mutation status was a meaningful risk factor for CBC events according to univariate analysis (HR = 6.580, P < 0.0001) and multivariate analysis (HR = 6.242, P < 0.0001).
Discussion
In our previous study, with a median follow-up period of 53.6 months, we demonstrated the high prevalence of BRCA1/2 mutation in unselected Korean TNBC patients and the higher incidence rate of CBC in BRCA1/2 mutation carriers compared to non-carriers16. With a median follow-up duration of 80.9 months, this long-term follow-up study showed a consistently increased CBC incidence rate in BRCA1/2 mutation carriers compared to non-carriers. There were no significant differences in disease-free survival (DFS), OS, BCSS, and DMFS between the two groups. Additionally, BRCA1/2 mutation was a significant risk factor for CBC occurrence with an HR of 6.242. Among patients with CBC recurrence, ~80% had TNBC-type as recurred CBC, and over 60% resumed chemotherapy.
We observed significantly younger age of onset and higher tumor nuclear grade for BRCA1/2 mutation carriers compared to non-carriers. In a study of 774 TNBC patients, Wong-Brown et al. also reported similar characteristics of younger age of onset for BRCA1 mutation carriers within their TNBC study group8. Brekelmans et al. demonstrated that BRCA1 mutation carriers had more histologic grade III tumors compared to sporadic breast cancer patients10. These characteristics of BRCA1/2 mutation carriers may have been one of the factors affecting DFS, although not statistically significant, lower 150-month DFS was observed (63.8% for BRCA1/2 mutation carrier group vs. 76.5% for non-carrier group).
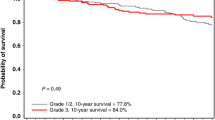
There have been many studies comparing the prognosis of BRCA1/2 mutation carriers to non-carriers. The common point of these study’s results was that there were no significant differences in survival according to BRCA1/2 mutation status17,18. This was consistent in our study as well, demonstrating no significant differences in DFS, OS, and BCSS between BRCA1/2 mutation carriers and non-carriers. Notably, we observed good survival rates at 10 years in both groups (88% vs. 84.5% DMFS, 88.8% vs. 82.4% BCSS and 84.4% vs. 79.3% OS). The Prospective Outcomes in Sporadic versus Hereditary breast cancer (POSH) study reported the 10-year overall survival of 558 TNBC patients with or without BRCA1/2 mutations. In this prospective study, TNBC patients with BRCA mutation showed a 10-year overall survival of 72% and TNBC patients without BRCA mutation showed 69% 10-year overall survival18.
A prospective cohort study including 2213 BRCA1/2 mutation carriers eligible for CBC analysis revealed that CBC risk was lower when the first breast cancer was diagnosed at age 40 years or higher19. We analyzed BRCA1/2 mutation carriers with a cut-off age of 40 and 50 years to investigate the differences in CBC incidence. Both age cut-offs revealed no significant difference in CBC incidence (P = 0.9023 and 0.6199, respectively). Giannakeas et al. reported the annual risk and 25-year cumulative risk of CBC using the surveillance, epidemiology, and end results (SEER) database. With an annual risk of ~0.4% per year, the cumulative CBC risk at 12.5 years was 4.6% in invasive breast cancer patients20. Although our study cohort only included TNBC patients, the cumulative CBC risk was comparable in the BRCA1/2 mutation non-carrier group (5.2% at 12.5 years). However, cumulative CBC risk in BRCA1/2 mutation carriers was 25.5% at 12.5 years. In another study including 223 breast cancer patients with BRCA1 mutation, CBC incidence was higher in the BRCA1 mutation group compared to sporadic breast cancer patients10. This result supports our study’s findings. Additionally, we revealed that BRCA1/2 mutation status was a significant risk factor for CBC incidence in a multivariate analysis.
The aggressive characteristics of TNBC are well reviewed in previous articles1,11. In a study that evaluated the recurrence patterns and outcomes of early-stage breast cancer, 310 TNBC patients were included. These patients had a higher risk of breast cancer-free interval events in the first 4 years after diagnosis3. Our study also investigated the recurrence patterns of TNBC including early- and late-recurrences. Although the majority of events occurred within the first 5 years, 13% of all recurrence events were observed after 5 years of initial diagnosis. Especially, CBC event rate was consistently higher in the BRCA1/2 mutation carriers in both early-, and late recurrences. Therefore, long-term active surveillance may be beneficial in the early detection of recurrences or distant metastasis.
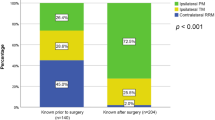
There have been several reports regarding the impact of BRCA1/2 mutation results on decision-making for risk-reducing mastectomy (RRM). Chiba et al. reported an 82.5% rate of RRM in patients with known BRCA mutation prior to surgery compared to 29% in those unaware of the BRCA mutation status at the time of surgery21. Similar rates were shown in a study of 220 BRCA mutation-associated breast cancer patients (76.4% in the group aware of their BRCA mutation vs. 14.7% in the group unaware of their BRCA mutation)22. These factors were also compared in a study of 344 BRCA mutation breast cancer patients, which was conducted in our institution. The results demonstrated a 45% rate of RRM in the group aware of their BRCA mutation vs. 2% in the group unaware of their BRCA mutation23. Our study revealed that BRCA1/2 mutation carriers have an increased risk of CBC occurrence and will require another systemic chemotherapy. Therefore, confirming the BRCA1/2 mutation status prior to surgery is vital.
In Korea, BRCA1/2 mutation testing is performed based on the Korean clinical practice guidelines for breast cancer. Our study included patients included TNBC patients from 2008 to 2016. At that time, the 6th clinical practice guideline was applied, in which TNBC patients were not candidates for BRCA1/2 mutation testing24. The low rate of mastectomy observed in our study’s cohort can be explained by this fact. The Korean health insurance policy was updated in July 2020, to include TNBC patients aged 60 or under for BRCA1/2 mutation testing, and the currently applied 10th Korean clinical practice guideline for breast cancer has identical BRCA1/2 mutation testing recommendations for TNBC patients25.
To the best of our knowledge, this report is the first single large institution study to analyze the long-term outcomes of unselected TNBC patients according to BRCA1/2 mutation. Furthermore, we investigated the recurrence patterns including early- and late-recurrence patterns. We hope our results may help in surgical decision-making and surveillance strategies.
Our study does have limitations. Since it was a non-randomized retrospective study, it included incomplete patient data, which introduces the possibility of other confounding variables, whether measured or unmeasured. The study included patients from 2008 to 2016; a period during which treatment guidelines and adjuvant treatments evolved, potentially affecting the results. Additionally, since the collected data is relatively old, there may be unclear or missing information that may have affected the results. BRCA1/2 mutation was retrospectively tested in our study. Although the cohort was ‘unselected’ for age or family history, only patients with available biobank tissue were included, making potential for unintentional selection bias. Additionally, BRCA1/2 mutation testing was performed using the Sanger and NGS methods in our study. These methods have limitations in detecting large deletions/duplications or copy number variations (CNVs). In such cases, multiplex ligation-dependent probe amplification (MLPA) should be performed. However, since the Korean population has a lower frequency of CNVs and due to the Korean insurance policy of testing BRCA1/2 mutation26,27, our testing methods still have an acceptable value.
In conclusion, our study identified that BRCA1/2 mutation was a significant risk factor for CBC occurrence in TNBC patients. Notably, approximately 80% of patients experiencing CBC were again diagnosed with the TNBC subtype, and over 60% underwent chemotherapy following recurrence. These findings highlight the critical importance of conducting BRCA1/2 genetic testing prior to initial surgical intervention and engaging in thorough discussions with patients regarding the option of contralateral RRM.
Moreover, our analysis revealed that the event rate for CBC remains significantly elevated beyond the 5-year mark, particularly among BRCA1/2 mutation carriers. This observation highlights the necessity for prolonged surveillance of TNBC patients with BRCA1/2 mutations, especially those who have not received RRM. Implementing such measures may potentially improve patient outcomes by facilitating early detection and intervention.
Methods
Study population
A schematic diagram of the study population is shown in Fig. 2. The details of the study population have been described in our previous study16. Briefly, unselected TNBC patients with samples available for BRCA1/2 genetic testing were included for analysis. Next-generation DNA sequencing (NGS) method was used for genetic testing. Within this cohort, 103 patients, who met the criteria for Korean genetic screening at that time, had been examined by the Sanger method. ‘Unselected’ patients are referred to as patients not selected for age at diagnosis of a family history of breast and/or ovarian cancer.
In this follow-up study, patients with a follow-up duration of less than 12 months, and patients with distant metastasis at initial presentation were excluded from analysis. Patients who deceased within 12 months after the first diagnosis were included in the analysis. Overall, the remaining 953 patients were included in this study. 91 patients (9.5%) were BRCA1 mutation carriers, 32 patients (3.4%) were BRCA2 mutation carriers, and one patient had both BRCA1/2 mutations. Long-term oncologic outcomes including CBC incidence, recurrence patterns, and survival according to BRCA1/2 mutation status were analyzed.
Data collection
We collected the following clinical information: BRCA1/2 genetic test results, age at breast and/or ovarian cancer diagnosis, menopausal status, and family history of breast cancer and/or ovarian cancer. We collected the following pathologic information: diagnosis, tumor size, lymph node (LN) metastasis (number of LNs in metastasis), hormone receptor status (ER, PR), HER-2 status, histologic grade, nuclear grade, and pathologic stage according to the 7th edition of the American Joint Committee on Cancer classification. We collected the following treatment information: information on surgery, date of operation(s), adjuvant chemotherapy, neo-adjuvant chemotherapy, adjuvant radiation therapy, and/or anti-hormone therapy.
Staining of 1% of cells or more was considered positive for ER and PR28. Only membrane staining intensity and pattern were evaluated using the recommendations of the 2013 American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP)29. TNBC was immunohistochemically defined as ER/PR-negative and lacking overexpression of HER-2. Recurrence or metastasis status, as well as survival data (including the date of occurrence), were also collected. DFS was defined as the interval from the date of diagnosis to recurrence, distant metastasis, or death or censored at the last follow-up date. CBCFS was defined as the interval from the date of diagnosis to CBC occurrence of censored at the last follow-up date. OS was defined as the interval from the date of diagnosis to death from any cause or censored at the last follow-up date. DMFS was defined as the interval from the date of diagnosis to distant metastasis or censored at the last follow-up date. BCSS was defined as the interval from the date of diagnosis to death caused by breast cancer progression or censored at the last follow-up date. Recurrence patterns (locoregional recurrence, distant metastasis, CBC incidence) were classified into early- and late recurrences based on a 5-year time frame.
Statistical analysis
The patient characteristics were compared using an independent sample t-test for continuous variables and Chi-square or Fisher’s exact test for categorical variables. DFS, OS, BCSS, and CBCFS were estimated using the Kaplan–Meier method, and the P-values of the log-rank test were used to assess significance. Univariate and multivariate analyses were performed using the Cox regression model. Variables that were considered relevant or showed a P-value < 0.05 were included in the multivariate Cox regression model. All P-values were two-sided and a P-value < 0.05 was considered statistically significant in our study. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R3.4.0 (Vienna, Austria; http://www.R-project.org).
Ethics
This study adhered to the ethical tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of SMC in Seoul, Korea (IRB number: 2022-05-062). The need for informed consent was waived because of the low risk posed by this investigation.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data sets generated during and/or analyzed during the present study are available from the corresponding author on reasonable request. Reference sequences for BRCA1 and BRCA2 DNA numbering were GenBank accession numbers NM_007294.2 and NM_000059.3, respectively16.
References
Dai, X. et al. Breast cancer intrinsic subtype classification, clinical use and future trends. Am. J. Cancer Res. 5, 2929–2943 (2015).
Lin, N. U. et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 118, 5463–5472 (2012).
Metzger-Filho, O. et al. Patterns of recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: results from International Breast Cancer Study Group Trials VIII and IX. J. Clin. Oncol. 31, 3083–3090 (2013).
Foulkes, W. D., Smith, I. E. & Reis-Filho, J. S. Triple-negative breast cancer. N. Engl. J. Med. 363, 1938–1948 (2010).
Xie, Y., Gou, Q., Wang, Q., Zhong, X. & Zheng, H. The role of BRCA status on prognosis in patients with triple-negative breast cancer. Oncotarget 8, 87151–87162 (2017).
Gonzalez-Angulo, A. M. et al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin. Cancer Res. 17, 1082–1089 (2011).
Hartman, A. R. et al. Prevalence of BRCA mutations in an unselected population of triple-negative breast cancer. Cancer 118, 2787–2795 (2012).
Wong-Brown, M. W. et al. Prevalence of BRCA1 and BRCA2 germline mutations in patients with triple-negative breast cancer. Breast Cancer Res. Treat. 150, 71–80 (2015).
Huzarski, T. et al. Ten-year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. J. Clin. Oncol. 31, 3191–3196 (2013).
Brekelmans, C. T. et al. Survival and prognostic factors in BRCA1-associated breast cancer. Ann. Oncol. 17, 391–400 (2006).
Rennert, G. et al. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N. Engl. J. Med. 357, 115–123 (2007).
Goodwin, P. J. et al. Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: an International Prospective Breast Cancer Family Registry population-based cohort study. J. Clin. Oncol. 30, 19–26 (2012).
Johannsson, O. T., Ranstam, J., Borg, A. & Olsson, H. Survival of BRCA1 breast and ovarian cancer patients: a population-based study from southern Sweden. J. Clin. Oncol. 16, 397–404 (1998).
Yadav, S. et al. Impact of BRCA mutation status on survival of women with triple-negative breast cancer. Clin. Breast Cancer 18, e1229–e1235 (2018).
Geyer, C. E. Jr. et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann. Oncol. 33, 1250–1268 (2022).
Ryu, J. M. et al. Prevalence and oncologic outcomes of BRCA 1/2 mutations in unselected triple-negative breast cancer patients in Korea. Breast Cancer Res. Treat. 173, 385–395 (2019).
Lee, L. J. et al. Clinical outcome of triple negative breast cancer in BRCA1 mutation carriers and noncarriers. Cancer 117, 3093–3100 (2011).
Copson, E. R. et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 19, 169–180 (2018).
Kuchenbaecker, K. B. et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 317, 2402–2416 (2017).
Giannakeas, V., Lim, D. W. & Narod, S. A. The risk of contralateral breast cancer: a SEER-based analysis. Br. J. Cancer 125, 601–610 (2021).
Chiba, A. et al. Impact that timing of genetic mutation diagnosis has on surgical decision making and outcome for BRCA1/BRCA2 mutation carriers with breast cancer. Ann. Surg. Oncol. 23, 3232–3238 (2016).
Yadav, S., Reeves, A., Campian, S., Sufka, A. & Zakalik, D. Preoperative genetic testing impacts surgical decision making in BRCA mutation carriers with breast cancer: a retrospective cohort analysis. Hered. Cancer Clin. Pract. 15, 11 (2017).
Woo, J. et al. Preoperative diagnosis of BRCA1/2 mutation impacts decision-making for risk-reducing mastectomy in breast cancer patients. Sci. Rep. 11, 14747 (2021).
Korean Breast Cancer Society. The 6th Korean Clinical Practice Guideline for Breast Cancer (Korean Breast Cancer Society, 2015).
Korean Breast Cancer Society. The 10th Korean Clinical Practice Guideline for Breast Cancer (Korean Breast Cancer Society, 2023).
Cho, J. Y. et al. Large genomic rearrangement of BRCA1 and BRCA2 genes in familial breast cancer patients in Korea. Fam. Cancer 13, 205–211 (2014).
Kang, E. et al. The prevalence and spectrum of BRCA1 and BRCA2 mutations in Korean population: recent update of the Korean Hereditary Breast Cancer (KOHBRA) study. Breast Cancer Res. Treat. 151, 157–168 (2015).
Hammond, M. E. et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 28, 2784–2795 (2010).
Wolff, A. C. et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 31, 3997–4013 (2013).
Acknowledgements
We would like to thank the Biomedical Statistics Center, Research Institute for Future Medicine, Samsung Medical Center, and all investigators of the KOHBRA study. This study was supported by the National R&D Program for Cancer Control through the National Cancer Center (NCC) funded by the Ministry of Health & Welfare, Republic of Korea (HA23C0144).
Author information
Authors and Affiliations
Consortia
Contributions
Woong Ki Park and Soo Yeon Chung had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: All authors. Acquisition, analysis, or interpretation of data: You Jin Jung, Woong Ki Park, Soo Yeon Chung. Drafting of the manuscript: Jai Min Ryu, Seok Jin Nam, Seok Won Kim. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Soo Yeon Chung. Obtained funding: Jai Min Ryu. Administrative, technical, or material support: Woong Ki Park, Changhee Ha, Jong-Won Kim, Jonghan Yu, Byung Joo Chae, Jeong Eon Lee. Supervision: Jai Min Ryu.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, W.K., Chung, S.Y., Jung, Y.J. et al. Long-term oncologic outcomes of unselected triple-negative breast cancer patients according to BRCA1/2 mutations. npj Precis. Onc. 8, 96 (2024). https://doi.org/10.1038/s41698-024-00559-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41698-024-00559-0