Abstract
Cyclophilin A (CypA) is a novel renal inflammation biomarker, with levels altered in various diseases, particularly in patients with diabetes mellitus (DM) and kidney damage. This study aimed to investigate the correlation between urinary cyclophilin A (uCypA) and chronic kidney disease (CKD) conditions with and without type 2 diabetes mellitus (T2DM) using an in-house enzyme-linked immunoassay (ELISA) method. A uCypA strip-test prototype was also developed. An indirect ELISA was performed to determine the uCypA levels. A 0.48 µg/mL uCypA cutoff differentiated healthy patients from those with early-stage CKD (stages I and II). The uCypA levels were significantly increased in patients with progression of renal deterioration, especially in the T2DM with late-stage CKD group, compared to the control group. Fasting blood sugar (FBS), estimated glomerular filtration rate (eGFR), albumin/creatinine ratio, and metformin use were associated with uCypA levels. Multinomial logistic regression analysis revealed an association between uCypA levels and T2DM diagnosed for over five years and early-stage CKD. This finding shows that uCypA could be used as a biomarker for distinguishing early-stage CKD as well as T2DM complications, which is beneficial for patients to be aware of their health status and change their behavior to slow kidney deterioration.
Similar content being viewed by others
Introduction
In 2017, chronic kidney disease (CKD) was included in the top ten global burden of disease with a prevalence of 843.6 million worldwide1. CKD is also a significant public health concern in Thailand, affecting 4.6–17.5% of the Thai population2. Persistence of kidney damage or loss of kidney function over 3 months is employed to discriminate CKD from acute conditions. Moreover, clinical criteria including an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2, albuminuria, presence of abnormal urinary sediment, and abnormal renal ultrasound are used for staging categorization3. Early screening with robust methods is beneficial for the development of effective treatment regimens. Thus, markers with proper sensibility and specificity are necessary to recognize CKD early. Although different markers have been reported and applied in routine practice, such can mostly diagnose CKD in advanced disease stages4.
Cyclophilin A (CypA) is an indicator of inflammation and oxidative stress5, and the association between CypA and diabetes mellitus (DM) has been described previously. Long-term hyperglycemia and dysregulation of the metabolic environment trigger the production of advanced glycation end products, activation of cellular signaling pathways, and the generation of free radicals6,7. In particular, monocytes secrete CypA, resulting in increased plasma CypA levels8. The secretion of CypA into the peripheral blood can also be observed in inflammatory conditions, infectious diseases, and solid organ damage, such as rheumatoid arthritis, asthma, and periodontitis9. Moreover, various reports have emphasized the strong relationship between macrophages and diabetic nephropathy (DN)10,11. As CypA is freely filtered from the glomeruli, elevation of urinary CypA (uCypA) levels may reflect an increase in plasma levels or directly reflect renal injury itself. Rather than causing phenotypic damage to resident kidney cells, activated macrophages release profibrotic cytokines, which increase cell proliferation and matrix volume expansion, and induce fibrosis12,13. More than 20% of patients with DM are estimated to develop CKD, with a 3% incidence 10–20 years after diabetes onset7.
A previous report has associated plasma CypA levels with DN and the severity of kidney damage. Urine as the less-invasive sample is the choice for CypA investigation. Thus, uCypA may reflect the occurrence at the kidney regional site. This study aimed to develop an enzyme-linked immunoassay (ELISA)-based method to identify uCypA cutoff levels for early-stage CKD. To simplify the detection procedure, an in-house CypA detection method was established using a lateral flow system. Furthermore, the correlation between uCypA and common noncommunicable diseases, including DM, DN, and CKD, was investigated.
Results
Characteristics of the study groups
Laboratory and clinical parameters of the participants of the study are shown as means and standard deviation (SD), while demographics, behaviors, and medicinal implementation are presented as numbers and percentages in Table 1. Among the 357 patients, patients with T2DM, hypertension, and hyperlipidemia condition accounted for 40.1%, 44%, and 71.2%, respectively. The highest levels of fasting blood sugar (FBS) and hemoglobin A1c (HbA1c) were found in patients with type 2 diabetes mellitus (T2DM) and early-stage CKD. In patients with late-stage CKD with and without T2DM, the higher creatinine levels corresponded to the lower eGFR, which was less than 60 mL/min/1.73 m2. The highest albumin creatinine ratio (ACR) was found in patients with late-stage CKD with T2DM, followed by those with late-stage CKD and early-stage CKD with T2DM.
The biochemical parameters stratified by groups (T2DM < 5 y, T2DM ≥ 5 y, T2DM + early CKD, T2DM + late CKD, early-stage CKD and late-stage CKD) were compared to the control group using the Kruskal–Wallis test, as shown in Supplementary Table S1. Compared to parameters indicating kidney function, significant differences in urine microalbumin (umAlb) and ACR values were observed comparing the control groups with the remaining groups, except for T2DM < 5 y. Interestingly, among the seven study groups, the levels of uCypA ranged from 0.02 to 39.60 µg/mL. Significant differences in uCypA levels were observed between the control and remaining groups (p-value < 0.001).
The association of uCypA levels and other parameters
The association between uCypA and other characteristic factors was explored using linear regression analysis. uCypA was associated with age, FBS, low density lipoprotein (LDL), eGFR, umAlb, ACR, and metformin levels (Table 2). Consequently, multiple linear regression analysis was performed, identifying an association between uCypA and FBS, eGFR, ACR, and use of metformin (p < 0.003, R2 = 0.530). uCypA was increased by 0.021 and 0.156 µg/mL for each increase of 1 mg/dL in FBS and decrease of 1 mL/min in eGFR, respectively.
The association of uCypA with T2DM and CKD
uCypA was significantly increased under T2DM with and without CKD conditions, and CKDs. The association of increasing uCypA levels was most prominent in patients with T2DM > 5 years and in the early-stage CKD group compared with the control group (Table 3). The aORs describe that for each 1-unit increase in uCypA, the higher the probability of T2DM ≥ 5 years and early-stage CKD, accounting for 1.817 and 1.808 times compared to the control group, respectively.
The accuracy of uCypA for CKD diagnosis
The capacity of uCypA levels (µg/mL) for CKD diagnosis was evaluated using receiver operating characteristic (ROC) curve analysis. The area under curve (AUC) showed a high predictive ability to differentiate the early-stage CKD group (n = 85) from the control group (n = 90; Fig. 1; Table 4). A cutoff value of 0.48 µg/mL provided 98.82% sensitivity and 92.21% specificity with an AUC at 0.982 (95% confidence interval [CI] 0.989–1.000). Moreover, a cutoff of 2.89 µg/mL of uCypA discriminated early-stage (n = 85) and late-stage CKD (n = 96) with 80.21% sensitivity and 50.59% specificity with an AUC of 0.777 (95% CI 0.709–0.845) (Table 5).
Establishment of a uCypA rapid screening method
To establish a rapid screening test for CKD, lateral flow strip (LFS) platform was developed to monitor CypA levels in urine samples. uCypA levels in the control, T2DM with early-stage CKD, and early-stage CKD groups were evaluated using LFS. A reddish band was observed on the control line in the control group (Fig. 2a). Meanwhile, the appearance of a red band in both the control and test lines was detected in T2DM with early-stage CKD and early-stage CKD groups. Additionally, quantitative detection of uCypA by LFS was performed using a LFS reader (Fig. 2b). The results showed that the intensities of the test line in the control group were 3.2 times lower than those in the T2DM with early-stage CKD group and 2.4 times lower than those in the early-stage CKD group.
LFS detection results of uCypA levels. (a) Images of LFS detection of uCypA levels in urine samples, including the running buffer, control group, T2DM with early-stage CKD group, and early-stage CKD group. (b) Corresponding test line intensity for uCypA levels in urine samples, including the running buffer, control group, T2DM with early-stage CKD group, and early-stage CKD group. LFS lateral flow system, uCypA urinary cyclophilin A, T2DM type 2 diabetes mellitus, CKD chronic kidney disease.
Discussion
CKD is defined as abnormalities in kidney structure or function that are present for more than three months. Other clinical manifestations, including eGFR < 60 mL/min/1.73 m2, presence of pathological findings, markers of kidney damage, or abnormal imaging tests may occur14. To evaluate renal function, clinical biochemical markers, such as eGFR, are assessed. eGFR is based on creatinine or cystatin C levels, which are freely filtered at the glomeruli15. Moreover, a combination of surrogate factors, including age, sex, ethnicity, and body size, are included in the eGFR equation. Elevation of serum creatinine (sCr) in patients with late-stage kidney disease reflects kidney malfunction, in which by-products cannot be excreted from the blood circulation. The decrease in eGFR values in patients with late-stage CKD (36.3 ± 17.3) and late-stage CKD with T2DM (36.3 ± 15.6) was associated to the staging criteria of CKD3. Additionally, the increase in umAlb and ACR levels in late-stage CKD and late-stage CKD with T2DM can be explained by a defect in glomerular permeability. To predict the severity and progression of the disease, > 30 mg/g ACR was designated as the CKD criterion3. Moreover, > 30 mg/g of ACR in patients with T2DM ≥ 5 y may reflect an abnormality of glomerular filtration in patients with poorly controlled DM and a probability of CKD progression. Besides, elevated uCypA and normal eGFR value can be observed in DM < 5 y. It can be described by the production of oxidative stress and inflammatory cytokines resulting from metabolic alteration and persistent low-grade inflammation under chronic hyperglycemic condition16,17,18. These evidences triggered vascular complication and impacted the local organs. The pathological changes of kidney such as glomerular basement membrane thickening, glomerular ECM production, fibrosis, nephrosclerosis and podocyte apoptosis were occurred prior to the functional changes18,19. It was evinced by normal/increase eGFR, and normal/intermittent albuminuria in the early DM, while the long-term DM condition led to decline of eGFR and albuminuria20,21,22 (Fig. 3).
The schematic of pathology related to diabetes and renal complications. Under hyperglycemic conditions, imbalanced glucose homeostasis and persistent low-grade inflammation generated oxidative stress and inflammatory cytokines, which caused vascular complications and impacted the local organs16,17,18. At the kidney site, oxidative stress by-products and inflammatory cytokines triggered pathological changes such as glomerular basement membrane thickening, glomerular ECM production, fibrosis, nephrosclerosis, and podocyte apoptosis18,19. Normal/increased eGFR and normal/intermittent albuminuria can be observed during the structural changes, while secretion of inflammatory biomarkers such as CypA can also be observed5,20,21,22. The long-term effects of these matters have led to functional changes in the kidney, resulting in microalbuminuria, proteinuria, and a decline in eGFR. Not only the regional site but also peripheral blood monocytes can secrete CypA as a result of an inflammatory response. Secreted CypA was then freely filtered through glomeruli and observed in urine. Chronic kidney damage or injury also triggered the CypA secretion through the ROS-Rho pathway. It can perform both autocrine and paracrine functions, stimulating cell proliferation, migration, and inflammatory cascade and promoting cytokines and chemokines release5. ECM extra cellular matrix,eGFR estimated glomerular filtration rate,CypA cyclophilin A,ROS reactive oxygen species.
The elevation of uCypA levels in CKD-associated conditions can be explained by the presence of both local and systemic inflammation23. The inflammatory response exerts paracrine and autocrine effects by secreting extracellular Cyclophilin A (eCypA). According to kidney injury, increased expression of CD147 bound with circulating eCypA activates the ERK/NF-kB pathway, leading to an inflammatory cascade24,25. Moreover, eCypA exerts a chemotactic effect, resulting in the infiltration of various inflammatory cells at regional sites5. In summary, increased uCypA levels were observed in patients since early-stage CKD. Moreover, uCypA was predominantly found in late-stage CKD, as well as in patients with T2DM with and without late-stage CKD; such is evidenced by responses to inflammatory stimuli such as hyperglycemia and oxidative stress. eCypA secreted from peripheral blood monocytes increases plasma eCypA level8. High glucose levels can also activate the STAT3-CypA interaction, which induces inflammation, oxidative stress, and apoptosis in podocytes26. Since circulating eCypA is freely filtered through the glomeruli, high levels of uCypA reflect elevated eCypA secretion from responsive cells and/or increased glomerular filtration or leakage27,28,29. It can be emphasized that the elevation of uCypA reflects the increase of circulation CypA as well as localized inflammation.
The correlation between uCypA and CKD can be explained by the exposure to generated inflammatory mediators in the vascular system and kidneys5. An association between eCypA and nephrotic conditions, including CKD, DN, and acute kidney injury (AKI) has been reported25,28,30. Long-term inflammation activation impairs renal function, limits tissue regeneration after cellular injury, and leads to progressive decline in renal function23. The use of uCypA as a biomarker for early-stage CKD may play an important role in providing effective treatments for patients with early-stage CKD. A cutoff value of 0.48 for patients in the early-stage CKD and control groups resulted in over 90% sensitivity and specificity, with an AUC of 0.982. A previous report found a significant difference in serum CypA levels between patients with non, early stage, and late-stage CKD31. Another cross-sectional study on T2DM also reported high accuracy of CypA in the early diagnosis of DN, with an AUC of 0.91432. Although routinely employed markers such as blood urea nitrogen, cystatin C, creatinine, and albuminuria have been established for CKD diagnosis, such cannot be used to distinguish the early stages of CKD33,34,35. The eGFR, which is the gold standard for determining kidney function and disease progression, might not be specific under certain conditions, such as extrarenal cystatin C or creatinine metabolism in patients with human immunodeficiency virus (HIV) infection, chronic liver disease, and cardiovascular diseases36,37. Moreover, a lack of association between eGFR and albuminuria has been observed in patients with end-stage renal disease (ESRD)38,39,40. Implementation of routine markers combined with proteomic or metabolomic markers may provide effective screening outcomes41,42. However, the high cost of biomarker detection and inconsistent detection methods limit their clinical application43. The development of a rapid screening method for uCypA using LFS could facilitate access to screening methods for the general population. Moreover, application of the lateral flow system provided impressive results for screening early-stage CKD with and without T2DM in our study. To improve detection sensitivity, capture systems should be developed.
The promising high sensitivity and specificity at a 0.48 µg/mL uCypA cutoff could be crucial for early CKD screening. uCypA levels also elucidate the renal complications of DM, indicating a strong association with other kidney and glycemic parameters, including eGFR, ACR, and FBS. Rather than palliative treatment and health expenditures, the addition of uCypA in routine practices may enable the initiation of effective treatment regimens for patients with early-stage CKD. For applying uCypA in predictive/prognostic approach, an extension of deteriorated renal subjects should be implied. In addition, the T2DM durational effect of the patient with and without CKD should be a concern. Exclusion of poor glycemic control patient group should be considered for further study.
Materials and methods
Sample population
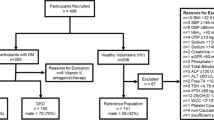
A total of 375 patients with metabolic syndrome were recruited from the Chronic Kidney Disease Northeast Thailand (CKDNET) project. The patient with waist circumference ≥ 90 cm for male or ≥ 80 cm for female, and the presence of any two criteria as follows: (1) hypertriglyceridemia (≥ 150 mg/dL), (2) low HDL (< 40 mg/dL in male or < 50 mg/dL in female), (3) elevated blood pressure (SBP ≥ 130 mmHg and DBP ≥ 85 mmHg or drug treatment for hypertension), and (4) fasting blood sugar ≥ 100 mg/dL or drug treatment for diabetes mellitus, was diagnosed as metabolic syndrome44. The study protocol conformed to the ethical guidelines based on the Declaration of Helsinki was approved by the Human Ethics Committee of Khon Kaen University (HE 601035), and all participants provided written informed consent before the study. Patients with inflammation, infection, liver disease, malignancy, or autoimmune diseases were excluded from the study. Additionally, the patient with serious conditions regarding the diabetes complications were also excluded. The remaining 357 participants were divided into the following seven groups:
Group I
control group (subjects who were free from underlying diseases, inflammation, infection, and medicinal treatment; n = 90);
Group II
patients with T2DM diagnosed less than five years prior (T2DM < 5 y; n = 45);
Group III
patients with T2DM diagnosed within and more than five years prior (T2DM ≥ 5 y; n = 41);
Group IV
patients with T2DM and early-stage CKD, i.e., stages I and II (T2DM + early CKD; n = 38);
Group V
patients with T2DM and late-stage CKD, i.e., stages III, IV and V (T2DM + late CKD; n = 52);
Group VI
patients with early-stage CKD, i.e., stages I and II (early CKD; n = 47);
Group VII
patients with late-stage CKD, i.e., stages III, IV, and V (late CKD; n = 44).
CKD staging classification was indicated using eGFR level from KDIGO guideline3. According to various CKD treatment guidelines, the indication for a nephropathic referral depends on eGFR, which is less than 60 mL/min/1.73 m245,46. Moreover, intensive monitoring was undertaken in the patient with a particular eGFR value. Therefore, 2 stages were categorized in this study, including early stage (stage I and II; eGFR ≥ 60 mL/min/1.73 m2), and late stage (stage III, IV and V; eGFR < 60 mL/min/1.73 m2). As per the clinical stages describing diabetic nephropathy progression20,47, 2 subgroups of patients without CKD were designated. Group II represented stage II DN describing the silent progression of kidney defect with less than 5 years after DM onset. Meanwhile group III illustrated stage III DN with more than 5 years after DM onset and prior for CKD diagnoses, coherent with various cohort studies reporting the estimating median time of 5–13 years for developing microalbumin and ESRD after DM onset21,48.
Baseline measurements
Fasting blood samples were collected from all the participants. Laboratory parameters including FBS, HbA1c, LDL cholesterol, alanine transaminase (ALT), uric acid, serum creatinine (sCr), and eGFR were determined. Morning urine was collected to examine urinary creatinine (uCr) and microalbumin (umAlb) levels and then calculate the ACR. Clinical examinations assessed the blood pressure (BP), including systolic blood pressure (SBP) and diastolic blood pressure (DBP). All data were collected twice within three months. A questionnaire inquired about demographics (age and sex), behaviors (exercise, current water consumption, smoking, and consumption of high-purine compounds), and use of medicines (metformin, glipizide, gliben, mixtard, Nph, amlodipine, enalapril, losartan, doxazosin, simvas, and atorvas).
ELISA for determining uCypA
uCypA levels were analyzed using an indirect ELISA method. Urine samples were diluted in a carbonate buffer (pH 9.5). A total 50 µL of the diluted urine was coated on the microplate (Thermo Scientific, MA, USA) and incubated overnight at 4 °C. The unbound antigen was washed three times with phosphate-buffered saline with Tween 20 (PBST). The wells were blocked with 100 µL of 3% skim milk-PBST for 2 h at 37 °C and washed three times with PBST. One hundred microliters of primary antibody (1:250; mouse monoclonal anti-human CypA antibody, Thermo Scientific, MA, USA) was added and then incubated for 2 h at 37 °C. After washing three times with PBST, 100 µL of conjugated anti-mouse immunoglobulin G (IgG) linked with alkaline phosphatase (Thermo Scientific, MA, USA) was added and incubated at 37 °C for 2 h. The plates were washed three times with PBST, followed by three washes with phosphate-buffered saline (PBS). Color development was executed by adding 100 µL of p-nitro-phenyl phosphate substrate solution and the reaction was then measured with an ELISA reader (PerkinElmer, MA, USA) at 405 nm. Calibration curves of CypA concentration levels at 0.001, 0.01, 0.1 and 1 ug/mL were utilized for calculation.
Statistical analyses
All statistical analyses were performed using the STATA statistical software package, version 13.0 (StataCorp LLC, TX, USA). Characteristic data for each variable were reported as means and standard deviations (SD) for continuous variables, and counted numbers and percentages for categorical variables. Normality testing using Kolmogorov–Smirnov test was performed for each biochemical parameter prior to comparison among the groups. Analysis of variance (ANOVA) was used to compare parametric data, whereas the Kruskal-Wallis test was applied for non-parametric data. The associations between uCypA levels and all characteristic factors were determined using multiple logistic regression analysis, and potential confounders (demographics, laboratory and clinical parameters, behaviors, and use of various drugs) were controlled. The association of uCypA among the groups was analyzed using multinomial logistic regression. Statistical significance was set at a p-value < 0.05.
Preparation of the LFS for uCypA detection
The LFS consisted of four parts: a sample pad (C048, Millipore Corporation, Billerica, MA, USA), a glass microfiber filter conjugate pad (GF33, Whatman Schleicher & Schuell, Dassel, Germany), a nitrocellulose membrane (UniSart® CN140, Sartorius Stedim Biotech SA, Goettingen, Germany), and an absorbent pad (Whatman ABS PAD #470, Sigma-Aldrich, MA, USA). The anti-CypA polyclonal antibody (Thermo Scientific, MA, USA) conjugated gold nanoparticle in a suspension buffer (10 mM phosphate buffer pH 7 + 1% bovine serum albumin (BSA) + 20% sucrose + 0.05% sodium azide) at an optical density of ∼5 was dropped onto the conjugated pad until reaching a volume of 20 µL before being dried at 37 °C for 2 h. Then, 1 mg/mL of mouse monoclonal anti-human CypA antibody (Thermo Scientific, MA, USA) and 1 mg/mL of goat anti-mouse IgG polyclonal antibody (Lampire Biological Laboratories, Pipersville, PA, USA) were dispensed (1 µL/cm) onto the nitrocellulose membrane as the test and the control line, respectively, by using a lateral flow dispenser (XYZ3000 Dispensing Platform; BioDot Inc., Irvine, CA, USA). Next, the membranes were dried at 37 °C for 1 h. The samples and absorbent pads were used without any treatment. Subsequently, all four parts were assembled on a backing card with a 2 mm overlap between successive components. Finally, the LFS was cut into narrower 4 × 60 mm strips using a guillotine cutter (BioDot Inc., Irvine, CA, USA).
LFS analytical procedure
To detect uCypA levels using the LFS platform, urine samples from the control (n = 5), T2DM + early CKD (n = 5), and early-stage CKD (n = 5) groups were randomly selected. Urine samples (10 µL) were diluted with 100 µL of running buffer (100 mM phosphate buffer + 1% Tween20 + 2% BSA), and the 110 uL mixture was subsequently released onto the sample pad and the sample was allowed to flow laterally for 15 min. After the assay was completed, test line intensities were visualized and quantified using a RapidScan ST5-W Lateral Flow Test Strip Reader (Pacific Image Electronic Co., Ltd., Taipei, Taiwan).
Data availability
The datasets generated or analysed during the current study are available from the corresponding author on reasonable request.
References
Kovesdy, C. P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2011(12), 7–11 (2022).
Cha’on, U. et al. High prevalence of chronic kidney disease and its related risk factors in rural areas of Northeast Thailand. Sci. Rep. 12, 18188 (2022).
Kdigo, K. Clinical practice guideline for the evaluation and management of chronic kidney disease. Off. J. Int. Soc. Nephrol. 3 (2013).
Zhang, W. R. & Parikh, C. R. Biomarkers of acute and chronic kidney disease. Annu. Rev. Physiol. 81, 309–333 (2019).
Hadpech, S. & Thongboonkerd, V. Current update on theranostic roles of cyclophilin A in kidney diseases. Theranostics 12, 4067–4080 (2022).
Chawla, A., Chawla, R. & Jaggi, S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum?. Indian J. Endocrinol. Metab. 20, 546–551 (2016).
Sulaiman, M. K. Diabetic nephropathy: Recent advances in pathophysiology and challenges in dietary management. Diabetol. Metab. Syndr. 11, 7 (2019).
Ramachandran, S. et al. Plasma level of cyclophilin A is increased in patients with type 2 diabetes mellitus and suggests presence of vascular disease. Cardiovasc. Diabetol. 13, 38 (2014).
El-Fattah, M. E. A., El-abaseri, T. B. & Mohamed, H. M. A. E. plasma cyclophilin A as a novel biomarker in chronic nephropathy. Int. J. Biochem. Res. Rev. 63–72 (2020).
Nguyen, D. et al. Macrophage accumulation in human progressive diabetic nephropathy. Nephrology (Carlton) 11, 226–231 (2006).
Yonemoto, S. et al. Correlations of tissue macrophages and cytoskeletal protein expression with renal fibrosis in patients with diabetes mellitus. Clin. Exp. Nephrol. 10, 186–192 (2006).
Thomas, M. C. et al. Diabetic kidney disease. Nat. Rev. Dis. Primers 1, 15018 (2015).
Agarwal, R. Pathogenesis of diabetic nephropathy. In Chronic Kidney Disease and Type 2 Diabetes (American Diabetes Assoc., 2021).
Levey, A. S. & Coresh, J. Chronic kidney disease. Lancet 379, 165–180 (2012).
Treacy, O., Brown, N. N. & Dimeski, G. Biochemical evaluation of kidney disease. Transl. Androl. Urol. 8(Supplement 2), S214–S223 (2019).
Ferroni, P. et al. Type 2 Diabetes and breast cancer: The interplay between impaired glucose metabolism and oxidant stress. Oxid. Med. Cell. Longev. 1–10 (2015).
Berbudi, A., Rahmadika, N., Tjahjadi, A. I. & Ruslami, R. Type 2 diabetes and its impact on the immune system. Curr. Diabetes Rev. 16, 442–449 (2020).
Wu, T., Ding, L., Andoh, V., Zhang, J. & Chen, L. The mechanism of hyperglycemia-induced renal cell injury in diabetic nephropathy disease: An update. Life 13, 539 (2023).
Ohiagu, F. O., Chikezie, P. C. & Chikezie, C. M. Pathophysiology of diabetes mellitus complications: Metabolic events and control. Biomed. Res. Ther. 8, 4243–4257 (2021).
Usman, M. S. The interplay between diabetes, cardiovascular disease, and kidney disease. In Chronic Kidney Disease and Type 2 Diabetes Vol. 2021 (ed. Weir, M. R.) 13–18 (American Diabetes Association, 2021).
Natesan, V. & Kim, S.-J. Diabetic nephropathy—A review of risk factors, progression, mechanism, and dietary management. Biomol. Ther. 29, 365–372 (2021).
Forbes, J. M. & Cooper, M. E. Mechanisms of diabetic complications. Physiol. Rev. 93, 137–188 (2013).
Zhang, R. et al. End-stage renal disease is different from chronic kidney disease in upregulating ROS-modulated proinflammatory secretome in PBMCs—A novel multiple-hit model for disease progression. Redox Biol. 34, 101460 (2020).
Qu, X., Wang, C., Zhang, J., Qie, G. & Zhou, J. The roles of CD147 and/or cyclophilin A in kidney diseases. Mediators Inflam. 2014, 728673 (2014).
Chiu, P. F. et al. Cyclophilin A and CD147 associate with progression of diabetic nephropathy. Free Radic. Res. 52, 1456–1463 (2018).
Zhang, X., Zhu, Y., Zhou, Y. & Fei, B. Interleukin 37 (IL-37) reduces high glucose-induced inflammation, oxidative stress, and apoptosis of podocytes by inhibiting the STAT3–cyclophilin A (CypA) signaling pathway. Med. Sci. Monit. 26, e922979 (2020).
Tsai, S. F. et al. Novel findings of secreted cyclophilin A in diabetic nephropathy and its association with renal protection of dipeptidyl peptidase 4 inhibitor. Clin. Chim. Acta 463, 181–192 (2016).
Cabello, R. et al. Urinary cyclophilin A as marker of tubular cell death and kidney injury. Biomedicines 9, 217 (2021).
Lee, C. C. et al. Diagnostic performance of cyclophilin A in cardiac surgery-associated acute kidney injury. J. Clin. Med. 9, 108 (2019).
Liu, M. C. et al. Cyclophilin A is associated with peripheral artery disease and chronic kidney disease in geriatrics: The Tianliao old people (TOP) study. Sci. Rep. 5, 9937 (2015).
Somdee, T. et al. Elevated concentration of serum cyclophilin A in patients with chronic. Kidney Dis. 27 (2020).
Abdel Ghafar, M. T. et al. Assessment of two novel renal tubular proteins in type 2 diabetic patients with nephropathy. J. Investig. Med. 68, 748–755 (2020).
Shlipak, M. G. et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann. Intern. Med. 145, 237–246 (2006).
Earley, A., Miskulin, D., Lamb, E. J., Levey, A. S. & Uhlig, K. Estimating equations for glomerular filtration rate in the era of creatinine standardization: A systematic review. Ann. Intern. Med. 156, 785–795 (2012).
Bidin, M. Z., Shah, A. M., Stanslas, J. & Seong, C. L. T. Blood and urine biomarkers in chronic kidney disease: An update. Clin. Chim. Acta 495, 239–250 (2019).
Stevens, L. A. & Levey, A. S. Measured GFR as a confirmatory test for estimated GFR. J. Am. Soc. Nephrol. 20, 2305–2313 (2009).
Alaini, A. et al. Establishing the presence or absence of chronic kidney disease: Uses and limitations of formulas estimating the glomerular filtration rate. World J. Methodol. 7, 73–92 (2017).
Tramonti, G. & Kanwar, Y. S. Review and discussion of tubular biomarkers in the diagnosis and management of diabetic nephropathy. Endocrine 43, 494–503 (2013).
Kramer, H. J., Nguyen, Q. D., Curhan, G. & Hsu, C. Y. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 289, 3273–3277 (2003).
MacIsaac, R. J. et al. Nonalbuminuric renal insufficiency in Type 2 diabetes. Diabetes Care 27, 195–200 (2004).
Fassett, R. G. et al. Biomarkers in chronic kidney disease: A review. Kidney Int. 80, 806–821 (2011).
Rysz, J., Gluba-Brzózka, A., Franczyk, B., Jabłonowski, Z. & Ciałkowska-Rysz, A. Novel biomarkers in the diagnosis of chronic kidney disease and the prediction of its outcome. Int. J. Mol. Sci. 18, 1702 (2017).
Yan, Z., Wang, G. & Shi, X. Advances in the progression and prognosis biomarkers of chronic kidney disease. Front. Pharmacol. 12, 785375 (2021).
Misra, A. et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J. Assoc. Physicians India 57, 163–170 (2009).
Nordheim, E. & Geir Jenssen, T. Chronic kidney disease in patients with diabetes mellitus. Endocr. Connect. 10, R151–R159 (2021).
National kidney foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification and stratification. Am. J. Kidney Dis. 39(suppl 1), S1–S266 (2002).
Poloni, J. A. T. & Rotta, L. N. Diabetic kidney disease: pathophysiological changes and urinalysis contribution to diagnosis—A narrative review. J. Lab. Precis. Med. 7, 3 (2022).
Jiang, G. et al. Progression of diabetic kidney disease and trajectory of kidney function decline in Chinese patients with type 2 diabetes. Kidney Int. 95, 178–187 (2019).
Acknowledgements
This research was financially supported by the National Science Research and Innovation Fund (NSRF) through Fundamental Fund of the Khon Kaen University, and the samples and clinical information were supported by CKDNET. We would like to express our gratitude to Professor Sakda Daduang for the laboratory support. We are also grateful to Associate Professor Udomsak Mahaweerawat for comments on the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization, R.K., T.S., N.S., P.T. and S.A.; methodology, T.S., S.N.; validation, P.C., T.S., S.N., P.T. and R.K.; investigation, P.C., P.T., T.S., S.N., M.W., N.S., S.A. and R.K.; resources, R.K.; writing—original draft preparation, T.S., P.C. and P.T.; writing—review and editing, P.C., P.T. and R.K.; supervision, R.K., P.T., N.S. and S.A.; project administration, R.K.; funding acquisition, R.K. and P.T. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chatchawal, P., Tippayawat, P., Somdee, T. et al. Urinary cyclophilin A as an early marker of chronic kidney disease with underlying type 2 diabetes. Sci Rep 14, 23207 (2024). https://doi.org/10.1038/s41598-024-73994-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-73994-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.