Abstract
Implant-related infections pose significant challenges to orthopedic surgeries due to the high risk of severe complications. The widespread use of bioactive prostheses in joint replacements, featuring roughened surfaces and tight integration with the bone marrow cavity, has facilitated bacterial proliferation and complicated treatment. Developing antibacterial coatings for orthopedic implants has been a key research focus in recent years to address this critical issue. Researchers have designed coatings using various materials and antibacterial strategies. In this study, we fabricated 3D-printed porous titanium rods, incorporated vancomycin-loaded mPEG750-b-PCL2500 gel, and coated them with a PCL layer. We then evaluated the antibacterial efficacy through both in vitro and in vivo experiments. Our coating passively inhibits bacterial biofilm formation and actively controls antibiotic release in response to bacterial growth, providing a practical solution for proactive and sustained infection control. This study utilized 3D printing technology to produce porous titanium rod implants simulating bioactive joint prostheses. The porous structure of the titanium rods was used to load a thermoresponsive gel, mPEG750-b-PCL2500 (PEG: polyethylene glycol; PCL: polycaprolactone), serving as a novel drug delivery system carrying vancomycin for controlled antibiotic release. The assembly was then covered with a PCL membrane that inhibits bacterial biofilm formation early in infection and degrades when exposed to lipase solutions, mimicking enzymatic activity during bacterial infections. This setup provides infection-responsive protection and promotes drug release. We investigated the coating’s controlled release, antibacterial capability, and biocompatibility through in vitro experiments. We established a Staphylococcus aureus infection model in rabbits, implanting titanium rods in the femoral medullary cavity. We evaluated the efficacy and safety of the composite coating in preventing implant-related infections using imaging, hematology, and pathology. In vitro experiments demonstrated that the PCL membrane stably protects encapsulated vancomycin during PBS immersion. The PCL membrane rapidly degraded at a lipase concentration of 0.2 mg/mL. The mPEG750-b-PCL2500 gel ensured stable and sustained vancomycin release, inhibiting bacterial growth. We investigated the antibacterial effect of the 3D-printed titanium material, coated with PCL and loaded with mPEG750-b-PCL2500 hydrogel, using a rabbit Staphylococcus aureus infection model. Imaging, hematology, and histopathology confirmed that our composite antibacterial coating exhibited excellent antibacterial effects and infection prevention, with good safety in trials. Our results indicate that the composite antibacterial coating effectively protects vancomycin in the hydrogel from premature release in the absence of bacterial infection. The outer PCL membrane inhibits bacterial growth and prevents biofilm formation. Upon contact with bacterial lipase, the PCL membrane rapidly degrades, releasing vancomycin for antibacterial action. The mPEG750-b-PCL2500 gel provides stable and sustained vancomycin release, prolonging its antibacterial effects. Our composite antibacterial coating demonstrates promising potential for clinical application.
Similar content being viewed by others
Introduction
Orthopedic implants are crucial for the management of fracture fixation and joint replacement surgeries. However, they are associated with a high risk of postoperative implant-related infections, among which prosthetic joint infection (PJI) is a prevalent and severe complication1,2,3,4. The resilience of PJI primarily stems from the difficulty in eradicating bacterial biofilms from the implant surface. These biofilms exhibit resistance to systemic antibiotics and can evade the immune response of the host5. Consequently, patients often require additional surgeries, which cause considerable physical, psychological, and economic challenges6. Although antibiotic regimens can be effective in early biofilm formation stages, the detection of bacterial conglomerates is often difficult, highlighting the need for proactive antibacterial measures such as specialized implant coatings7,8,9,10,11,12. Currently, coatings for bacterial infections are classified into: (1) passive coatings that prevent bacterial attachment and (2) active coatings that kill or inhibit bacterial growth13. Recent developments in antimicrobial implant coatings, particularly antibiotic ones, have highlighted their effectiveness against implant-associated infections. However, achieving sustained and effective antibiotic release from these coatings over extended periods represents an ongoing challenge, which requires the development of innovative coatings that can actively respond to infection cues and facilitate antibiotic release14,15,16.
To address this challenge, our research exploits recent advances in three-dimensional (3D) printing technologies and harnesses the biocompatibility of titanium17. Titanium and titanium alloys are commonly used for implants, owing to their good mechanical properties, biocompatibility, and corrosion resistance18,19,20. 3D bioprinting is a rapidly evolving manufacturing process that can be employed to develop advanced and sophisticated scaffolds for tissue engineering applications21. Porous titanium alloys are bioinert materials, and it is hard to protect them from implant-associated infection22. Implant-associated infections caused by bacteria can lead to osteomyelitis and implant failure4. Hence, these implants are strengthened with antimicrobial coatings loaded with antibiotics that react upon infection detection. In this study, we employed polycaprolactone (PCL) for its bacterial-responsive properties, high biocompatibility, and controllable degradation through bacterial lipase23,24,25. Furthermore, our coatings included polyethylene glycol (PEG), a hydrophilic and thermoresponsive polymer promoting gradual and steady drug delivery26,27. Our approach involved the fabrication of porous titanium rods covered with a vancomycin-loaded mPEG750-b-PCL2500 gel, followed by an evaluation of both the drug release and PCL coating degradation kinetics in a simulated infectious medium. This research focuses on designing a smart implant system capable of hindering bacterial proliferation and preventing biofilm formation, with substantial implications for clinical applications (Fig. 1).
Materials and methods
Materials
3D printing porous titanium rod (Wuxi Shaxinna New Material Technology Co., LTD.); High purity titanium mesh (purity: Ti ≥ 99.96%, Shenglong metal); PCL (molecular weight 30,000) and mPEG750-b-PCL2500 were provided by Changchun Institute of Applied Chemistry, Chinese Academy of Sciences. Lipase (CAS No. 9001-62-1, Shanghai McLean Company); Staphylococcus aureus (ATCC25923, Wenzhou Kangtai Biotechnology Co., LTD.); Dichloromethane (analytical pure, Tianjin Yongda Chemical Preparations Co., LTD.); Field emission surround Scanning electron Microscope (LM2-031, Oxford Instruments, UK); Human osteosarcoma cell line MG-63 was purchased from the cell bank of the Chinese Academy of Sciences.
Fabrication of 3D-printed titanium rod
High-grade titanium powder (Purity: Ti ≥ 99.96%, Shenglong metal) was used to fabricate our implant materials. The rods measured 2.5 mm in diameter and 20 mm in length, with a target pore size of approximately 500 μm (Fig. 2B). These pore dimensions have been shown to enhance osteogenesis and facilitate osteointegration28,29. Interconnected pores were included to optimize drug delivery. The 3D porous titanium structures were precisely synthesized by Wuxi Sha Xinna New Material Technology Co., Ltd. Post-fabrication, the structural stability and characteristics of the titanium rods were evaluated using advanced microcomputed tomography (Micro-CT) imaging, confirming the tailored structural properties essential for their intended medical applications.
Preparation of PCL coatings
We dissolved PCL in a dichloromethane solvent at three different concentrations (0.1, 0.2, and 0.3 g/mL) and dipped the implant material into the obtained solutions2. The thickness of the PCL coatings could be adjusted by changing the concentration of PCL, the dipping time of the material, and the number of dipping cycles. This is an important factor to enable PCL to rapidly break down in the presence of bacteria and effectively release the drug. To evaluate the PCL breakdown speed, we used flat titanium meshes with 500 μm pores (Fig. 3A) (similar to our 3D-printed titanium rods) and coated them with PCL. After preparing PCL solutions following the procedure described above, we stirred them until PCL dissolution, and waited for any bubbles to disappear. The titanium meshes were washed with 95% ethanol, ultrasonically cleaned in distilled water to remove impurities, and then dried. Next, we dipped the meshes into the different PCL solutions for 10 min and left them to dry at room temperature. Once the dichloromethane had fully evaporated, as indicated by a constant weight, we examined the meshes using scanning electron microscopy (SEM) to check their coverage. Then, the lowest PCL concentration that still fully covered the mesh was selected for further experiments, aiming to keep the PCL layer as thin as possible.
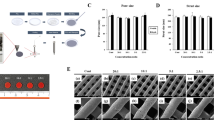
(A) Appearance of titanium cylinders used in in vitro experiments. (B) Appearance of various types of drug-loaded titanium rods. (C) Coverage of PCL membranes at different concentrations. When the concentration of PCL was 0.1 g/mL, the membrane coverage was incomplete, whereas a PCL concentration of 0.2 g/mL resulted in a complete film with moderate thickness, and a PCL concentration is 0.3 g/mL produced a complete thick film. (D) SEM images showing degradation of PCL at a concentration of 0.2 g/mL in the presence of lipase over time. The membrane structure was destroyed over time, and its morphology disappeared after 36 h. Morphology of PCL membrane after immersion in PBS solution for 10 weeks: although the morphology became uneven, the structure was still intact. (E) Drug release rate of drug-loaded titanium rods in each groups in lipase solution with a concentration of 0.2 mg/mL.
Evaluation of PCL coatings
Following a bacterial infection, lipase is secreted in varying concentrations depending on the infection’s severity30,31. An ideal PCL film should rapidly degrade in response to the lowest concentration of lipase present at the infection site. We prepared PCL films using the minimum concentration of PCL in dichloromethane that could still form a film. According to literature, the maximum yield of staphylococcal lipase can reach 5 mg/mL32,33, To simulate an infection environment, we used 5 mL of lipase solution at 0.2 mg/mL, and 5 mL of PBS solution as a control. Both sets were then placed in the same controlled environment at 37 °C and shaken a speed of 80 rpm. The meshes were removed, dried, and weighed at specific intervals (after 1, 3, 24, and finally 36 h) until the weight stopped changing. To monitor the degradation process over time, SEM was used to inspect changes in the PCL coating, both before and during its immersion in the lipase solution.
Preparation of mPEG750-b-PCL2500 gels loaded with vancomycin
To tackle the problem of rapid drug release from implants with antimicrobial coatings, we used the temperature-sensitive mPEG750-b-PCL2500 gel as drug carrier. Vancomycin, a widely-used antibiotic to prevent infections, was employed to test this system After preparing solutions with different amounts of gel, we monitored their transformation from liquid to gel and back to liquid as the temperature was gradually increased. Based on these observations, we found that the optimal concentration for the gel to work at body temperature was 25% (Fig. 2C). Vancomycin was then mixed with this gel solution. Vancomycin was weighed at a 1:10 mass ratio to mPEG750-b-PCL2500, and PBS was added at a 20% mass fraction of mPEG750-b-PCL2500. The rods were further subjected to ultrasonic vibration in sterile water for 10 min to remove surface impurities, followed by high-temperature sterilization in an oven. A high pressure was applied to ensure that the gel penetrated deeply into the rod pores. For comparison, we also loaded the vancomycin powder directly onto some rods without the gel. After calculation, we confirmed that each coated rod contained 2 mg of vancomycin, ensuring an even spread throughout the rod pores.
Evaluation of drug release properties in vitro
The titanium rods were divided into five groups prior to the laboratory tests (Fig. 4D): (1) Group A: Ti + vancomycin. (2) Group B: Ti + vancomycin + PCL. (3) Group C: Ti + vancomycin-loaded mPEG750-b-PCL2500 gel. (4) Group D: Ti + vancomycin-loaded mPEG750-b-PCL2500 gel + PCL. (5) Control group: Ti + vancomycin-loaded mPEG750-b-PCL2500 gel + PCL (Figs. 2A, 3B). Groups A–D were placed in a solution of the lipase enzyme (0.2 mg/mL) to mimic an infection scenario, with the purpose of assessing how well the coatings held and released the drug under these stress conditions. Moreover, the control group was simply soaked in PBS to simulate typical body conditions. All groups were placed in a controlled environment with consistent temperature and shaking conditions, mimicking the environment that they would experience inside the body. Over various intervals, ranging from 1 to 96 h, we collected the surrounding solutions (termed “degradation solutions”) and stored them in a fridge. For each time interval, we replaced the solutions with fresh ones. Ultraviolet (UV) spectrophotometry was used to measure the amount of vancomycin released into these solutions, with a pre-prepared standard vancomycin solution as a reference.
(A) The results of MTT test (OD570 nm, \(\overline{{\text{x}}}\) ± SD, n = 6). (B) SEM images of materials in each group. a: surface of titanium material, showing no MG63 cell adhesion. b: PCL-coated titanium material, basically maintaining the 3D structure of titanium, and showing cell adhesion on it. c,d: magnified images of single MG63 cell, showing good pseudopod extension and some broken pseudopodia. (C) Staphylococcus aureus inhibition test by filter paper method (Group D: filter paper soaked in degradation liquid for 12 and 96 h; Group C: filter paper soaked in degradation liquid for 12 and 48 h). (D) Pocket creation in erector spinae muscle. (E) Photographs of tissue sections. No obvious abnormality was found in liver/renal sections of each group (HE × 200). The tissue sections around the material showed different degrees of fibrous capsular wall formation, and there were differences in muscle. Fibroblasts, lymphocytes, and macrophages were infiltrated to a certain extent, with fibroblasts as the dominant cells.
Biological characterization
In vitro cell culture
To evaluate their suitability for medical purposes, we fabricated 3D-printed titanium cylinders with 10 mm width and 2 mm high (Fig. 3A). To ensure that they were clean for the following tests, the cylinders were shaken in a strong alcohol solution, followed by under sonication with water and sound waves to remove any remaining particles. Afterward, the cylinders were dried in a heated chamber. Then, the titanium cylinders were divided into three different sets for comparison: Group A(Ti); Group B(Ti + PCL); Group C( Ti + mPEG750-b-PCL2500);Group D(Ti + mPEG750-b-PCL2500 + PCL). After that, we placed the titanium cylinders into a gel substance in vacuum to ensure that the gel filled up all the holes. The cylinders were coated with a PCL layer by dipping into the PCL solution and air-dried. The solvent used was fully evaporated. Finally, a gentle gas sterilization method was used to prepare the cylinders for medical use. An MG-63 human osteosarcoma cell line was obtained from the Chinese Academy of Sciences cell repository. We cultured the cells using DMEM/F-12 medium, facilitating their growth and division in a controlled environment until they were actively proliferating. After a sterilization treatment that included ultraviolet irradiation, we selected vigorously growing cells for further tests. Upon sterilization, each set of materials was rinsed and placed in the individual wells of a culture plate, and a specific amount of cells (2 × 105) was carefully layered on top of each well. The cells were left to attach for 3 h before being submerged in additional culture medium.
MTT assay
The MTT assay, measuring cell metabolic activity, assessed cell viability at multiple intervals over a 2-week period. After the viability assays, cell cultivation was stopped, and samples were washed and fixed with glutaraldehyde at low temperatures. Samples were then dehydrated with ethanol solutions, desiccated, gold-coated, and inspected by scanning electron microscopy to analyze cell structures. Examination of 3D-printed titanium rods with PEG hydrogels and PCL antimicrobial layers relied on cell growth and adherence visualized by SEM (Oxford Instruments, UK). This analysis evaluated the material’s biocompatibility, crucial for its potential medical application.
In vivo compatibility evaluation
We used the 3D printed titanium rod as described above. The mPEG750-b-PCL2500 hydrogel and PCL membrane were prepared as previously described. Adult healthy Japanese big-eared white rabbits, of mixed gender and weighing 2.5 to 3 kg, were acquired from the Animal Laboratory of Jilin University. The rabbits were divided into four experimental groups: Group A: control group with sutured incisions; Group B: received titanium implants; Group C: received titanium and mPEG750-b-PCL2500 hydrogel implants; Group D: received titanium, mPEG750-b-PCL2500 hydrogel, and PCL membrane implants. Surgical procedures created pockets in the back muscles for implanting the respective materials. The surgical procedure and implant placement are shown in Fig. 4D. After the surgery, each rabbit received a series of antibiotic injections to prevent infection. One month after the operation, the rabbits were humanely euthanized, and the implant sites were examined. This included checking for the formation of fibrous cyst walls, tissue irritation, or swelling, as well as the harvesting of the material and nearby muscle tissue for further analysis. Additionally, broad assessments of the livers and kidneys of the rabbits were conducted post-mortem to look for signs of surface irregularities or tissue damage. The excised tissues were then preserved in a formalin solution for potential histological examination.
Evaluation of antibacterial efficacy
Antibacterial test in vitro
Filter paper discs with a diameter of 6 mm were sterilized on both sides using UV irradiation. Then, the discs were soaked in 1 mL of the degradation solutions from Group D, collected at 12 and 96 h after mesh immersion, and labeled as D12 and D96, respectively. Similarly, discs soaked in 1 mL of the degradation solutions from Group C, collected at 12 and 48 h after immersion, were labeled as C12 and C48, respectively. The filter papers were immersed in the respective solutions for 1 min to absorb any vancomycin present in the solution. After absorption, the soaked filter papers were allowed to dry and then sterilized under UV light. A suspension of Staphylococcus aureus (ATCC 25923; 1 × 108 CFU/mL) was uniformly applied to agar-filled Petri dishes. Each filter paper was placed on a separate section of the S. aureus culture and incubated in a constant-temperature incubator at 37 °C for 24 h. After incubation, the filter papers were removed and the size of the inhibition ring, if present, was measured to determine the effect of the bacteriostatic.
Establishment of animal model
Healthy adult Japanese big-eared white rabbits (2.5–3 kg) were procured from the Animal Laboratory of Jilin University. Before surgery, the rabbits were fasted and deprived of water for 8 h. Japanese large-eared white rabbits were weighed and restrained in rabbit clips. They were then anesthetized with an intravenous injection of 3% pentobarbital sodium at 1 mL/kg. Under anesthesia, the rabbits were positioned on their backs, and the area over their right knee was prepared for surgery with hair removal and disinfection. After local iodine application, we surgically exposed the femur by moving the patella aside. At the midpoint of the femoral condyle, a small wire was used to drill into the bone marrow cavity to extract bone marrow fluid. A known concentration (1 × 107 CFU/mL, 0.1 mL) of Staphylococcus aureus was injected into the upper femur. The entry point was sealed with sterile bone wax to prevent bacterial fluid leakage. After the procedure, the area was thoroughly cleaned and disinfected, followed by multilayer suturing of the ligaments, fascia, and skin. Post-surgery, pure iodine was applied daily for three days to the incision site. Incision healing, mobility, feeding behavior, and signs of local inflammation and limb swelling were monitored. Four weeks post-procedure, under sterile conditions, the rabbits were euthanized and their femurs removed. The bones were sliced longitudinally to examine inner tissue, then fixed, decalcified, paraffin-embedded, sectioned, and stained with hematoxylin and eosin (HE) for infection assessment. At the 4-week review, the rabbits showed clear signs of infection, such as swelling of the soft tissues in the operated limbs, with some developing sinus tracts. Skin incisions revealed significant subcutaneous pus accumulation (Fig. 5A). Pathological assessments showed extensive inflammatory cell infiltration, confirming the successful establishment of the osteomyelitis model.
Antibacterial test in vivo
Forty-eight healthy adult rabbits were randomly assigned to four groups of 12: Group A: Ti implants; Group B: Ti implants + vancomycin + PCL; Group C: Ti implants + vancomycin in mPEG750-b-PCL2500 gel; Group D: Ti + vancomycin in mPEG750-b-PCL2500 gel + PCL. During the operation, under anesthesia (The method of anesthesia is mentioned in 2.8.2), a 1 mm Kirschner wire was used to drill into the bone marrow cavity at the femoral condyle midpoint. The entry point was gradually enlarged to 3 mm to aspirate bone marrow fluid. Staphylococcus aureus (1 × 107 CFU/mL, 0.1 mL) was administered using a sterile syringe, and materials were implanted as per group assignments (Fig. 5A). Aseptic bone wax sealed the entry point to prevent bacterial fluid leakage. On days 1, 3, and 5, and weeks 1, 2, 4, and 8 post-surgery, selected rabbits were evaluated for white blood cell (WBC) count, serum C-reactive protein (CRP) levels, and other serum indicators. Data from each rabbit group and time point were statistically analyzed. Radiographic examinations of the affected limbs were performed immediately post-surgery and at 1, 2, 4, and 8 weeks. Radiographs were evaluated using the Norden osteomyelitis grading score, and osteomyelitis severity was quantified statistically. Daily postoperative monitoring included assessments of the incision site, activity level, diet, local inflammation, limb swelling, and overall mobility. The surgical incision healing process was recorded photographically. At 8 weeks post-operation, rabbits were humanely euthanized via air embolization of the auricular vein. In an aseptic environment, the entire femur was extracted and longitudinally dissected, and the implanted materials were excised. The samples were fixed, decalcified, paraffin-embedded, and processed for histological analysis. Tissue sections were stained with HE to evaluate infection presence and extent.
Statistical analysis
All data are presented as mean ± standard deviation (SD). Statistical analysis was performed using the SPSS 26.0 software. Multiple group comparisons were conducted using one-way ANOVA and Kruskal–Wallis rank sum test, while the t-test was employed to assess differences between specific groups. A P value < 0.05 was considered statistically significant.
Results
Preparation and degradation of PCL membranes
To develop an optimal PCL coating fully covering the surface of the implant, while maintaining a thin profile for rapid infection response, we performed a series of experiments involving 2D titanium meshes. The meshes were soaked in PCL–dichloromethane solutions with different concentrations, followed by a drying phase to assess film formation. Our findings indicated that a homogeneous coverage with a minimum film thickness (20 µm; see Fig. 3C) could be obtained at a 0.2 g/mL PCL concentration. Further tests exposed the meshes to a 0.2 g/mL lipase concentration for a period of 1 h, leading to a clear disruption in the film integrity. The appearance of evident breaks within the film cover demonstrated the degradation process induced by the lipase activity, as shown through SEM analysis. With increasing time, the degradation of the PCL films became more intense, with almost complete disappearance after 36 h. In contrast, in the control experiment where the PCL film was immersed in pure PBS solution, its integrity remained unchanged, displaying no mass loss even after an extended immersion for 10 weeks (Fig. 3D).
Evaluation of drug release properties in vitro
The in vitro drug release tests showed that, when immersed in a 0.2 mg/mL lipase PBS solution, the vancomycin in group D began to be released within the first 1 h. The release process proceeded slowly over the initial 6 h, followed by a rapid increase in release rate between 6 and 12 h. Approximately 18% and 35% of the total loaded drug was released in the first 12 h and within the first full day, respectively. The release pattern then changed to a stable release rate continuing up to the 96th hour. In contrast, group C exhibited a gradually increasing release rate, with the drug fully released within 36 h. Group B displayed an abrupt release of the drug within the first 24 h. However, the release profile remained more consistent than that of group A thereafter, maintaining a steady rate until 60 h after the initial surge. On the other hand, the drug release for group A occurred within the first 1 h. The drug release profile for each group is shown in Fig. 3E. In Control Group, the drug release was tested in a PBS solution intended to mimic a normal physiological environment; the test showed a slow release rate of less than 1% after 96 h, and ~ 10% of the total drug load was released over a period of 10 weeks.
In vitro biocompatibility
The co-culture tests with the studied materials indicated that MG63 cells proliferated vigorously in all tested conditions, exhibiting remarkable cellular adhesion and pseudopod extension. The cell viability was quantitatively assessed using the MTT assay to measure the absorbance at various intervals; the results are detailed in Table 1 and Fig. 4A. The analysis of the MTT absorbance data revealed a notable increase in cell viability in group A on the 14th day compared to groups B, C and D, with a statistically significant difference (P < 0.05). On the other days, group A displayed no substantial differences in cell viability compared to groups B, C and D (P > 0.05). Furthermore, the absorbance levels of groups B and C were statistically indistinguishable at all measured time points (P > 0.05), highlighting the biocompatibility of the PCL membrane.
Following a 2-week co-culture period with the studied materials, the MG63 cells were imaged using SEM. The obtained images evidenced strong cellular adhesion to the PCL membrane, characterized by cells with widely extended pseudopodia. In contrast, no MG63 cells were detected on the titanium surface, as shown in Fig. 4B.
In vitro antibacterial test
Significant inhibition rings were observed on filter papers D12 and C12, which were soaked in the titanium rod solutions of group D and group C, respectively, after 12 h in the presence of lipase. Moreover, a noticeable antibacterial ring was observed on filter paper D96, which was soaked in the titanium rod solution of group D after 96 h in lipase. However, the antibacterial ring on filter paper C48, soaked in the titanium rod solution of group C, almost disappeared after 48 h in lipase (Fig. 4C). These findings suggest that the mPEG750-b-PCL2500 gel served as an effective drug carrier, enabling sustained and stable drug release.
In vivo biocompatibility
On the day following the surgical procedure, the experimental rabbits returned to their normal dietary habits and activity levels. Moreover, the surgical sites remained free of hyperemia, edema, or infection, showing signs of satisfactory healing, as shown in Fig. 4D. The liver and kidney surfaces were considerably smooth, with no obvious signs of edema, congestion, or hemorrhage. No prominent fibrous sac encasing the material was observed for groups B, C, and D. Additionally, the muscle tissue across all groups retained its normal appearance, without signs of hyperemia, edema, infection, or necrosis, and there was no evidence of cartilage or bone development. The histological examination of liver and kidney tissues did not show signs of necrosis, edema, congestion, or infiltration by inflammatory cells in the experimental rabbits of any group. These findings closely matched those of normal tissues. Examination of the tissue sections adjacent to the material revealed various degrees of fibrocystic wall formation. There was varying degrees of infiltration by fibroblasts, lymphocytes, and macrophages in the muscle tissue, with fibroblasts being the predominant cell type (Fig. 4E).
In vivo antibacterial test
After the surgical procedure, the experimental rabbits presented various degrees of limb claudication and soft tissue edema. A noticeable reduction in dietary consumption was observed in the immediate postoperative period, which showed a gradual improvement over time. It is important to highlight that all other rabbits employed in the experiment survived until the end of the observation period. The postoperative healing of the incisions is illustrated in the photographs shown in Fig. 5C. Notably, during the initial week post-surgery, rabbits in group A exhibited significant erythema and edema localized to the knee joint area, with subsequent development of purulent exudate and sinus tract formation. At the beginning of the second week, the group B rabbits started to exhibit similar inflammatory signs at the knee joint to those observed in group A. In contrast, groups C and D showed promising healing trajectories, with suture lines gradually disappearing.
X-ray analysis
The severity of osteomyelitis in each experimental group was classified using the established Norden osteomyelitis criteria34 (Table 2). Radiographic assessments conducted at weeks 1, 2, 4, and 8 post-surgery are shown in Fig. 5D. Upon examination at the 8-week postoperative mark, the Norden scores revealed no statistically significant difference between groups A and B (P < 0.05) or between groups C and D (P > 0.05). In contrast, a statistically significant difference was found between groups A and C, as well as between groups B and D (P < 0.01) (Fig. 5B).
Hematological examination and evaluation
The groups are as follows: Group A: Ti implants; Group B: Ti implants + vancomycin + PCL; Group C: Ti implants + vancomycin in mPEG750-b-PCL2500 gel; Group D: Ti + vancomycin in mPEG750-b-PCL2500 gel + PCL. Leukocyte counts in a predetermined subset of experimental rabbits were systematically evaluated at multiple time points post-surgery: days 1, 3, and 5, and weeks 1, 2, 4, and 8. The data indicated that group A consistently exhibited higher WBC counts compared to the other groups. Moreover, WBC counts in group B exhibited fluctuations and exceeded those of groups C and D starting from the second postoperative week. Importantly, by week 8, the leukocyte counts in groups C and D had returned to baseline preoperative levels. Statistical evaluation at the 8-week postoperative mark confirmed significant differences in leukocyte counts among the different groups (Fig. 6A). In addition to the leukocyte assessment, a selected group of rabbits underwent serum CRP level measurements at the same postoperative time points. The results revealed an initial surge in CRP levels across all groups, followed by a decreasing trend. Group A persistently presented higher CRP levels than groups B, C, and D throughout the study period. On day 3 post-surgery, the CRP concentration in group B was lower than that in groups C and D; however, an increasing trend was observed thereafter. The CRP levels in groups C and D remained significantly lower than those of groups A and B. Statistical analysis showed significant differences in CRP levels among the groups at weeks 2, 4, and 8 following surgery (Fig. 6B). Additionally, serum interleukin-6 (IL-6) levels were measured in a specific subset of rabbits at identical postoperative time points. The IL-6 levels of Group A were markedly higher than those of groups B, C, and D. On postoperative day 5, the IL-6 levels of group B exceeded those of groups C and D. Interestingly, on the 56th day post-surgery, group C exhibited marginally higher IL-6 levels compared to group D, whereas the IL-6 levels of group D peaked at day 7 and then decreased. Significant differences in IL-6 levels among the groups were statistically confirmed at weeks 4 and 8 after surgery (Fig. 6C).
(A) Changes in WBC levels in each group; data are presented as mean ± standard deviation (n = 12). *p < 0.05. (B) Changes in CRP levels in each group; data are presented as mean ± standard deviation (n = 12). *p < 0.05. (C) Changes in IL-6 levels in each group; data are presented as mean ± standard deviation (n = 12). *p < 0.05. (D) Pathological sections obtained at 8 weeks after operation in each group (white arrow: fibrous tissue hyperplasia; white double arrow: contact interface; black arrow: bone; black double arrow: necrotic tissue; black triangular arrow: material–bone marrow interface).
Histopathological assessment
At 8 weeks post-operation, we assessed infection in the intramedullary tissue surrounding the implant using HE staining. Due to the disruption of the contact surface between the material and the bone marrow during extraction, we chose to examine the intramedullary tissue and perform HE staining. We also observed bone ingrowth on the surface of the retrieved implants in all groups. These findings are shown in Fig. 6D and Table 3. Pathological sections obtained at the 8-week time point (stained with HE, magnified 40 times) showed that the distal medullary cavity of the implant was closely adjacent to the cortical bone. In groups A and B, a fibrotic medulla was identified beneath the cortical bone (as indicated by black arrows in Fig. 6D), with the absence of normal medulla. Conversely, groups C and D predominantly exhibited normal bone marrow.
Discussion
Implant-related infections pose significant challenges to orthopedic surgeries due to the high risk of severe complications. The widespread use of bioactive prostheses in joint replacements, featuring roughened surfaces and tight integration with the bone marrow cavity, has facilitated bacterial proliferation and complicated treatment. Developing antibacterial coatings for orthopedic implants has been a key research focus in recent years to address this critical issue. Researchers have designed coatings using various materials and antibacterial strategies. In this study, we fabricated 3D-printed porous titanium rods, incorporated vancomycin-loaded mPEG750-b-PCL2500 gel, and coated them with a PCL layer. We then evaluated the antibacterial efficacy through both in vitro and in vivo experiments. Our coating passively inhibits bacterial biofilm formation and actively controls antibiotic release in response to bacterial growth, providing a practical solution for proactive and sustained infection control.
The present in vitro experiments showed that the thermosensitive mPEG750-b-PCL2500 gel exhibited an improved drug release kinetics and reduced drug burst release24. To increase the drug load, we used the 3D printing technology to fabricate porous titanium rods, thus increasing the surface area of the coating. Additionally, the incorporation of a bacterial-responsive PCL outer coating prevented drug release in a non-infectious simulated environment. In an infectious environment, the PCL barrier degraded, releasing antibiotics even at 0.2 mg/mL lipase concentration. This intelligent coating system shows potential for preventing implant-related infections.
Orthopedic implants require biocompatibility, bone conductivity, and bone induction. In vitro cell culture is crucial for assessing material biocompatibility. We used MG63 cells for their ease of culture and similarity to osteoblasts, making them suitable for evaluating orthopedic implant biocompatibility35. Titanium is commonly used for orthopedic implants due to its mechanical properties, biocompatibility, low elastic modulus, ease of processing, and corrosion resistance36. The PCL coating on titanium is a biodegradable aliphatic polyester25 known for its semicrystalline nature. PCL exhibits excellent biocompatibility, and recent research showed favorable MG63 cell growth on PCL37,38.
MG63 cells were co-cultured with titanium materials coated with PCL and with uncoated titanium. Except on day 14, the PCL film did not significantly affect MG63 cell growth compared to uncoated titanium. On day 14, more MG63 cells were found on uncoated titanium than on PCL-coated titanium. The reason for this discrepancy is unclear. The PCL membrane might limit growth space for adherent cells, causing the differences seen on day 14. Although not statistically significant, cell numbers on PCL-coated titanium were consistently lower than on uncoated titanium from day 1. This difference could be due to cytotoxicity from residual organic solvents. To mitigate this, Fuchs proposed using medical-grade PCL scaffolds, suppressing cytotoxic effects of organic solvents39. SEM analysis revealed that MG63 cells did not adhere to uncoated titanium. This lack of adhesion is attributed to the inert nature of titanium40,41. Additionally, cells respond to microscale surface features such as shape, position, and polarization changes, a phenomenon known as contact guidance42. Different levels of surface roughness induce various cellular responses. For instance, micro-rough structures promote cell attachment, whereas nano-rough structures enhance cell differentiation, protein synthesis, and gene expression43. Currently, clinical joint prostheses often feature porous coatings and hydroxyapatite sprays on bone contact surfaces to promote bone ingrowth. The lack of MG63 cell adhesion to titanium in our study is likely due to the fact that the 3D-printed titanium rods were not micro-roughened. The smooth surface, combined with the inherent inertness of titanium, prevented cell adhesion. Cell growth and pseudopod extension were observed on the PCL membrane. After implantation, the material surface initially accumulates blood and protein, followed by cell adhesion, proliferation, and differentiation44,45. During this process, implant and body fluid interactions primarily involve micro-electrochemical reactions. Local damage may be caused by small molecules or toxic metal ions from corrosion or degradation. These species can enter the circulatory system and cause systemic damage. Muscle growth occurred on uncoated titanium and titanium coated with mPEG750-b-PCL2500 hydrogel and PCL. Pathological examination revealed significant fibroblast accumulation around all materials, with no acute or chronic inflammatory cell infiltration. No abnormalities were observed in liver and kidney histopathological sections. In vivo and in vitro cytocompatibility experiments showed that 3D-printed titanium coated with mPEG750-b-PCL2500 hydrogel and PCL film exhibited excellent biocompatibility. No adverse reactions were observed in vivo.
Orthopedic implants require good biocompatibility, bone conductivity, bone induction, and mechanical properties. Titanium, as an inert metal, is highly suitable for orthopedic implants. 3D-printed titanium materials provide advantages like uniform pore size and distribution. The pore size used (500 μm) promotes cell proliferation, new bone growth46,47, a and drug release. Pores in 3D-printed materials provide higher drug loading capacity compared to conventional coatings. The drug carrier, thermosensitive mPEG750-b-PCL2500 hydrogel, exhibits excellent biocompatibility, biodegradability, and low immunogenicity and drug release48. Pores in 3D-printed materials provide higher drug loading capacity compared to conventional coatings. The drug carrier, thermosensitive mPEG750-b-PCL2500 hydrogel, exhibits excellent biocompatibility, biodegradability, and low immunogenicity49,50. Studies showed PCL membrane remains intact after 10 weeks in PBS, with only ~ 10% drug release. This suggests PCL remains stable in the body without bacterial infection. However, bacterial infection around the implant attracts inflammatory cells. Cholesterol esterase from inflammatory cells degrades the PCL membrane51, releasing encapsulated antibiotics. This “intelligent” release mechanism helps control early infection.
A limitation of this study is using lipase to mimic bacteria. Most studies using bacterial-secreted lipase are primarily qualitative52. Further investigation is needed to explore PCL degradation by specific bacteria. Future research should focus on in vivo experiments and clinical validation.
Staphylococcus aureus is the most common pathogen in PJI in clinical settings53,54. Studies show that using Staphylococcus aureus concentrations of 1 × 106 to 1 × 108 CFU/mL in a rabbit osteomyelitis model results in a 100% infection rate55. Other studies indicate that the bacterial concentration needed to induce peri-implant infection is 2.5–40 times lower with porous metal surfaces compared to smooth ones. We established an infection model in experimental rabbits by injecting 0.1 mL of Staphylococcus aureus at a concentration of 1 × 107 CFU/mL into the femoral marrow cavity. By week 8, X-rays showed soft tissue swelling, bone destruction, osteosclerosis, and other infection signs in the pure titanium group.
We compared the therapeutic effects of four materials based on rabbit blood parameters (WBC, CRP, IL-6) and pathological observations at different time points. Results showed that long-term therapeutic efficacy of titanium with mPEG750-b-PCL2500 hydrogel (group C) and PCL coating (group D) was superior to titanium + vancomycin + PCL (group B). The inflammatory reaction in group D was more intense than in group C on days 1, 3, 5, and 7. This suggests that (i) mPEG750-b-PCL2500 hydrogel prevents sudden drug release, and (ii) PCL membrane degradation takes time. However, these experiments used lipase to degrade the PCL membrane, which differs from in vivo conditions. Thus, the exact time for PCL membrane degradation and antibiotic release could not be determined.
Pathological sections showed fibrous tissue proliferation at the interface between bone marrow and material in groups B and D (PCL-coated). We believe the issue may be related to the disruption of the marrow cavity during surgery, as bone ingrowth is associated with increased fibroblast activity. In this study, we established a bone marrow infection model in rabbits, where the inflammatory response might contribute to fibrous tissue proliferation. Additionally, this may be due to PCL’s hydrophobic nature and its lower biocompatibility compared to hydrophilic materials56,57. As a result, fibrous tissue proliferation occurred between the intramedullary tissue and the material. To address PCL biocompatibility, researchers have employed various strategies: (1) Advanced Production Techniques: For instance, PCL produced using a combination of unidirectional freeze-drying and electrospinning techniques has been shown to significantly enhance cell proliferation and activity in vitro, thereby improving PCL’s biocompatibility58. (2) Incorporation of Antioxidants: Abdulhameed et al. demonstrated that PCL films combined with Vitamin C (PCL-Vit C) effectively improved the biocompatibility of PCL59. Several other studies incorporating antioxidants into PCL have also reported positive outcomes60. (3) Chemical Modification of Polymers: Adding chemical groups to the polymers to increase their biocompatibility61. These recent advancements in improving PCL biocompatibility are promising. Although biocompatibility remains a critical issue to be resolved before clinical application, rapid progress in research has led to an increasing number of effective solutions. This progress paves the way for future clinical applications. For future applications, combining these approaches could effectively enhance biocompatibility, reduce patient risk, and improve satisfaction.
Challenges remain for the effective translation and application of biomaterials with drug delivery systems (BDDS) into clinical practice. Sterilizing drug carriers after assembly is an unresolved issue62. We used pre-assembly sterilization for individual components before combining them into drug carriers in a sterile environment. This method requires strict sterile conditions, posing contamination risks during large-scale production. The diverse compositions of materials enable their application in complex conditions; for instance, metal materials may be sterilized using high-pressure sterilizers, ultraviolet irradiation, ethylene oxide, reactive ion plasma, and heating systems. However, these sterilization methods may damage drug delivery materials, organic coatings, or drugs63. Hydrogels and other organic polymer materials hold significant potential for clinical applications. However, a critical sterilization process must be employed to bring these materials to market. Common sterilization methods, such as heat and irradiation, can degrade or cross-link polysaccharides, negatively affecting hydrogel performance. Therefore, resolving the sterilization issue is a priority64,65. Recently, Yuan et al. used electron beam (EB) irradiation innovatively for the sterilization of freeze-dried hydrogels. This method effectively sterilizes hydrogels while preserving their properties, facilitating the clinical translation of related materials66.We recommend that each component be subjected to appropriate sterilization methods and assembled under strictly sterile conditions to ensure the absolute sterility of the implants67. Additionally, given the nature of the drugs and hydrogel coatings, materials should be transported and stored under low-temperature and light-protected conditions to maintain the effectiveness of the antimicrobial coatings.
The application scenarios of orthopedic implants vary by type. Titanium, the most common orthopedic material, has excellent biocompatibility and osseointegration, enabling its wide use in joint prostheses. Our research is based on titanium’s superior osseointegration properties. Thus, this study focused on the drug release performance and antibacterial properties of drug-loaded materials. The impact of polymer coatings on osseointegration after titanium coating needs further exploration. We plan to design various titanium materials with polymer coatings for specific applications. PJI is one of the most catastrophic complications following joint replacement surgery, making early prevention and diagnosis critical for patient outcomes. According to the literature, the highest risk period for PJI is within the first two years post-surgery, accounting for 60% to 70% of all cases53,68. Furthermore, the rough surface of joint prostheses facilitates bacterial colonization and biofilm formation, leading to antibiotic resistance and reduced antibiotic efficacy69. In this study, we believe our composite material effectively addresses these challenges. First, it provides an early and timely antibacterial response, effectively controlling infection. Second, the presence of polymers and antibiotics can prevent or slow bacterial biofilm formation, improving conditions for other treatments. We will use suitable animal models to investigate osseointegration and antibacterial properties, aiming to develop orthopedic materials with excellent osseointegration and antibacterial properties for clinical use.
Conclusion
-
1.
3D-printed titanium materials coated with mPEG750-b-PCL2500 hydrogel and PCL film exhibit high biocompatibility, enabling their use as effective drug carriers.
-
2.
Our findings indicate that the material achieves stable antibiotic release in response to bacterial infection, inhibiting bacterial growth.
-
3.
In future clinical applications, this approach could offer a novel antibacterial strategy for joint prostheses. It has the potential to inhibit or eliminate pathogens early after joint replacement surgery, preventing PJI. This would reduce both economic costs and physiological burden for patients.
Data availability
The data are available from the corresponding author upon reasonable request.
References
Xie, H. et al. Recent advances in prevention, detection and treatment in prosthetic joint infections of bioactive materials. Front. Bioeng. Biotechnol. 10, 1053399 (2022).
Zhou, Z. et al. Effects of poly (ε-caprolactone) coating on the properties of three-dimensional printed porous structures. J. Mech. Behav. Biomed. Mater. 70, 68–83 (2017).
Mortazavi, S. M. J. et al. Failure following revision total knee arthroplasty: Infection is the major cause. Int. Orthop. 35(8), 1157–1164 (2011).
Arciola, C. R., Campoccia, D. & Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 16(7), 397–409 (2018).
Arciola, C. R. et al. Biofilm-based implant infections in orthopaedics. Adv. Exp. Med. Biol. 830, 29–46 (2015).
Hellebrekers, P. et al. Effect of a standardized treatment regime for infection after osteosynthesis. J. Orthop. Surg. Res. 12(1), 41 (2017).
Pérez-Anes, A. et al. Bioinspired titanium drug eluting platforms based on a poly-β-cyclodextrin-chitosan layer-by-layer self-assembly targeting infections. ACS Appl. Mater. Interfaces 7(23), 12882–12893 (2015).
Lv, H. et al. Layer-by-layer self-assembly of minocycline-loaded chitosan/alginate multilayer on titanium substrates to inhibit biofilm formation. J. Dent. 42(11), 1464–1472 (2014).
Shi, X. et al. Electrical signals guided entrapment and controlled release of antibiotics on titanium surface. J. Biomed. Mater. Res. A 101(5), 1373–1378 (2013).
Raphel, J. et al. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 84, 301–314 (2016).
Visai, L. et al. Titanium oxide antibacterial surfaces in biomedical devices. Int. J. Artif. Org. 34(9), 929–946 (2011).
Cyphert, E. L. et al. Recent advances in the evaluation of antimicrobial materials for resolution of orthopedic implant-associated infections in vivo. ACS Infect. Dis. 7(12), 3125–3160 (2021).
Li, B. et al. Implants coating strategies for antibacterial treatment in fracture and defect models: A systematic review of animal studies. J. Orthop. Transl. 45, 24–35 (2024).
Goodman, S. B. et al. The future of biologic coatings for orthopaedic implants. Biomaterials 34(13), 3174–3183 (2013).
Besinis, A. et al. Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings. Nanotoxicology 11(3), 327–338 (2017).
Campoccia, D., Montanaro, L. & Arciola, C. R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 34(34), 8533–8554 (2013).
Hizal, F. et al. Impact of 3D hierarchical nanostructures on the antibacterial efficacy of a bacteria-triggered self-defensive antibiotic coating. ACS Appl. Mater. Interfaces 7(36), 20304–20313 (2015).
Sandler, N. & Preis, M. Printed drug-delivery systems for improved patient treatment. Trends Pharmacol. Sci. 38(3), 317 (2017).
Alhnan, M. A. et al. Emergence of 3D printed dosage forms: Opportunities and challenges. Pharm. Res. 33(8), 1817–1832 (2016).
Huang, H. et al. In vitro application of drug-loaded hydrogel combined with 3D-printed porous scaffolds. Biomed. Mater. 17, 6 (2022).
Adhikari, J. et al. Development of hydroxyapatite reinforced alginate–chitosan based printable biomaterial-ink. Nano-Struct. Nano-Obj. 25, 100630 (2021).
Chen, Z.-Y. et al. Antibacterial biomaterials in bone tissue engineering. J. Mater. Chem. B 9(11), 2594–2612 (2021).
García-Alvarez, R., Izquierdo-Barba, I. & Vallet-Regí, M. 3D scaffold with effective multidrug sequential release against bacteria biofilm. Acta Biomater. 49, 113–126 (2017).
Shahi, R. G. et al. Novel bioactive tetracycline-containing electrospun polymer fibers as a potential antibacterial dental implant coating. Odontology 105(3), 354–363 (2017).
Shirzaei Sani, I. et al. Preparation and characterization of polycaprolactone/chitosan-g-polycaprolactone/hydroxyapatite electrospun nanocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 182, 1638–1649 (2021).
Xiao, Y. et al. Functional poly(ε-caprolactone) based materials: Preparation, self-assembly and application in drug delivery. Curr. Top. Med. Chem. 14(6), 781–818 (2014).
Oladapo, B. I., Oshin, E. A. & Olawumi, A. M. Nanostructural computation of 4D printing carboxymethylcellulose (CMC) composite. Nano-Struct. Nano-Obj. 21, 100423 (2020).
Frosch, K.-H. et al. Growth behavior, matrix production, and gene expression of human osteoblasts in defined cylindrical titanium channels. J. Biomed. Mater. Res. A 68(2), 325–334 (2004).
Hara, D. et al. Bone bonding strength of diamond-structured porous titanium-alloy implants manufactured using the electron beam-melting technique. Mater. Sci. Eng. C Mater. Biol. Appl. 59, 1047–1052 (2016).
Hedström, S. A. & Kronvall, G. Phage group, lipase activity and protein A content of Staphylococcus aureus strains from cases of chronic osteomyelitis. Scand. J. Infect. Dis. 4(3), 203–207 (1972).
Nehal, F. et al. Biochemical and molecular characterization of a lipase from an Algerian isolated Staphylococcus aureus strain. J. Basic Microbiol. 57(3), 253–264 (2017).
Sayari, A. et al. Biochemical and molecular characterization of Staphylococcus simulans lipase. Biochimie 83(9), 863–871 (2001).
Mosbah, H. et al. Biochemical and molecular characterization of Staphylococcus xylosus lipase. Biochim. Biophys. Acta Gen. Subj. 1723(1), 282–291 (2005).
Norden, C. W., Myerowitz, R. L. & Keleti, E. Experimental osteomyelitis due to Staphylococcus aureus or Pseudomonas aeruginosa: A radiographic-pathological correlative analysis. Br. J. Exp. Pathol. 61(4), 451–460 (1980).
Wu, Y.-H.A. et al. 3D-printed bioactive calcium silicate/poly-ε-caprolactone bioscaffolds modified with biomimetic extracellular matrices for bone regeneration. Int. J. Mol. Sci. 20, 4 (2019).
Jiang, P. et al. Advanced surface engineering of titanium materials for biomedical applications: From static modification to dynamic responsive regulation. Bioact. Mater. 27, 15–57 (2023).
Harikrishnan, P., Islam, H. & Sivasamy, A. Biocompatibility studies of nanoengineered polycaprolactone and nanohydroxyapatite scaffold for craniomaxillofacial bone regeneration. J. Craniofac. Surg. 30(1), 265–269 (2019).
Sharifi, F. et al. Polycaprolactone/carboxymethyl chitosan nanofibrous scaffolds for bone tissue engineering application. Int. J. Biol. Macromol. 115, 243–248 (2018).
Fuchs, A. et al. Medical-grade polycaprolactone scaffolds made by melt electrospinning writing for oral bone regeneration—A pilot study in vitro. BMC Oral Health 19(1), 28 (2019).
Head, W. C., Bauk, D. J. & Emerson, R. H. Titanium as the material of choice for cementless femoral components in total hip arthroplasty. Clin. Orthop. Relat. Res. 311, 85–90 (1995).
Szczęsny, G. et al. A review on biomaterials for orthopaedic surgery and traumatology: From past to present. Materials 15, 10 (2022).
Yoshinari, M. et al. Solubility control of thin calcium-phosphate coating with rapid heating. J. Dent. Res. 76(8), 1485–1494 (1997).
von Wilmowsky, C. et al. Implants in bone: part I. A current overview about tissue response, surface modifications and future perspectives. Oral Maxillofac. Surg. 18(3), 243–57 (2014).
Bächle, M. & Kohal, R. J. A systematic review of the influence of different titanium surfaces on proliferation, differentiation and protein synthesis of osteoblast-like MG63 cells. Clin. Oral Implants Res. 15(6), 683–692 (2004).
Stoilov, M. et al. Effects of different titanium surface treatments on adhesion, proliferation and differentiation of bone cells: An in vitro study. J. Funct. Biomater. 13, 3 (2022).
Cheong, V. S. et al. Novel adaptive finite element algorithms to predict bone ingrowth in additive manufactured porous implants. J. Mech. Behav. Biomed. Mater. 87, 230–239 (2018).
Mygind, T. et al. Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials 28(6), 1036–1047 (2007).
Theerasilp, M. et al. Glucose-installed biodegradable polymeric micelles for cancer-targeted drug delivery system: Synthesis, characterization and in vitro evaluation. J. Mater. Sci. Mater. Med. 29(12), 177 (2018).
Labet, M. & Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 38(12), 3484–3504 (2009).
Coudane, J. et al. Poly(ε-caprolactone)-based graft copolymers: Synthesis methods and applications in the biomedical field: A review. Molecules 27, 21 (2022).
Hiraishi, N. et al. Susceptibility of a polycaprolactone-based root canal filling material to degradation using an agar-well diffusion assay. Am. J. Dent. 21(2), 119–123 (2008).
Quave, C. L. & Horswill, A. R. Flipping the switch: Tools for detecting small molecule inhibitors of staphylococcal virulence. Front. Microbiol. 5, 706 (2014).
Pulido, L. et al. Periprosthetic joint infection: The incidence, timing, and predisposing factors. Clin. Orthop. Relat. Res. 466(7), 1710–1715 (2008).
Hao, L. et al. Direct detection and identification of periprosthetic joint infection pathogens by metagenomic next-generation sequencing. Sci. Rep. 13(1), 7897 (2023).
Mariani, B. D. et al. Polymerase chain reaction molecular diagnostic technology for monitoring chronic osteomyelitis. J. Exp. Orthop. 1(1), 9 (2014).
Gupta, D. et al. Modelling and optimization of NaOH-etched 3-D printed PCL for enhanced cellular attachment and growth with minimal loss of mechanical strength. Mater. Sci. Eng. C Mater. Biol. Appl. 98, 602–611 (2019).
Zargarian, S. S. et al. Surfactant-assisted-water-exposed versus surfactant-aqueous-solution-exposed electrospinning of novel super hydrophilic polycaprolactone-based fibers: Cell culture studies. J. Biomed. Mater. Res. A 107(6), 1204–1212 (2019).
Feng, P. Y. & Jing, X. Novel shish-kebab structured nanofibrous decorating chitosan unidirectional scaffolds to mimic extracellular matrix for tissue engineering. J. Mech. Behav. Biomed. Mater. 158, 106677 (2024).
Abdulhameed, E. A. et al. Managing oxidative stress using vitamin C to improve biocompatibility of polycaprolactone for bone regeneration in vitro. ACS Omega 9(29), 31776–31788 (2024).
Wang, Y. et al. Preparation and characterization of polycaprolactone (PCL) antimicrobial wound dressing loaded with pomegranate peel extract. ACS Omega 8(23), 20323–20331 (2023).
Darroch, C. et al. Melt electrowriting of poly(ϵ-caprolactone)-poly(ethylene glycol) backbone polymer blend scaffolds with improved hydrophilicity and functionality. Biomed. Mater. 19, 5 (2024).
Kunrath, M. F., Shah, F. A. & Dahlin, C. Bench-to-bedside: Feasibility of nano-engineered and drug-delivery biomaterials for bone-anchored implants and periodontal applications. Mater. Today. Bio 18, 100540 (2023).
França, A. et al. Sterilization matters: Consequences of different sterilization techniques on gold nanoparticles. Small 6(1), 89–95 (2010).
Ways, T. M., Lau, W. & Khutoryanskiy, V. V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 10, 3 (2018).
Dureja, H., Tiwary, A. K. & Gupta, S. Simulation of skin permeability in chitosan membranes. Int. J. Pharm. 213(1–2), 193–198 (2001).
Yuan, H. et al. Clinical applicable carboxymethyl chitosan with gel-forming and stabilizing properties based on terminal sterilization methods of electron beam irradiation. ACS Omega 9(16), 18599–18607 (2024).
Duddeck, D. U. et al. On the cleanliness of different oral implant systems: A pilot study. J. Clin. Med. 8, 9 (2019).
Kurtz, S. M. et al. Prosthetic joint infection risk after TKA in the Medicare population. Clin. Orthop. Relat. Res. 468(1), 52–56 (2010).
Tande, A. J. & Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 27(2), 302–345 (2014).
Acknowledgements
This work was supported by the Jilin Provincial Science and Technology Department Project (Project No. 20240302070GX).
Author information
Authors and Affiliations
Contributions
Zheru Ma, Yao Zhao, Yao Zhang, and Zhe Xu contributed equally to this work and should be considered co-first authors. Conceptualization: Yao Zhang and Zhe Xu; Methodology: Yao Zhang and Zhe Xu; Investigation: Zhe Xu and Yao Zhang; Writing—Original Draft: Zheru Ma; Writing—Review & Editing: All authors; Funding Acquisition: Wei Feng; Resources: Wei Feng; Supervision: Wei Feng and Yao Zhao.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All institutional and national guidelines for the care and use of laboratory animals were followed. In vivo experiments were conducted in accordance with the Chinese Animal Experimentation Law and approved by the Ethics Committee of the first hospital of Jilin University. We confirm the study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org).
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ma, Z., Zhao, Y., Xu, Z. et al. 3D-printed porous titanium rods equipped with vancomycin-loaded hydrogels and polycaprolactone membranes for intelligent antibacterial drug release. Sci Rep 14, 21749 (2024). https://doi.org/10.1038/s41598-024-72457-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72457-1
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.