Abstract
Tuberculous meningitis (TBM)—the extrapulmonary form of tuberculosis, is the most severe complication associated with tuberculosis, particularly in infants and children. The gold standard for the diagnosis of TBM requires cerebrospinal fluid (CSF) through lumbar puncture—an invasive sample collection method, and currently available CSF assays are often not sufficient for a definitive TBM diagnosis. Urine is metabolite-rich and relatively unexplored in terms of its potential to diagnose neuroinfectious diseases. We used an untargeted proton magnetic resonance (1H-NMR) metabolomics approach to compare the urine from 32 patients with TBM (stratified into stages 1, 2 and 3) against that from 39 controls in a South African paediatric cohort. Significant spectral bins had to satisfy three of our four strict cut-off quantitative statistical criteria. Five significant biological metabolites were identified—1-methylnicotinamide, 3-hydroxyisovaleric acid, 5-aminolevulinic acid, N-acetylglutamine and methanol—which had no correlation with medication metabolites. ROC analysis revealed that methanol lacked diagnostic sensitivity, but the other four metabolites showed good diagnostic potential. Furthermore, we compared mild (stage 1) TBM and severe (stages 2 and 3) TBM, and our multivariate metabolic model could successfully classify severe but not mild TBM. Our results show that urine can potentially be used to diagnose severe TBM.
Similar content being viewed by others
Introduction
Tuberculous meningitis (TBM) is a devastating and severe form of extrapulmonary tuberculosis (TB) that affects the central nervous system, especially the meninges that surround the brain1,2. TBM causes substantial mortality and morbidity worldwide, with sub-Saharan Africa being by far the most severely affected region globally. Infants and children are the most vulnerable group to TBM, resulting in death or neurological deficits in those who are fortunate enough to survive3,4,5,6,7. In South Africa, the Western Cape Province has a high incidence of tuberculosis (TB) (681 per 100,000) in infants and children under the age of 14 years8,9,10. The diagnosis of TBM is a major clinical challenge since this insidious infection often masks nonspecific symptoms that overlap with a spectrum of neurological complications that mimic other forms of meningitis11. Despite the availability of effective treatments, TBM remains a severe disease in progressive stages with slow onset, particularly in resource-constrained settings. The vague clinical manifestations of TBM make the initial distinction of this inflammatory disease a formidable task, especially in the early stages7. A study showed an outcome difference between stages (mortality stage I: 1%, stage II: 10% and stage III: 34% with morbidity of approximately 27% stage I, 41% stage II and 71% stage III)12, and the lack of a definitive diagnostic gold standard in children makes the diagnosis even more complex13. One of the mainstays of TBM diagnosis is the detection of Mycobacterium tuberculosis (M.tb) in cerebrospinal fluid (CSF), which requires a lumbar puncture—an invasive procedure. Traditional diagnostic methods for TBM, such as microscopy with acid-fast staining and culture, are characterized by their limited sensitivity and the long time required to obtain results7,11,14. Polymerase chain reaction (PCR) is a valuable diagnostic tool; however, a meta-analysis of the commonly used Xpert MTB/RIF in TBM showed high accuracy but was unable to rule out TBM15. Similarly, Xpert MTB/RIF Ultra improves the sensitivity of Xpert MTB/RIF but is also unable to exclude TBM16. Currently, no single diagnostic test is sufficient for accurate differential diagnosis of TBM; hence, a combination of multiple diagnostic tests has been proposed.
Biomarkers can be used to diagnose, monitor, or predict the progression of a disease17. However, currently, there are no highly specific diagnostic biomarkers for TBM. Hence, it remains a priority to identify markers that allow timely and accurate diagnosis for optimal treatment outcomes. Efforts to overcome these diagnostic challenges in TBM continue to progress, and researchers are working to improve and refine diagnostic approaches to allow for the earlier identification and subsequent treatment of TBM patients. Urine-based diagnostic tests are becoming increasingly useful for diagnosing TB because they are noninvasive and easy to perform. Urinary TB lipoarabinomannan (TB-LAM) assay detects the presence of LAM, a cell wall protein of M.tb, especially in HIV-infected people with low CD4 counts, and Xpert MTB/RIF Ultra also detects the presence of M.tb DNA in urine18,19,20; however, the results of TB-LAM detection have been suboptimal, and there is a paucity of M.tb DNA in urine. Metabolomics is the study of the complete set of small molecule metabolites in a biological system21. Metabolites are the end products of metabolic pathways, and their levels can be affected by a variety of factors, including diseases22. From our urinary metabolomics studies on TBM, we described the biochemical characterization of the urinary profile of TBM23 and identified markers of dysbiosis24, paving the way for the use of urinary metabolomics for diagnostic purposes. In this investigation, we used 1H-NMR spectroscopy because it is a rapid, highly repeatable, and robust metabolomics analytical platform. By highlighting the diagnostic difficulties associated with TBM, this study aimed to identify biomarkers of TBM with high precision and specificity using noninvasively collected urine.
Materials and methods
Sampling
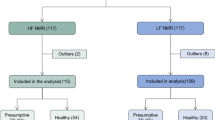
This study included infants and children (13 years or younger) from the Western Cape province of South Africa, a region with a high burden of TB, particularly in children8,9,25,26. Children with clinical symptoms and signs suggestive of meningitis who initially arrived at basic-level and regional health centres were referred to the Department of Paediatrics and Child Health at Tygerberg Hospital in the Western Cape Province of South Africa. According to the universal research case definition criteria for TBM7, all participants in our study (n = 32) were diagnosed with definite TBM and staged according to the revised British Medical Research Council (BMRC) criteria, which were used to classify TBM severity as follows: stage 1, Glasgow Coma Scale (GCS) of 15, without focal neurological deficit; stage 2, GCS of 15 with focal neurological deficit or GCS of 11–14 with or without focal neurological deficit; and stage 3, GCS < 11 with or without focal neurological deficit27. TBM diagnosis was based on the identification of M.tb in CSF by microscopy, culture, and/or commercial nucleic acid amplification tests. All patients with TBM were stabilized in the Tygerberg Hospital Paediatric Neurology ward and treated with a short-intensified anti-M.tb drug regimen that included high-dose rifampicin, isoniazid, pyrazinamide, and ethionamide given for 6 months if HIV uninfected and 9 months if HIV co-infected28,29. Urine samples from TBM patients were collected upon discharge from the hospital; the median time from admission to discharge in the study setting over the past 38 years was 16 days, the interquartile range was 12–23 days24. HIV co-infection considerably complicates an already complex metabolic profile; hence, participants with a positive or unknown HIV status were excluded from this investigation.
Sample collection
This study compared the urine of a control group (n = 39) with that of an experimental group of paediatric patients (n = 32). The controls, anonymously collected with written and informed consent, were classified as ‘normal’ paediatric patients who were admitted to Tygerberg Hospital. Here, ‘normal’ is based on the following benchmark: paediatric patients without meningitis, without neurological symptoms, and from the same geographic location as the patients with TBM. No further clinical data on the controls were available due to ethical constraints. The group of patients with TBM was subdivided into stage 1 TBM (n = 8), stage 2 TBM (n = 10) and stage 3 TBM (n = 14). Clinical symptoms included headache, fever, nausea/vomiting, photophobia, meningeal irritation, and/or neck stiffness9. The first urine sample after hospital discharge was obtained and classified as time point 1 (T1). Table 1 provides a more detailed description of the demographics and clinical information of the TBM patients.
Sample transport, storage, and handling
The urine samples were stored at − 80 °C in a dedicated freezer at Stellenbosch University's Division of Molecular Biology and Human Genetics. These samples were then collectively transferred overnight—frozen on dry ice—to the BSL-3 Laboratory at Human Metabolomics North‒West University, Potchefstroom campus and stored at − 80 °C. On the day that the metabolomics analyses were performed, the samples were allowed to thaw in a biological safety cabinet. A pooled quality control (QC) urine sample was created by aliquoting 50 µL of each sample and pooling them into a single tube.
Sample preparation and 1H NMR analysis
Prior to analysis, all urine samples were thawed to room temperature. To remove any particulates and macromolecules, 600 µL of urine was centrifuged at 12,000 g for 5 min. A 540 µL volume of supernatant was added to 60 µL of NMR buffer solution [1.5 M potassium phosphate solution in deuterium oxide with internal standard trimethylsilyl-2,2,3,3-tetradeuteropropionic acid (TSP); pH 7.4]. The sample was vortexed and centrifuged at 12,000 g for 5 min, and 540 µL of the supernatant was transferred to a 5 mm NMR glass tube and capped. All the samples were loaded in random order onto the NMR autosampler, with the QC samples periodically interspaced. The QC samples were used as a quality assurance method to monitor the analytical variation. The instrument used was a Bruker Avance III HD 500 MHz NMR spectrometer with a triple-resonance inverse (TXI) 1H (15N, 13C) probe head and x, y, and z gradient coils. The 1H spectra were captured as 128 transients at 32 K data points with a spectral width of 12,000 Hz. The sample temperature was kept constant at 300 K, and the H2O resonance was pre-saturated using single-frequency irradiation with a 4 s relaxation delay and an 8 μs excitation pulse. The deuterium signal was used to automatically shim the samples. The resonance line widths of half of the TSP peak were less than 1 Hz, indicating good resolution. Automatic Fourier transformation and phase and baseline correction were performed. The NMR data were processed using Bruker Topspin (V3.5). Bruker AMIX (V3.9.14) was used for identification and quantification30.
Data pre-processing
The 1H NMR spectral output was binned at set widths of 0.02 ppm, and probabilistic quotient normalization31 was applied to account for dilution differences in the urine samples. A data matrix was constructed, with bins in columns and samples in rows and data entries as the spectral data intensity. The bins affected by the suppressed water peak at ~ 4.72 ppm were removed. The coefficient of variation (CV) was checked in all bins for the QC samples, and any bin with a QC CV > 10% was removed. Therefore, all remaining bins were considered reliable (< 10% analytical variation) for biological interpretation.
Statistical analysis
All the statistical analyses were performed with Microsoft Excel, MetaboAnalyst 5.0, an online metabolomics tool (www.metaboanalyst.ca/), and GraphPad Prism 10. The data were log transformed and Pareto scaled for all multivariate analyses [principal component analysis (PCA), orthogonal partial least squares discriminant analysis (OPLSDA), and random forest (RF) alongside OPLSDA to support the results (discriminatory variables were compared)], while all univariate statistics were analysed using nonparametric tests. Unsupervised PCA was used to obtain a visual overview of the data—qualitative assessment of group differentiation and/or clustering. Subsequently, four quantitative statistical tests were performed with defined and strict critical cut-off criteria. A spectral bin was considered important for further investigation of its diagnostic potential if it met at least three of the following four criteria: 1) OPLSDA with a VIP > 1.5 (supported by an RF out-of-the-bag (OOB) value < 0.05), 2) a Wilcoxon t test with an FDR p value < 0.01, 3) a fold change > 5.0, or 4) Hedge’s effect size g value > 1.0. The metabolites within the important bins were identified based on 1D and 2D 1H-NMR pure compound spectral libraries, and the metabolite concentrations were calculated as mmol/mol creatinine. Subsequently, violin plots were used to illustrate the distribution of the frequency of the concentrations of these important metabolites, as well as their medians, interquartile ranges, and statistical significance (p < 0.01, with multiple test corrections). Receiver operating characteristic (ROC) curves of the most important biological variables were generated to assess their diagnostic potential, and correlation analysis was performed to assess whether the biological diagnostic markers and unknowns had any strong correlations (r > 0.6), with statistical significance (p < 0.05) with the metabolites of the medication. Figure 1 illustrates the overall experimental design.
Ethics approval and consent to participate
All research was performed in accordance with relevant guidelines/regulations, and informed consent was obtained from all participants and/or their legal guardians. All research was performed in accordance with the Declaration of Helsinki. Ethical approval was obtained from the Stellenbosch University Health Research Ethics Committee (HREC) (ethics approval number: N16/11/142 and N11/03/061 for TBM cases), the Western Cape Department of Health and Wellness, and the HREC of North‒West University, Potchefstroom campus (ethics approval number: NWU-00063-18-A1-01).
Results and discussion
Quality assurance
A total of 21 QC samples were run at regular intervals throughout the 1H-NMR analysis. Quantitatively, the coefficient of variation (CV) was calculated in Excel for all spectral bins, and the bins with a QC CV greater than 10% (i.e., more than 10% analytical variation, likely due to horizontal shifting in the 1H-NMR spectra in regions that contain peaks that are sensitive to slight pH differences) were removed from the entire data matrix. Hence, we can assure the quality of the remaining data and that we will assess biological variances with negligible analytical variation present in the data. Furthermore, qualitatively, an evaluation of the PCA scores plot (Figure S1) showed that the QCs clustered closely together; therefore, no experimental drift of the analytical pipeline was observed in this study.
Qualitative overview: multivariate statistics
The unsupervised PCA scores plots show natural differentiation between controls and all stages of TBM (Fig. 2A), as well as between individual comparisons (Figs. 2B-D). These qualitative results provide confidence in proceeding with additional quantitative statistical analyses—OPLSDA with RF and univariate assessment.
PCA score plots of (A) Controls vs. all three TBM stages, (B) Controls vs. Stage 1 TBM, (C) Controls vs. Stage 2 TBM, (D) Controls vs. Stage 3 TBM. These PCA scores plots give qualitative confirmation that natural differentiation exists between the control group and each TBM stage, supporting the use of quantitative multivariate statistical analysis.
Quantitative statistical evaluation
Four quantitative statistical tests, with strict critical cut-off criteria, were evaluated in our binned 1H-NMR data set: 1) OPLSDA with a VIP > 1.5 (supported by RF with similar discriminatory bins and OOB values < 0.05–Fig. 3), 2) Wilcoxon t test with FDR p value < 0.01, 3) fold change > 5.0, and 4) Hedge’s effect size g value > 1.0. Comparisons were made between the controls and each stage of the TBM individually. If a bin achieved at least three of the four critical cut-off criteria, then that bin was considered important for the investigation of diagnostic markers. Table 2 shows a summary of these statistically significant quantitative results. As expected, several anti-TB drugs and their metabolites were identified as significant in differentiating TBM patients on treatment from controls. Of interest to our study were the biological elements identified as significant: N-acetylglutamine, succinic acid, citric acid, 5-aminolevulinic acid, 1-methylnicotinamide, and quinolinic acid. Furthermore, 14 unknown compounds that were not found in our spectral library databases were identified as important.
Random forest outputs supporting the OPLSDA results. The results are shown in the left, centre, and right panels for the Stage 1 TBM, Stage 2 TBM, and Stage 3 TBM, respectively. The top part of each panel shows that similar discriminating bins were found in comparison to the OPLSDA results, and the out-of-bag (OOB) values for each panel are < 0.05, supporting the OPLSDA model. The bottom part of each panel shows the Random forest classification: stage 1 TBM cases were misclassified 25% of the time as controls, and no misclassifications occurred for stage 2 TBM, while stage 3 TBM cases were misclassified 14.3% of the time as controls.
Correlations
We also performed a correlation analysis of all the significant variables identified in Table 2 for all patients with TBM (Fig. 4). Of the five biological diagnostic metabolic markers, only 3-hydroxyisovaleric acid showed any strong correlations (r > 0.6), with statistical significance (p-value < 0.05), with other significant variables, namely, propylene glycol and unknowns 4, 6 and 9. Hence, only 3-hydroxyisovaleric acid showed some correlation with a known medication compound (propylene glycol). The other four diagnostic markers, 1-methylnicotinamide, 5-aminolevulinic acid, N-acetylglutamine and methanol, did not show strong and/or statistically significant correlations with other significant variables. These data support our argument that 1-methylnicotinamide, 5-aminolevulinic acid, and N-acetylglutamine are diagnostic markers of TBM and are not directly associated with medication.
Plot showing correlations with an r value greater than 0.6 and a p value < 0.05 for all important quantified variables in Table 2 for all the TBM cases.
Evaluation of diagnostic markers for TBM
Since most of the significant variables annotated in Table 2 were medications and their known metabolites, we reran the statistical pipeline on a reduced data set in which all the bins related to known medications were removed. Furthermore, Fig. 2 and Table 2 show that TBM stages 2 and 3 exhibit similar characteristics. Therefore, to improve the power of the analysis, TBM stages 2 and 3 were combined and labelled ‘severe TBM’, and the remaining cases of stage 1 TBM were labelled ‘mild TBM’. Figure 5 shows the PCA score plots for this revised data set—controls vs mild TBM show some overlap, and controls vs severe TBM show complete natural differentiation. Random Forest showed that mild TBM could not be successfully classified using the current multivariate metabolic model (71.4% misclassified and OOB = 0.109). However, the random forest misclassified only one severe TBM case (4% misclassification) and had an OOB value < 0.05. Therefore, our multivariate metabolic model can successfully classify severe but not mild TBM. Furthermore, compared with the OPLSDA results, the random forest results showed similar discriminating bins.
Based on univariate statistics, using the Wilcoxon t test with an FDR p value < 0.01 and Hedge’s effect size g value > 2.0, several bins were identified as statistically and practically significant in differentiating controls from mild and severe TBM patients. Table 3 shows these quantitative statistical values, along with the annotations of the metabolites.
Five biological metabolites from Table 3 (1-methylnicotinamide, 3-hydroxyisovaleric acid, 5-aminolevulinic acid, N-acetylglutamine and methanol) were quantified (mmol/mol creatinine); the violin plots are presented in Fig. 6. ROC curve analysis of the diagnostic potential of these five metabolites was carried out based on the area under the curve (AUC), sensitivity, and specificity (Fig. 7 and Table 4).
Violin plots depicting the five significant metabolites (1-methylnicotinamide, 3-hydroxyisovaleric acid, 5-aminolevulinic acid, N-acetylglutamine, and methanol) in mild and severe TBM. The concentration is given as mmol/mol creatinine in the y-axis. Statistical significance (FDR p < 0.01) is indicated by *.
As shown in Fig. 7, all five metabolites have diagnostic potential. 1-Methylnicotinamide had AUCs of 0.99 and 0.95 for mild TBM and severe TBM, respectively; 3-hydroxyisovaleric acid had AUCs of 0.89 and 0.99 for mild TBM and severe TBM, respectively; 5-aminolevulinic acid had AUCs of 0.94 and 0.99 for mild TBM and severe TBM, respectively; N-acetylglutamine had AUCs of 0.99 and 0.99 for mild TBM and severe TBM, respectively; and methanol had AUCs of 0.92 and 0.98 for mild TBM and severe TBM, respectively. Table 4 shows the sensitivity of the specific cut-off concentrations, all with a specificity of 97.5% and a 95% confidence interval of 87%-100%.
1-Methylnicotinamide and N-acetylglutamine have 100% sensitivity as early diagnostic markers of mild TBM and > 90% sensitivity for severe TBM, while 3-hydroxyisovaleric acid has greater sensitivity for severe TBM, and 5-aminolevulinic acid has equivalent sensitivity as a diagnostic marker for both mild and severe TBM. Therefore, 1-methylnicotinamide, 3-hydroxyisovaleric acid, 5-aminolevulinic acid and N-acetylglutamine show potential as diagnostic markers of TBM but do not have sufficient power to differentiate mild-stage from severe-stage TBM. Methanol showed statistical significance (Fig. 6) but lacked diagnostic sensitivity for use as a diagnostic marker for TBM.
Biological context for the four TBM diagnostic metabolites
Three (1-methylnicotinamide, 3-hydroxyisovaleric acid, and N-acetylglutamine) of the four urinary metabolic markers of TBM, as described above, have been discussed in terms of their biological context in our previous study—see23 for more details. Here, we report 5-aminolevulinic acid as being a significant urinary metabolite in TBM for the first time. 5-Aminolevulinic acid has been defined as a non-proteinogenic amino acid, meaning it is not used in the synthesis of proteins. This endogenous metabolite is biosynthesized in the body by the condensation of glycine and succinyl-CoA, catalysed by the enzyme 5-aminolevulinate synthase. 5-Aminolevulinic acid is required in the metabolism of heme in the body and it is involved in maintaining normal mitochondrial function.
To the best of our knowledge, this is the first time that 5-aminolevulinic acid has been identified in any type of sample matrix collected from TBM patients. However, it is important to state that 5-aminolevulinic acid is not a specific marker of TBM. 5-Aminolevulinic acid has been identified in the urine of patients following an acute attack of porphyria32, as well as in patients diagnosed with the tyrosinemia type I33. Moreover, due to its anti-inflammatory and immunoregulatory properties34, 5-aminolevulinic acid has been identified as a novel therapeutic for inflammatory bowel disease35, and has potential in treating type II diabetes mellitus36, and COVID-1937.
Hence, 5-aminolevulinic acid is likely an endogenous metabolic marker of the immune response to severe inflammation (severe and chronic neuroinflammation is a classic symptom of TBM). But, it is also important to note that some studies38 have identified 5-aminolevulinic acid as being neurotoxic. Therefore, future studies should examine the levels of this metabolite in the brain of TBM patients.
TBM stage differentiation
In addition to identifying potential diagnostic markers in each TBM stage (comparing TBM patients with controls), the TBM stages were also compared to each other to determine whether any urinary metabolic marker could differentiate the TBM stages. Based on the PCA score plot (Figure S2), no qualitative differentiation could be detected between the stages of TBM (i.e., the metabolic urinary 1H-NMR profiles could not differentiate the stage of TBM). Quantitative statistical data were checked and did not show statistically significant differences. Therefore, no urinary metabolites could differentiate the stages of TBM.
Unknown compounds—Biological or medication related?
Ten significant unknown compounds were identified, as shown in Table 3, and quantified. Based on the correlation data (Fig. 4), none of the unknowns were correlated with the identified anti-TB medications. However, unknowns 6, 9 and 10 were significantly correlated with propylene glycol and may be related to other forms of medication. The other unknowns (1, 2, 3, 4, 5, 7 and 8) had no correlation with medication and may be of biological origin.
Speculation of the identities of unknowns
Based on the 2D JRES and COSY NMR spectral data, a doublet at 1.55 ppm (Unknown 2) was correlated with a quartet at 5.19 ppm (Unknown 8) (see Figure S3 in the supplementary information for more details). This 1H-NMR chemical shift information implies that a terminal methyl group is present next to a double bond CH component. This chemical information suggests that one of the unknowns is likely to have a chemical structure similar to that of a short-chain unsaturated carboxylic acid. The 1H-NMR spectra of several suspected short-chain unsaturated carboxylic acids were investigated, including propionic acid (C3), butyric acid (C4), and isobutyric acid (C4). However, while the patterns were similar, none of these pure compound spectra matched the unknown spectra sufficiently. We suspect that the unknown compound that contains a doublet at 1.55 ppm and a quartet at 5.19 ppm is a short branched-chain organic acid that is methylated. Short branched-chain organic acids originate from micro-organisms, and these unknowns unique to the TBM cases could be degradation products of the unique cell wall of M.tb. It is our recommendation that additional cohorts of urine samples collected from treated TBM patients should undergo targeted gas chromatography‒mass spectrometry (GC‒MS) metabolomics analyses, with a focus on short and branched-chain organic acids that are methylated.
Conclusions
The identification of urinary biomarkers for TBM is a promising area of research. Our multivariate metabolic model can successfully classify severe but not mild TBM. The four metabolites (1-methylnicotinamide, 3-hydroxyisovaleric acid, 5-aminolevulinic acid and N-acetylglutamine) identified in this study show good diagnostic potential for severe TBM (stage 2 and 3 TBM patients combined), but they have not yet been established as definitive diagnostic tools. Future studies are needed to validate these biomarkers, including the significant unknowns identified in this study, to determine their clinical utility and explore their specificity and sensitivity for the diagnosis of TBM. Further research is needed, but we believe that urine from TBM patients will potentially aid in the earlier diagnosis and treatment of this disease.
Data availability
All metabolomics research data associated with this project will be made available online in a data repository once the project is completed and all the data are published. On special request, immediate subsets of data from the study published here can be made accessible via email: nmr.nwu@gmail.com.
References
Hektoen, L. The vascular changes of tuberculous meningitis, especially the tuberculous endarterities. J. Exp. Med. 1(1), 112–163 (1896).
Wolzak, N. K. et al. The Changing profile of pediatric meningitis at a referral centre in Cape Town. S. Afr. J. Trop. Pediatr. 58(6), 491–495 (2012).
Kotzbauer, D. et al. Etiology and laboratory abnormalities in bacterial meningitis in neonates and young infants. Clin. Pract. 7(2), 943 (2017).
Hristeva, L. et al. Prospective surveillance of neonatal meningitis. Arch. Dis. Child. 69((1 Spec No)), 14 (1993).
Okike, I. O. et al. Incidence, etiology, and outcome of bacterial meningitis in infants aged & lt;90 days in the united kingdom and republic of Ireland: Prospective, enhanced national population-based surveillance. Clin. Infect. Dis. 59(10), e150–e157 (2014).
van den Bos, F. et al. Tuberculous meningitis and miliary tuberculosis in young children. Trop. Med. Int. Health 9(2), 309–313 (2004).
Marais, S. et al. Tuberculous meningitis: A uniform case definition for use in clinical research. Lancet Infect. Dis. 10(11), 803–812. https://doi.org/10.1016/S1473-3099(10)70138-9 (2010).
Donald, P. et al. Pediatric meningitis in the Western cape province of South Africa. J. Trop. Pediatr. 42(5), 256–261. https://doi.org/10.1093/tropej/42.5.256 (1996).
van Toorn, R. & Solomons, R. Update on the diagnosis and management of tuberculous meningitis in children. Semin. Pediatr. Neurol. 21(1), 12–18. https://doi.org/10.1016/j.spen.2014.01.006 (2014).
Van Rie, A. et al. Childhood tuberculosis in an urban population in South Africa: Burden and risk factor. Arch. Dis. Child. 80(5), 433 (1999).
Foppiano Palacios, C. & Saleeb, P. G. Challenges in the diagnosis of tuberculous meningitis. J. Clin. Tuberc. Other Mycobact. Dis. 20, 100164 (2020).
Chiang, S. S. et al. Treatment outcomes of childhood tuberculous meningitis: A systematic review and meta-analysis. Lancet Infect. Dis. 14(10), 947–957 (2014).
Handryastuti, S. et al. Development of clinical-based scoring system to diagnose tuberculous meningitis in children. Arch. Dis. Child. 108(11), 884–888 (2023).
Thwaites, G. E., van Toorn, R. & Schoeman, J. Tuberculous meningitis: More questions, still too few answers. Lancet Neurol. 12(10), 999–1010 (2013).
Hernandez, A. V. et al. Diagnostic accuracy of Xpert MTB/RIF for tuberculous meningitis: Systematic review and meta-analysis. Trop. Med. Int. Health. 26(2), 122–132 (2021).
Donovan, J. et al. Xpert MTB/RIF ultra for the diagnosis of tuberculous meningitis: A small step forward. Clin. Infect. Dis. 71(8), 2002–2005 (2020).
Ahmad, A., Imran, M. & Ahsan, H. Biomarkers as biomedical bioindicators: Approaches and techniques for the detection, analysis, and validation of novel biomarkers of diseases. J. Pharm. 15(6), 1630 (2023).
Simieneh, A. et al. Combination of Xpert® MTB/RIF and DetermineTM TB-LAM Ag improves the diagnosis of extrapulmonary tuberculosis at Jimma university medical center, Oromia Ethiopia. PloS One 17(2), e0263172 (2022).
Suwanpimolkul, G. et al. Utility of urine lipoarabinomannan (LAM) in diagnosing tuberculosis and predicting mortality with and without HIV: Prospective TB cohort from the Thailand Big City TB research network. Int. J. Infect. Dis. 59, 96–102 (2017).
Peter, J. et al. Urine for the diagnosis of tuberculosis: Current approaches, clinical applicability, and new developments. Curr. Opin. Pulm. Med. 16(3), 262–270 (2010).
Wishart, D. S. Metabolomics for investigating physiological and pathophysiological processes. Physiol. Rev. 99(4), 1819–1875 (2019).
Qiu, S. et al. Small molecule metabolites: Discovery of biomarkers and therapeutic targets. Sig. Transduct. Target Ther. 8(1), 132 (2023).
Isaiah, S. et al. Urinary metabolic characterization of advanced tuberculous meningitis cases in a South African paediatric population. Front. Mol. Biosci. https://doi.org/10.3389/fmolb.2024.1253983 (2024).
Isaiah, S. et al. Urinary markers of Mycobacterium tuberculosis and dysbiosis in paediatric tuberculous meningitis cases undergoing treatment. Gut Pathog. 16(1), 14 (2024).
Kanabus, A., “Information about Tuberculosis”, GHE, 2020, https://www.tbfacts.org/about-us/ (2020).
Mandalakas, A. M. et al. Tuberculosis prevention in children: A prospective community-based study in South Africa. Eur. Respir. J. 57(4), 2003028. https://doi.org/10.1183/13993003.04438-2020 (2021).
BMRC. STREPTOMYCIN treatment of tuberculous meningitis. Lancet 1(6503), 582–596 (1948).
van Toorn, R. et al. Value of different staging systems for predicting neurological outcome in childhood tuberculous meningitis. Int. J. Tuberc. Lung Dis. 16(5), 628–632 (2012).
World Health Organization. https://www.who.int/news/item/26-08-2021-who-issues-rapid-communication-on-updated-guidance-for-the-management-of-tb-in-children-and-adolescents (2021).
Erasmus, E. et al. A laboratory approach for characterizing chronic fatigue: What does metabolomics tell us?. Metabolomics 15(12), 1–11. https://doi.org/10.1007/s11306-019-1620-4 (2019).
Dieterle, F. et al. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures application in 1H NMR metabonomics. J. Anal. Chem. 78(13), 4281–4290 (2006).
Marsden, J. T. & Rees, D. C. Urinary excretion of porphyrins, porphobilinogen and δ-aminolaevulinic acid following an attack of acute intermittent porphyria. J. Clin. Pathol. 67(1), 60–65 (2014).
Mitchell, G. et al. Neurologic crises in hereditary tyrosinemia. N. Engl. J. Med. 322(7), 432–437 (1990).
Fujino, M. et al. 5-Aminolevulinic acid regulates the inflammatory response and alloimmune reaction. Int. Immunopharmacol. 37, 71–78 (2016).
Yadav, V. et al. 5-Aminolevulinic acid as a novel therapeutic for inflammatory bowel disease. Biomedicines 9(5), 578 (2021).
Al-Saber, F. et al. The safety and tolerability of 5-aminolevulinic acid phosphate with sodium ferrous citrate in patients with type 2 diabetes mellitus in Bahrain. J. Diabetes Res. 2016(1), 8294805 (2016).
Sakurai, Y. et al. 5-amino levulinic acid inhibits SARS-CoV-2 infection in vitro. Biochem. Biophys. Res. Commun. 545, 203–207 (2021).
Emanuelli, T. et al. Inhibition of adenylate cyclase activity by 5-aminolevulinic acid in rat and human brain. Neurochem. Int. 38(3), 213–218 (2001).
Funding
Open access funding provided by North-West University. Funding for this work was provided by the National Research Foundation (NRF) of South Africa, the Dutch Organization for Internationalization in Education (NUFFIC) [grant number UID: 120209], and Mr. Willem Backhuys Roozeboomstichting in the Netherlands.
Author information
Authors and Affiliations
Contributions
Conceptualization: Shayne Mason; Data curation: Simon Isaiah and Shayne Mason; Formal analysis: Simon Isaiah, Johan Westerhuis and Shayne Mason; Funding acquisition: Martijn van der Kuip and A. Marceline Tutu van Furth; Investigation: Simon Isaiah; Methodology: Simon Isaiah, Johan Westerhuis, Du Toit Loots, Sabine van Elsland and Shayne Mason; Project administration: Martijn van der Kuip and A. Marceline Tutu van Furth; Resources: Regan Solomons; Supervision: Du Toit Loots, Martijn van der Kuip and A. Marceline Tutu van Furth and Shayne Mason; Writing – original draft: Simon Isaiah; Writing – review & editing: Simon Isaiah, Johan Westerhuis, Du Toit Loots, Regan Solomons, A. Marceline Tutu van Furth, Sabine van Elsland, Martijn van der Kuip and Shayne Mason.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Isaiah, S., Westerhuis, J.A., Loots, D.T. et al. The diagnostic potential of urine in paediatric patients undergoing initial treatment for tuberculous meningitis. Sci Rep 14, 19471 (2024). https://doi.org/10.1038/s41598-024-70419-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70419-1
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.