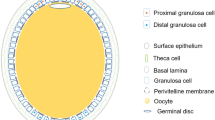
Abstract
Much remains unknown about the reproductive physiology of southern white rhinoceros (SWR) and the effect of ovarian stimulation prior to ovum pickup (OPU) have not been fully elucidated. Granulosa cells (GC) provide valuable insight into follicle growth and oocyte maturation status. The goals of this study were to evaluate transcriptomic changes in GC from three stages of follicle development and to identify biomarkers possibly associated with follicular growth and maturation as a result of ovarian stimulation. GC collected from SWRs following OPU were assigned stages based upon follicle size. Total RNA was isolated, and cDNA libraries were prepared and sequenced on a NovaSeq 6000. All bioinformatics analyses were performed utilizing the Galaxy web platform. Reads were aligned to CerSimCot1.0, and the manual curation was performed with EquCab3.0. Overall, 39,455 transcripts (21,612 genes) were identified across follicle stages, and manual curation yielded a 61% increase in gene identification from the original annotation. Granulosa cells from preovulatory follicles expressed the highest number of unique transcripts. The following seven biomarkers were determined based upon cluster analysis and patterns of expression: COL1A1, JMY, FBXW11, NRG1, TMPO, MACIR and COL4A1. These data can be used to potentially evaluate the effects of different ovarian stimulation protocols on follicle dynamics, improve OPU results, and support conservation efforts in this species.
Similar content being viewed by others
Introduction
Wild rhinoceroses are facing rapid population decline worldwide due to poaching and habitat loss. To preserve these keystone species, assisted reproductive technologies (ARTs) are indispensable. ARTs have been developed and applied to rhinoceros species with variable success rates due to the unique reproductive characteristics of each species1,2,3,4,5,6,7,8,9. In general, the success rate of ART for rhinoceros species is lower than that for domestic species10,11,12,13,14, mainly due to the lack of fundamental reproductive knowledge, challenges in adapting protocols for domestic species, and limited opportunities to collect gametes. In the past decade, considerable conservation effort has been directed toward the rescue of the near-extinct northern white rhinoceros (NWR), with successful application of ARTs and the production of blastocysts in vitro from the last two female NWRs1,5. The closely related subspecies, the southern white rhinoceros (SWR), has been key to the success of developing these techniques for the NWR2,6,15. Moreover, recent studies have shown that the NWR and SWR populations are distinct but genetically compatible for future efforts at genetic rescue; therefore, the less threatened SWR population is an ideal model for developing new reproductive strategies to help this species15.
Recent work has explored similarities and differences in the anatomy and reproductive physiology of the rhinoceros and the domestic horse due to their taxonomic proximity16. The mare and the rhinoceros both have bicornuate uteri and similar ovarian structures, such as ovarian and follicle sizes. In addition, the mare and rhinoceros share similar hormonal estrous cycle features (estradiol, progesterone and LH)16. Therefore, based upon their reproductive similarities, the mare is a valuable candidate model for rhinoceros reproductive questions.
With the recent success of ovum pick-up (OPU) in the SWR1,2,5,17, it is imperative to fully understand the estrous cycle to develop optimal ovarian stimulation protocols. Ovarian ultrasonography has enabled the identification of cycle lengths in the SWR3,4,18, but the mechanisms of follicle growth, oocyte maturation, ovulation, and embryo development are only partly understood2. Like in horses and cows, follicle growth begins with increasing FSH levels in response to pulsatile GnRH stimulation16,19. Once selection of a dominant follicle occurs, FSH levels decrease, and all nondominant follicles begin to undergo atresia. The dominant follicle becomes luteinizing hormone (LH)-dependent and will ultimately ovulate or plateau in the presence, or absence, of progesterone (i.e., the presence or absence of a corpus luteum). The developmental competence of an oocyte in vitro is strongly correlated with follicle development19. Therefore, proper ovarian stimulation prior to OPU is crucial for aspirating the oocytes with the highest developmental potential.
Ovarian stimulation is routinely performed in horses and cattle prior to OPU20,21. In horses, although not widely successful, stimulation to retrieve mature oocytes during OPU is performed22,23,24. This method of ovarian stimulation in horses utilizes GnRH analogs but results in only one or two preovulatory follicles. An alternative method of OPU for horses is to aspirate immature oocytes for in vitro maturation25. Due to the limited opportunities to perform OPU on SWRs, aspirating a single preovulatory follicle is not ideal; therefore, stimulation methods for producing multiple large follicles are necessary. In cattle, superstimulation protocols utilize both FSH and LH, depending on the desired outcome [multiple large follicles (OPU) or timed artificial insemination (ovulation)]26. For both methods, a CIDR that secretes progesterone may be used to synchronize estrus prior to stimulation27. After CIDR removal, for OPU, multiple FSH injections are given, resulting in an increased number of large follicles. For timed artificial insemination, after CIDR removal, injections of prostaglandin and GnRH are provided to synchronize ovulation26.
In the SWR, a single GnRH injection successfully induces ovulation when the follicle length reaches 30–35 mm18. In previous studies, the administration of the GnRH analog Histrelin increased follicle numbers with alternate day injections prior to OPU1,5. However, the effects of ovarian stimulation on follicle gene expression in rhinoceros are unknown. Given the influence of the follicle stage on oocyte meiotic competence, it is crucial to understand the gene expression patterns associated with these stages and how they are influenced by ovarian stimulation prior to OPU.
With limited access to rhinoceros oocytes, granulosa cells (GC) can provide insight into the physiologic status of follicles and oocytes as they undergo dynamic changes during exogenous ovarian stimulation28,29,30. Our previous study identified the transcriptomic signature of a pool of granulosa cells from the SWR, but to date, no studies have evaluated GC across follicle stages2. Little has been done in horses to understand gene expression patterns in GC across different follicle stages; hence, cattle are another valuable domestic model for providing comparative transcriptomic data generated from the SWR. A thorough analysis of bovine GC across different follicle stages and correlating gene expression patterns to oocyte and meiotic competence has been completed, and biomarkers for the physiologic status of the oocytes have been identified19,28,31,32.
It is especially important to determine biomarkers for these stages to develop appropriate ovarian stimulation protocols for ovum pickup (OPU). The aim of this study was to develop a stimulation protocol that results in the largest number of follicles at the appropriate stage for collecting oocytes capable of in vitro meiotic resumption after OPU. The goals of this study were to identify transcriptional changes across follicle development and develop a panel of biomarkers for use in future studies to evaluate transcriptional changes due to different ovarian stimulation protocols. First, we determined the transcriptional signatures of follicles in different stages of development in the SWR. This allowed us to identify gaps in knowledge of the reproductive physiology and ovarian function of the SWR, determine key drivers of follicle development and oocyte maturation in this species, and ultimately develop an in vitro system that best emulates in vivo conditions.
Results
Part 1. Additions to the NWR genome
The northern white rhinoceros (NWR) genome is newly assembled and annotated15 but still contains many gaps, primarily in the annotation. Manual curation of both the sequences identified in the annotation and those identified via de novo sequencing with Stringtie was performed to improve the understanding of the data generated with this study. Manual curation involved searching for the specific sequences identified in the dataset through NCBI BLAST33 and comparing the homology to the SWR and the domestic horse (as the genome is highly homologous and well annotated). If a sequence was at least 80% homologous to a gene identified via NCBI, that gene was assigned to that sequence. In this study, improvements were made to provide proper gene nomenclature, assignment of genes to scaffolds and sequences, and identification of genes that were not included in the original annotation. The original annotation included 14,274 transcripts that were expressed in granulosa cells according to this analysis. Throughout the annotation, the protein nomenclature was corrected and changed to gene nomenclature. Through manual curation, 6763 transcripts were corrected from protein to gene nomenclature, with an overall improvement in nomenclature of 47%. In addition, 70% of the transcript sequences were reassigned to different genes after being searched for gene homology. Through de novo sequencing, an additional 7429 genes were added to the original annotation, with an increase of 61% more genes from the original annotation (Fig. 1). These improvements in the annotation will be incorporated in a follow-up annotation to be made publicly available with the genome. Supplemental Table 1 contains all the sequences identified as a gene as well as their corresponding gene reference.
Part 2. Analysis of granulosa cells
Prior to analysis, principal component analysis (PCA) was performed to determine the variability of the samples within each follicle stage and between the different stages. Figure 2 shows that within each stage, the replicates had very little variability between the two samples at each follicle stage. In addition, there was clear clustering of each stage, independent of the other stages. A heatmap showed the same trend, with granulosa cells (GC) from preovulatory (P) follicles being in their own expression group and GC from growing (G) and dominant (D) follicles clustering closer together (Fig. 2).
Across all the samples (n = 6), 39,455 different transcripts were detected, which corresponded to 21,621 genes, with multiple genes expressing multiple transcripts (Supplemental Table 1). The first analysis was to determine the genes that were specific to different follicle stages (growing, dominant, or preovulatory). The growing follicles contained 427 unique transcripts (corresponding to 385 genes), with only 8% of the transcripts identified being unannotated. A total of 812 transcripts were specifically identified in the dominant follicle stage, resulting in a total of 677 genes, and 104 transcripts (13%) were unannotated. Preovulatory follicles contained the largest number of follicle stage-specific transcripts, 945, corresponding to 433 genes. Of the 945 transcripts identified, 484 (51%) were unannotated. Importantly, the most highly expressed genes specific to each follicle type were associated with different transcription factors. The canonical pathways most enriched in growing follicles were involved in the regulation of TP53 activity through phosphorylation, the regulation of TP53 expression and degradation, the signaling of VEGF, the cell cycle checkpoint, and HDR through homologous recombination or single-strand annealing. The genes in preovulatory follicles targeted the following canonical pathways: signaling via the insulin receptor, iron uptake and transport, the iron homeostasis signaling pathway, amino acid regulation of mTORC1, and the adrenergic receptor signaling pathway.
The next analyses evaluated the differentially expressed genes (DEG) between each follicle category. The comparison with the least number of differentially expressed transcripts was growing versus dominant, with 855 transcripts (resulting in 802 different genes). The genes annotated with the greatest significant differences were RNPC3 and ZDHHC5, both more highly expressed in growing follicles. Figure 3 shows the representative volcano plot for this analysis, highlighting the genes with the largest significant differences. Table 1 shows the top 10 genes differentially expressed between growing and dominant follicles.
Volcano plots representing the three primary comparisons: growing (G) vs. dominant (D), D vs. preovulatory (P), and G vs. P. The genes marked have the greatest significant differences between stages. In the comparison, the red dots are more highly expressed in the first stage, and the green dots are more highly expressed in the second stage.
When comparing growing and preovulatory follicles, the largest number of transcripts (4581) were significantly differentially expressed, corresponding to 3980 genes. The genes annotated with the greatest significant differences were CDH11 and TMEM51 (both of which were more highly expressed in growing follicles, as shown in Fig. 3). Table 2 lists the top 10 significantly differentially expressed transcripts.
The final comparison was dominant versus preovulatory follicles. There were 4315 transcripts differentially expressed within this comparison (3704 genes). The annotated genes most differentially expressed were HSPG2 and CFAP52, both of which are more highly expressed in dominant follicles. Table 3 lists the top 10 differentially expressed transcripts, and Fig. 4 shows the genes with the most differential expression. Supplemental Table 2 details all the differentially expressed transcripts identified in each comparison.
Top canonical pathways for each comparison. The threshold was set to − log(p-value) > 1.3, but the z score thresholds were varied due to the large number of canonical pathways. (A) Represents growing vs. dominant follicles with an absolute z score ≥ 2. (B) Represents growing vs. preovulatory follicles with an absolute z score ≥ 5. (C) Represents dominant vs. preovulatory follicles with an absolute z score ≥ 5.
Although not specific to any follicle type, MACIR was the most highly expressed gene in each follicle category. Table 4 contains the most highly expressed genes in each category, and interestingly, the same genes are present in multiple follicle types.
Further evaluation of the DEG in each comparison led to the identification of the most enhanced canonical pathways for each comparison. Table 5 shows the canonical pathways affected by the most transcripts in each category, and the canonical pathways with the greatest fold changes and z scores are shown in Fig. 4.
To understand the expression patterns across follicle types, a cluster analysis was performed, revealing seven unique patterns of expression (Fig. 5). Each cluster contained varying numbers of transcripts and differentially expressed transcripts within different comparisons, ranging from 173 transcripts (cluster four) to 1620 transcripts (cluster five). The expression of 173 transcripts in cluster four increased from G to D to P, with the largest number of differentially expressed transcripts occurring between the G and P follicle stages. Pathways associated with this cluster included focal adhesion, the T-cell receptor signaling pathway, the Pi3K-Akt signaling pathway, and the MAPK signaling pathway. This cluster closely resembled the gene expression patterns associated with follicle differentiation. Cluster five contained the largest number of transcripts (1620) whose expression largely decreased from the D to the P follicle stage. The pathways associated with these transcripts include cellular senescence, EGFR tyrosine kinase inhibitor resistance, and endocytosis.
Discussion
This is the first study evaluating the transcriptomic changes in growing (G), dominant (D) and preovulatory (P) follicles in southern white rhinoceros (SWR). This information is needed to advance assisted reproductive technologies (ARTs) by determining appropriate methods for manipulating ovarian function prior to oocyte collection to obtain oocytes with the highest developmental potential. Through this analysis, we generated candidate gene markers for the physiological status of the follicle at the time of OPU.
Although this study was performed on SWR granulosa cells (GC), the SWR genome is incomplete and contains large gaps in annotation. Therefore, the northern white rhinoceros (NWR) genome and currently available annotations are the only resources for generating transcriptomic data on white rhinoceroses, but extensive manual curation is still necessary15. In addition, the NWR and SWR are genetically similar at the chromosomal and mitochondrial genome levels, enabling the NWR genome to be used for this analysis15. The original annotation for the NWR contained 14,274 different transcripts (12,252 genes) that were expressed in the GC from this dataset. After adding the transcripts from de novo sequencing, a total of 39,455 different transcripts (21,621 genes) were expressed. This was an overall improvement of 119% in transcripts and 61% in genes compared to the original annotation. In addition, it is important to note that through manual curation, multiple transcripts were reassigned to different genes, and transcripts with unknown associations were identified. This approach resulted in a substantial improvement to the available annotation, and these new annotations will be available publicly in the future. Due to their scarcity, reproductive cells are often underrepresented when generating annotations for genomes. Many new genes were annotated in the granulosa cells due to the massive transcriptional changes that occurred within the cells over time and their dynamic behavior. This additional information to the annotation will benefit other researchers in reproductive physiology in this species, and it emphasizes the importance of using reproductive cells, such as granulosa cells, as a resource for conservation research and genome improvement and accuracy. One of our objectives was to identify the genes most highly expressed across the follicle stages. Interestingly, all follicle stages had the same top three most highly expressed genes. These three genes were involved in macrophage immunometabolism regulator (MACIR), MSTRG15261.1, and collagen type I alpha 1 chain (COL1A1). The most highly expressed gene, MACIR, formally known as C5ORF30, has a well-documented role in modulating the immune response and regulating macrophage function34,35. In addition, blocking MACIR enhances the expression of genes involved in regulating cell migration, adhesion, and angiogenesis36. In cattle GC, MACIR has been shown to be upregulated in follicles undergoing late-stage atresia37. This is a potential indicator that the follicles in this study were under stress and already progressed through advanced, irreversible atresia. The second most highly expressed gene, MSTRG15261.1, was found to be unannotated in the SWR and in the domestic horse. The third most highly expressed gene was COL1A1, which is the most abundant collagen in the mammalian ovary and is regulated by transforming growth factor beta 1 (TGF-β1)38. In addition, COL1A1 promotes the secretion of estrogen while also inhibiting the increase in progesterone, resulting in follicle growth39,40. Most importantly, COL1A1 plays an important role in oocyte maturation and embryo development through its association with the PI3K/Akt signaling pathway in mice and pigs41,42,43,44. Interestingly, the most highly expressed gene was involved in follicle death, and the third highest was involved in follicle growth. This could indicate that prior to OPU, follicles received mixed signals during ovarian treatment.
Cluster analysis was performed to generate gene expression patterns across the follicle stages to identify genes that follow conventional literature for these stages and those that are not. Due to the limited opportunities to perform OPU in SWRs, the current preferred approach is to stimulate the growth of multiple follicles to increase the oocyte recovery rate. Therefore, unstimulated animals were not considered for this elective procedure in our study. Due to the lack of an unstimulated rhinoceros for comparison, the gene expression patterns were compared to published literature generated from unstimulated cows28. The two main cluster types we focused on were patterns associated with follicle growth (clusters four and seven) and differentiation (clusters three, five, and six). For cluster three, the gene expression levels were consistent between G and D and increased from D to P; therefore, these genes are associated with follicle differentiation. Of the top 20 genes from this cluster, 13 have known expression patterns across follicle stages28; interestingly, five of those genes should be downregulated in P, yet their expression is increased in our dataset. COL4A1 (collagen type IV alpha 1 chain) was found to be differentially expressed in this cluster compared to cattle data28. In cattle, COL4A1 is a gene that stays in relatively low abundance throughout the follicle stages, with a decrease in P and an increase only observed in atretic follicles28. COL4A1 is directly regulated by forkhead box L2 (FOXL2), a transcription factor specifically expressed in granulosa cells45. High levels of FOXL2 decrease COL4A145, which is required for the proliferation of GC, allowing follicle remodeling, and should remain high during folliculogenesis to allow for proliferation and differentiation46. In our data, we observed a significant decrease in FOXL2 expression between D and P, which led to a significant increase in COL4A1 expression between D and P, a sign of late-stage atresia associated with follicular demise31. In addition, high levels of collagen in the follicle basement membrane have been linked to an imbalance in gonadotropin stimulation47. It is hypothesized that this delay in maturation due to increased collagen may lead to asynchrony during maturation and negative effects on oocyte developmental competence47. Therefore, we can hypothesize that high levels of COL4A1 in our SWRs could be caused by incomplete or suboptimal stimulation prior to OPU.
Junction mediating and regulatory protein p53 cofactor (JMY) is a transcriptional cofactor and an actin nucleation factor48,49. Actin nucleation occurs during oocyte polarization and affects the microtubule and microfilament cytoskeleton by activating Arp 2/3 (for actin remodeling) and the assembly of filaments in the cytoskeletal spindle48,50,51. If JMY is not present, the spindle fails to migrate to the cortex, and the oocyte arrests with a centrally located spindle, highlighting the crucial role of JMY during peripheral spindle migration during oocyte maturation49. In both mouse and porcine oocytes, JMY decreases during oocyte maturation49,52. This same pattern of expression was observed in cluster five of our dataset, with JMY being highly significant within that cluster.
Cluster four included the genes whose expression increased from G to D to P. A gene of interest in this pattern was F-box and WD repeat domain containing 11 (FBXW11) due to its regulation by estradiol, its role in meiotic resumption, and its normal pattern of expression compared to our data. FBXW11 is regulated by many different factors, one of which is helicase for meiosis 1 (HFM1)53. Low levels of HFM1 result in high levels of FBXW11, which corresponds to the pattern of expression we observed for both of these genes; however, according to the literature in mice, HFM1 expression should be high for oocytes to resume meiosis53. In addition, FBXW11 is regulated by estradiol, as indicated by high levels of estradiol (as found in late stage developing follicles), which results in low levels of FBXW1128,54. The primary function of FBXW11, formally known as β-TRCP2, is to degrade cell division cycle 25A (CDC25A), which is crucial for the resumption of meiosis (at the appropriate time)55,56. CDC25A must be downregulated for oocytes to be released from MII arrest57, which we observed in the rhinoceros data. Therefore, from the literature, it appears that the timing of the increase in FBXW11 is important in relation to the resumption of oocyte meiosis.
NRG1 (neuregulin 1) is a member of the EGF-like factor family that is induced by gonadotropins58,59. There is a dramatic increase in NRG1 expression due to LH induction, which regulates proper oocyte maturation and developmental competence60. Recent literature has shown that NRG1 supplementation during IVM can promote oocyte nuclear maturation and blastocyst development and hatching in cows60. During development, amphiregulin (AREG) and NRG1 work synergistically (AREG is an accelerator, and NRG1 is a brake) to ensure that the required temporal changes within the oocyte occur for proper meiotic resumption and competence59. It is important that these genes are balanced to ensure that oocytes have high developmental competence. According to our data, NRG1 is part of cluster five and remains consistent from G to D but decreases from D to P, which is the opposite of what the literature states regarding high levels of NRG1 being needed for oocyte competence acquisition28,60. NRG1 is a novel factor that may impact oocyte maturation; therefore, it should be considered a biomarker in GC for oocyte maturation competence and should be added to in vitro maturation medium.
Cluster six was classified as a cluster related to differentiation because there was a decrease in gene expression from G to D and a large increase from D to P. A gene that followed this cluster pattern but, based upon the literature should not be, was thymopoeitin (TMPO), also known as lamina-associated polypeptide 2 (LAP2)61. According to both bovine and porcine data, TMPO decreases as follicle progression progresses28,62, which we previously reported from G to D but subsequently deviates with an increase from D to P. A specific region in TMPO [regions 32–36 known as thymopentin (TP5)] has been studied extensively due to its role in the activation of the pluripotency marker LIN28a in oocytes63,64; therefore, high levels of TMPO result in high levels of LIN28a. LIN28a expression decreases until the 8-cell stage of embryo development, when a dramatic increase is then noted63. In contrast, LIN28a levels should be low until after resumption of meiosis, and high LIN28a levels in oocytes are associated with MII-arrested oocytes in mice63,65. High levels of LAP2 have also been associated with DNA damage in oocytes and programmed cell death in granulosa cells in mice61. Overall, these data indicate that the P follicles in this dataset may have DNA damage, potentially leading to oocyte arrest at MII.
Based upon all the cluster analyses, the largest digression from the literature appears to be in the D to P transition, corresponding to differentiation. The five main drivers of differentiation in cattle are TGF-β1, tumor protein 53 (TP53), estradiol, hepatocyte nuclear factor 4 alpha (HNF4A) and retinoic acid (RA)28. Our dataset included all but RA, and the expression of each gene significantly increased from D to P. RA has a positive regulatory effect on granulosa cell proliferation and oocyte maturation66. RA levels in growing follicles are required for the follicle to reach appropriate final maturation (differentiation) in response to gonadotropins and affect gonadotropin receptor expression in granulosa cells in cows67. It can therefore be hypothesized that with this ovarian treatment protocol, follicles are not receiving the proper stimuli to fully differentiate and produce oocytes with the highest developmental competence potential.
Based upon these results, it appears that the ovarian treatment protocol used on these animals did not result in GC or oocytes with the highest developmental competence potential. It is important to note that these comparisons are based primarily on data from unstimulated cows. We hypothesize that the gene expression changes we observed could be an outcome of the simulation protocol, but it is unknown whether some of these deviations are due to the unique expression pattern of the rhinoceros from other species. Because we performed oocyte group culture in vitro, we cannot directly correlate the in vivo GC analyzed with the corresponding oocyte maturation outcome; hence, in the future, we will match GC to the in vitro maturation outcome of oocytes by performing individual culture. Overall, for the animals treated with this stimulation protocol, we achieved an in vitro maturation rate of 33%, but none of those oocytes fertilized or developed into embryos. Although a percentage of retrieved oocytes were capable of extruding the first polar body and reaching metaphase II (MII) after in vitro maturation, we hypothesize that unpaired cytoplasmic maturation, erroneous chromosomal segregation, or morphological characteristics of atresia68 left the oocyte incapable of fertilization and becoming a zygote. Incomplete cytoplasmic maturation can lead to failed sperm decondensation, erroneous male pronuclear formation, and ultimately unsuccessful fertilization69. However, additional studies are needed to characterize the effects of ovarian stimulation on oocyte developmental competence acquisition/failure and cytoplasmic maturation in this species. A balance between the quantity and quality of oocytes obtained during OPU is suggested, and low, frequent doses of GnRH might be a valuable treatment.
With this dataset, we generated a list of potential biomarkers of the GC for the physiologic status of the follicle to determine which oocytes had the highest potential for developmental competence. These markers include COL1A1 (follicle growth)41,42,43,44, JMY and FBXW11 (oocyte preparedness to resume meiosis)49,53,57, NRG1 (oocyte maturation potential)60, TMPO (DNA damage)61, and MACIR and COL4A1 (markers for cell death and atresia)31,46. However, further testing will be needed to correlate these markers with the status of the follicles we collected during OPU and ultimately with oocyte maturation and cleavage.
It is important to note that one limitation in this study is grouping the follicle stages were grouped based upon follicle size at OPU. Nevertheless, for this study, we were not able to obtain follicles at all stages from the same animal, as there is no guarantee of a full range of follicles sizes at the time of OPU regardless of ovarian stimulation and the status of the estrous cycle prior to ovarian stimulation. We attempted to overcome this limitation by obtaining technical replicates from different granulosa cell aliquots of the preovulatory follicle. In addition, the animals in this study were not ultrasounded regularly prior to ovarian treatment and OPU; therefore, the trajectory of the follicle prior to OPU was unknown (i.e., was it increasing in size or was it already regressing and undergoing atresia). We attempted to overcome this by evaluating multiple follicles per stage, and in the future, we will attempt to monitor these animals prior to OPU.
In conclusion, this was the first study to evaluate the transcriptomic differences in GC between the different stages of follicle development in the SWR, an important chapter in the reproductive knowledge of this species. In this threatened species, much remains unknown regarding ovarian physiology and follicle activity; nevertheless, it is important to continue addressing these reproductive questions to support assisted reproductive technologies for rhinoceros species. Finally, these data provide multiple biomarkers to potentially improve OPU results. This body of work contains novel information on the SWR and enriches the reproductive knowledge of the species, which will support conservation efforts for this species.
Materials and methods
Animal management and ovum pickup (OPU)
All procedures, experiments, and methods were reviewed and approved by San Diego Zoo Wildlife Alliance’s Institutional Animal Care and Use Committee (IACUC; protocol number 18-018). In addition, guidelines set forth by ARRIVE (Animal Research: Reporting of in Vivo Experiments, https://arriveguidelines.org/arrive-guidelines) were followed. All methods for this study were performed in accordance with all relevant guidelines and regulations of the IACUC and ARRIVE. Three southern white rhinoceros females (aged 7, 11, and 24 at the time of OPU) were utilized for this study. While under the care of the SDZWA, all rhinoceroses have undergone regular body score analyses and health assessments to ensure that no reproductive pathologies, tumors, or cysts were present and were continually monitored. Complete information regarding each individual rhinoceros is included in Supplemental Table 3. All females were stimulated prior to transrectal OPU as previously described2,17, facilitating the in vivo collection of follicular fluid aspirate from follicles of distinct sizes. Prior to OPU, females received synthetic chlormadinone acetate (CMA) at 30 mg/day for 35 days (days 0–34). The day following CMA withdrawal (Day 35), all animals received 3 mg of deslorelin [gonadotropin-releasing hormone (GnRH) analog] followed by 2 mg of deslorelin every 48 h prior to OPU (Day 37 and 39; 7 mg total) via intramuscular injection. OPU was performed on day 41. All rhinoceroses were anesthetized using a combination of etorphine (2 mcg/kg), butorphanol (20–24 mcg/kg), medetomidine (23–25 mcg/kg), and azaperone (14–16 mcg/kg) administered intramuscularly using a remote drug delivery system. During initial positioning to facilitate intubation, propofol was administered intravenously (0.5 mg/kg). Following OPU procedures, anesthesia reversal was achieved using atipamezole delivered intramuscularly at a 5:1 ratio to medetomidine (116–125 mcg/kg) and naltrexone delivered intramuscularly at a target 50:1 ratio to etorphine (53–61 mcg/kg).
Following fecal removal, rinsing, and disinfection, OPU was achieved using a customized, ultrasound-guided probe accompanied by three double-lumen needles. The follicles were ablated, the contents were aspirated, and the samples were rinsed with a warm (37 °C) flushing solution (Vigro) containing 12.5 I.U./mL of heparin. During OPU, follicle diameters were measured via trans-rectal ultrasonography. Follicular fluid and granulosa cells were separated based upon follicle stage/size. The follicle stages were growing (11–17 mm), dominant (25–29 mm), and preovulatory (30–34 mm).
Granulosa cell collection and RNA isolation/quantification
After OPU and the oocyte was collected from the collection fluid, free-floating mural granulosa cells were collected at the follicle stage, pipetted directly into RNAlater (Thermo Fisher Scientific, Waltham, MA) for 24 h at 4 °C and subsequently transferred to − 80 °C until RNA isolation. Prior to isolation, the granulosa cells were thawed, mixed 1:1 with cold PBS (Sigma Aldrich, St. Louis, MO) and centrifuged at 3000×g for 10 min. The supernatant was discarded, and the pellet was resuspended in cold PBS. The samples were centrifuged again at 3000×g for 5 min to remove all remnants of RNAlater. Total RNA was isolated from granulosa cells using an Arcturus PicoPure RNA Isolation Kit (Thermo Fisher Scientific, Waltham, MA) per the manufacturer’s instructions. Two biological replicates (one sample from two separate individuals) were used for both growing and dominant follicles. Neither of these two animals had a preovulatory follicle present at the time of OPU, and a third animal provided two technical replicates (the GC were stored in numerous separate aliquots) for the preovulatory stage. In total, six samples were used for this study (n = 2 per follicle type). Granulosa cells were incubated with extraction buffer for 30 min before centrifugation to remove debris and extracellular material. The cell extract was incubated with ethanol, bound to, and washed on preconditioned purification columns. Total RNA was recovered into elution buffer and quantified using a Qubit 4 Fluorometer (Thermo Fischer Scientific, Waltham, MA. After quantification, the samples were evaluated on a 4150 TapeStation System (Agilent Technologies, Santa Clara, CA) to determine RNA integrity number (RIN) values. Only samples with a RIN greater than 6.0 were used for RNA-Seq analysis.
RNA sequencing
Library preparation and RNA sequencing were performed at the University of California San Diego Institute for Genomic Medicine Center. Following the manufacturer’s protocol, RNA-sequencing (cDNA) libraries were prepared using the Illumina TruSeq Stranded Total RNA Prep with Ribo-Zero Plus. A library was prepared for each sample using 50 ng of total RNA. Briefly, RNA was rRNA depleted and fragmented, first- and second-strand cDNA synthesis was performed, and adapters were ligated. Each sample was amplified with a specific barcoded PCR primer for sample identification purposes. The prepared libraries were sequenced as 100 base pair paired end reads on an Illumina NovaSeq 6000 (Illumina, San Diego, CA). The raw data files were uploaded to the Gene Expression Omnibus under accession number GSE261038.
Bioinformatic and statistical analysis
Bioinformatic analysis was performed on the Galaxy web platform using the public server usegalaxy.org70. Initial assessment of sequence quality was performed by FastQC, and the sequences were aggregated for comparison by MultiQC71,72. The adapter sequences and reads with quality scores below the quality threshold of 25 and with fewer than 20 base pairs were removed from analysis via Trimmomatic73. HISAT2 was used to align reads to the northern white rhinoceros (NWR) genome CerSimCot1.0 (GCA_021442165.1)15,74. The southern white rhinoceros genome (CerSimSim1.0; GCA_000283155.1) was not used for this analysis due to large gaps in the sequencing and extremely limited annotation. Transcript assembly and quantification for both annotated and unannotated transcripts were initially performed with Stringtie, and each animal sample was analyzed individually75. The resulting transcripts for individual samples were merged to create a final annotation file representing the intersection and union of all samples75. Stringtie was subsequently applied to the complete merged annotation to generate transcript read counts. Transcript counts were exported from Galaxy and imported into RStudio for statistical analysis76. Transcript read counts were analyzed between each follicle type utilizing DESeq2 within R77. The average expression value for replicates within a follicle stage was ≥ 3, except in the cases where the expression value was 0 for the replicates within a follicle type. When not expressed in a follicle type, the expression value for both replicates was 0. The data were normalized internally using the median ratio method of DESeq2. Benjamini Hochberg false discovery rate adjustment was used. Significance was assessed at P ≤ 0.05. Differentially expressed transcripts were grouped into different cluster patterns according to their expression profiles among the different developmental stages using the Mfuzz Bioconductor package78. Pathway analysis and visualization were completed using Qiagen Ingenuity Pathway Analysis79. Manual curation was required to assign sequences identified to corresponding genes and to ensure that the HGNC/VGNC naming conventions were maintained.
Data availability
The datasets generated and analyzed during the current study are available in the Gene Expression Omnibus (GEO) under accession number GSE261038.
References
Hildebrandt, T.B., Holtze, S., Colleoni, S., Hermes, R., Stejskal, J., Lekolool, I., et al. In vitro fertilization (IVF) program in white rhinoceros. Reproduction (Cambridge, England). REP-23-0087 (2023).
Ruggeri, E., Klohonatz, K., Sirard, M.-A., Durrant, B. & Coleman, S. Genomic insights into southern white rhinoceros (Ceratotherium simum simum) reproduction: Revealing granulosa cell gene expression. Theriogenol. Wild. 3, 100055 (2023).
Pennington, P. M., Marshall, K. L., Capiro, J. M., Howard, L. & Durrant, B. S. Pregnancies following long luteal phases in southern white rhinoceros (Ceratotherium simum simum). Zoo Biol. 39(2), 141–144 (2020).
Pennington, P. M. & Durrant, B. S. Assisted reproductive technologies in captive rhinoceroses. Mammal Rev. 49(1), 1–15 (2019).
Hildebrandt, T. B. et al. Embryos and embryonic stem cells from the white rhinoceros. Nat. Commun. 9(1), 2589 (2018).
Saragusty, J. et al. Rewinding the process of mammalian extinction. Zoo Biol. 35(4), 280–292 (2016).
Roth, T. L. A review of the reproductive physiology of rhinoceros species in captivity. Int. Zoo Yearbook. 40(1), 130–143 (2006).
Patton, M. L. et al. Reproductive cycle length and pregnancy in the southern white rhinoceros (Ceratotherium simum simum) as determined by fecal pregnane analysis and observations of mating behavior. Zoo Biol. 18(2), 111–127 (1999).
Roth, T.L. That was then, this is now–over two decades of progress in rhinoceros reproductive science and technology. Theriogenol. Wild. 4, 100065 (2023).
Ferre, L. B. et al. Review: Recent advances in bovine in vitro embryo production: Reproductive biotechnology history and methods. Animal. 14(5), 991–1004 (2020).
Chang, E. M., Song, H. S., Lee, D. R., Lee, W. S. & Yoon, T. K. In vitro maturation of human oocytes: Its role in infertility treatment and new possibilities. Clin. Exp. Reprod. Med. 41(2), 41–46 (2014).
Thomas, M. R., Sparks, A. E., Ryan, G. L. & Van Voorhis, B. J. Clinical predictors of human blastocyst formation and pregnancy after extended embryo culture and transfer. Fertil. Steril. 94(2), 543–548 (2010).
Hinrichs, K. The equine oocyte: Factors affecting meiotic and developmental competence. Mol. Reprod. Dev. 77(8), 651–661 (2010).
Galli, C., Colleoni, S., Duchi, R., Lagutina, I. & Lazzari, G. Developmental competence of equine oocytes and embryos obtained by in vitro procedures ranging from in vitro maturation and ICSI to embryo culture, cryopreservation and somatic cell nuclear transfer. Anim. Reprod. Sci. 98(1–2), 39–55 (2007).
Wang, G., Brändl, B., Rohrandt, C., Hong, K., Pang, A., Lee, J., et al. Chromosome-level genome assembly of the functionally extinct northern white rhinoceros (Ceratotherium simum cottoni). bioRxiv. 2021.12.11.472206 (2021).
Meuffels-Barkas, J., Wilsher, S., Allen, W.R.T., Ververs, C., Lueders, I. Comparative reproduction of the female horse, elephant and rhinoceros: Implications for advancing Assisted Reproductive Technologies (ART). Reprod. Fertil. 4(3) (2023).
Ruggeri, E. et al. Glucose consumption and gene expression in granulosa cells collected before and after in vitro oocyte maturation in the southern white rhinoceros (Ceratotherium simum simum). Reprod. Fertil. Dev 34, 875–888 (2022).
Pennington, P. M., Marshall, K. L., Capiro, J. M., Felton, R. G. & Durrant, B. S. Ovulation induction in anovulatory southern white rhinoceros (Ceratotherium simum simum) without altrenogest. Conserv. Physiol. 7(1), coz033 (2019).
Girard, A., Dufort, I., Douville, G. & Sirard, M. A. Global gene expression in granulosa cells of growing, plateau and atretic dominant follicles in cattle. Reprod. Biol. Endocrinol. 13, 17 (2015).
Squires, E. Current reproductive technologies impacting equine embryo production. J Equine Vet Sci. 89, 102981 (2020).
De Roover, R., Feugang, J. M., Bols, P. E., Genicot, G. & Hanzen, C. Effects of ovum pick-up frequency and FSH stimulation: A retrospective study on seven years of beef cattle in vitro embryo production. Reprod. Domest. Anim. 43(2), 239–245 (2008).
Carnevale, E. M. & Sessions, D. R. In vitro production of equine embryos. J. Equine Vet. Sci. 32(7), 367–371 (2012).
Carnevale, E. M. et al. Clinical use of intracytoplasmic sperm injection in horses. Proc. Am. Assoc. Equine Pract. 53, 560 (2007).
Carnevale, E. M. & Maclellan, L. J. Collection, evaluation, and use of oocytes in equine assisted reproduction. Vet. Clin. N. Am. Equine Pract. 22(3), 843–856 (2006).
Benammar, A., Derisoud, E., Vialard, F., Palmer, E., Ayoubi, J.M., Poulain, M., et al. The mare: A pertinent model for human assisted reproductive technologies? Animals (Basel). 11(8) (2021).
Bo, G. A. & Mapletoft, R. J. Superstimulation of ovarian follicles in cattle: Gonadotropin treatment protocols and FSH profiles. Theriogenology. 150, 353–359 (2020).
Mantovani, A. P. et al. Prolonged use of a progesterone-releasing intravaginal device (CIDR®) for induction of persistent follicles in bovine embryo recipients. Anim. Reproduct. (AR). 2(4), 272–277 (2018).
Khan, D. R. et al. Meta-analysis of gene expression profiles in granulosa cells during folliculogenesis. Reproduction. 151(6), R103–R110 (2016).
Cheng, J. et al. Circular RNA expression profiling of human granulosa cells during maternal aging reveals novel transcripts associated with assisted reproductive technology outcomes. PLoS One. 12(6), e0177888 (2017).
Li, Z. et al. scRNA-seq of ovarian follicle granulosa cells from different fertility goats reveals distinct expression patterns. Reprod. Domest. Anim. 56(5), 801–811 (2021).
Douville, G. & Sirard, M. A. Changes in granulosa cells gene expression associated with growth, plateau and atretic phases in medium bovine follicles. J. Ovarian Res. 7, 50 (2014).
Nivet, A. L., Vigneault, C., Blondin, P. & Sirard, M. A. Changes in granulosa cells’ gene expression associated with increased oocyte competence in bovine. Reproduction. 145(6), 555–565 (2013).
Camacho, C. et al. BLAST+: Architecture and applications. BMC Bioinform. 10, 421 (2009).
Dorris, E. R. et al. The autoimmune susceptibility gene C5orf30 regulates macrophage-mediated resolution of inflammation. J. Immunol. 202(4), 1069–1078 (2019).
Muthana, M. et al. C5orf30 is a negative regulator of tissue damage in rheumatoid arthritis. Proc. Natl. Acad. Sci. 112(37), 11618–11623 (2015).
Serwin, K. et al. Human macrophage immunometabolism regulator (MACIR) in patients with periodontitis. Immunobiology. 228(6), 152760 (2023).
Hatzirodos, N. et al. Transcriptome profiling of granulosa cells from bovine ovarian follicles during atresia. BMC Genom. 15, 40 (2014).
Li, H., Chang, H. M., Shi, Z. & Leung, P. C. K. The p38 signaling pathway mediates the TGF-beta1-induced increase in type I collagen deposition in human granulosa cells. FASEB J. 34(11), 15591–15604 (2020).
Huet, C., Monget, P., Pisselet, C. & Monniaux, D. Changes in extracellular matrix components and steroidogenic enzymes during growth and atresia of antral ovarian follicles in the sheep. Biol. Reprod. 56(4), 1025–1034 (1997).
Huet, C., Pisselet, C., Mandon-Pepin, B., Monget, P. & Monniaux, D. Extracellular matrix regulates ovine granulosa cell survival, proliferation and steroidogenesis: Relationships between cell shape and function. J. Endocrinol. 169(2), 347–360 (2001).
Shimada, M., Ito, J., Yamashita, Y., Okazaki, T. & Isobe, N. Phosphatidylinositol 3-kinase in cumulus cells is responsible for both suppression of spontaneous maturation and induction of gonadotropin-stimulated maturation of porcine oocytes. J. Endocrinol. 179(1), 25–34 (2003).
Hoshino, Y. et al. Phosphatidylinositol 3-kinase and Akt participate in the FSH-induced meiotic maturation of mouse oocytes. Mol. Reprod. Dev. 69(1), 77–86 (2004).
Hall, S. E., Upton, R. M., McLaughlin, E. A. & Sutherland, J. M. Phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) and Janus kinase/signal transducer and activator of transcription (JAK/STAT) follicular signalling is conserved in the mare ovary. Reprod. Fertility Develop. 30(4), 624–633 (2018).
Riley, J. K. et al. The PI3K/Akt pathway is present and functional in the preimplantation mouse embryo. Develop. Biol. 284(2), 377–386 (2005).
Du, H., Guo, Y., Wu, X. & Gong, Y. FOXL2 regulates the expression of the Col4a1 collagen gene in chicken granulosa cells. Mol. Reprod. Dev. 89(2), 95–103 (2022).
Marongiu, M. et al. Novel action of FOXL2 as mediator of Col1a2 gene autoregulation. Develop. Biol. 416(1), 200–211 (2016).
Adriaenssens, T., Mazoyer, C., Segers, I., Wathlet, S. & Smitz, J. Differences in collagen expression in cumulus cells after exposure to highly purified menotropin or recombinant follicle-stimulating hormone in a mouse follicle culture model. Biol. Reprod. 80(5), 1015–1025 (2009).
Zuchero, J. B., Coutts, A. S., Quinlan, M. E., Thangue, N. B. L. & Mullins, R. D. p53-cofactor JMY is a multifunctional actin nucleation factor. Nat. Cell Biol. 11(4), 451–459 (2009).
Sun, S.-C., Sun, Q.-Y. & Kim, N.-H. JMY is required for asymmetric division and cytokinesis in mouse oocytes. MHR Basic Sci. Reproduct. Med. 17(5), 296–304 (2011).
Brunet, S. & Verlhac, M. H. Positioning to get out of meiosis: The asymmetry of division. Hum. Reprod. Update. 17(1), 68–75 (2011).
Namgoong, S. & Kim, N. H. Roles of actin binding proteins in mammalian oocyte maturation and beyond. Cell Cycle. 15(14), 1830–1843 (2016).
Lin, Z., Xu, Y.-N., Namgoong, S. & Kim, N.-H. JMY functions as actin nucleation-promoting factor and mediator for p53-mediated DNA damage in porcine oocytes. Plos One. 9(10), e109385 (2014).
Pu, D. et al. Regulation of FUS ubiquitination and localization by HFM1 is essential for oocyte meiosis prophase I progression in mice. bioRxiv (2023).
Xie, J., Jin, Y. & Wang, G. The role of SCF ubiquitin-ligase complex at the beginning of life. Reproduct. Biol. Endocrinol. 17(1), 1–9 (2019).
Silverman, J. S., Skaar, J. R. & Pagano, M. SCF ubiquitin ligases in the maintenance of genome stability. Trends Biochem. Sci. 37(2), 66–73 (2012).
Isoda, M. et al. The extracellular signal-regulated kinase-mitogen-activated protein kinase pathway phosphorylates and targets Cdc25A for SCF beta-TrCP-dependent degradation for cell cycle arrest. Mol. Biol. Cell. 20(8), 2186–2195 (2009).
Oh, J. S., Susor, A., Schindler, K., Schultz, R. M. & Conti, M. Cdc25A activity is required for the metaphase II arrest in mouse oocytes. J. Cell Sci. 126(5), 1081–1085 (2013).
Chowdhury, I., Branch, A., Mehrabi, S., Ford, B. D. & Thompson, W. E. Gonadotropin-dependent neuregulin-1 signaling regulates female rat ovarian granulosa cell survival. Endocrinology. 158(10), 3647–3660 (2017).
Noma, N. et al. LH-induced neuregulin 1 (NRG1) type III transcripts control granulosa cell differentiation and oocyte maturation. Mol. Endocrinol. 25(1), 104–116 (2011).
Dellaqua, T. T. et al. Neuregulin 1 (NRG1) modulates oocyte nuclear maturation during IVM and improves post-IVF embryo development. Theriogenology. 195, 209–216 (2023).
Miao, X., Guo, R., Williams, A., Lee, C., Ma, J., Wang, P.J., et al. Replication Protein A1 is essential for DNA damage repair during mammalian oogenesis. bioRxiv. (2023).
Kulus, J., Kulus, M., Kranc, W., Jopek, K., Zdun, M., Jozkowiak, M., et al. Transcriptomic profile of new gene markers encoding proteins responsible for structure of porcine ovarian granulosa cells. Biology (Basel). 10(11) (2021).
Vogt, E. J., Meglicki, M., Hartung, K. I., Borsuk, E. & Behr, R. Importance of the pluripotency factor LIN28 in the mammalian nucleolus during early embryonic development. Development. 139(24), 4514–4523 (2012).
Liu, T. et al. Thymopentin alleviates premature ovarian failure in mice by activating YY2/Lin28A and inhibiting the expression of let-7 family microRNAs. Cell Prolif. 54(8), e13089 (2021).
Flemr, M., Moravec, M., Libova, V., Sedlacek, R. & Svoboda, P. Lin28a is dormant, functional, and dispensable during mouse oocyte-to-embryo transition. Biol. Reproduct. 90(6), 131, 1–9 (2014).
Kawai, T., Yanaka, N., Richards, J. S. & Shimada, M. De novo-synthesized retinoic acid in ovarian antral follicles enhances FSH-mediated ovarian follicular cell differentiation and female fertility. Endocrinology. 157(5), 2160–2172 (2016).
Damdimopoulou, P., Chiang, C. & Flaws, J. A. Retinoic acid signaling in ovarian folliculogenesis and steroidogenesis. Reprod. Toxicol. 87, 32–41 (2019).
Hinrichs, K. The relationship of follicle atresia to follicle size, oocyte recovery rate on aspiration, and oocyte morphology in the mare. Theriogenology. 36(2), 157–168 (1991).
Fulka, J. Jr., First, N. L. & Moor, R. M. Nuclear and cytoplasmic determinants involved in the regulation of mammalian oocyte maturation. Mol. Hum. Reprod. 4(1), 41–49 (1998).
Afgan, E. et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 46(W1), W537–W544 (2018).
Andrews, S. FastQC: A quality control tool for high throughput sequence data. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (2010).
Ewels, P., Magnusson, M., Lundin, S. & Kaller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 32(19), 3047–3048 (2016).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 30(15), 2114–2120 (2014).
Kim, D., Paggi, J. M., Park, C., Bennett, C. & Salzberg, S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 37(8), 907–915 (2019).
Pertea, M. et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 33(3), 290–295 (2015).
R Core Team R. R: A language and environment for statistical computing. (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15(12), 550 (2014).
Kumar, L. & Matthias, E. F. Mfuzz: A software package for soft clustering of microarray data. Bioinformation. 2(1), 5–7 (2007).
Kramer, A., Green, J., Pollard, J. Jr. & Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 30(4), 523–530 (2014).
Acknowledgements
We thank Dr. Candace Williams at the San Diego Zoo Wildlife Alliance and Dr. Ahmed Gad and Nico Menjivar at Colorado State University for aiding in the data analysis and visualization. In addition, we would like to acknowledge the Conservation Science Wildlife Health team, the Veterinary Services, and the Rhino Rescue Center team at San Diego Zoo Wildlife Alliance for aiding in animal management, care, and sample collection.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
E.R. and B.D. collected the samples. E.R. processed and prepared the samples for RNA sequencing. E.R. and K.K. performed the bioinformatic analyses and wrote the manuscript. M.A.S. aided in comparative data analysis and interpretation. B.D. aided in the writing and revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Klohonatz, K., Durrant, B., Sirard, MA. et al. Granulosa cells provide transcriptomic information on ovarian follicle dynamics in southern white rhinoceros. Sci Rep 14, 19321 (2024). https://doi.org/10.1038/s41598-024-70235-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70235-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.