Abstract
Ulcerative colitis (UC) is a chronic idiopathic inflammatory disease affecting the gastrointestinal tract. Although paeonol has been used for treating UC due to its anti-inflammatory and antioxidant effects, the underlying mechanisms remain unclear. In this study, we investigated the mechanisms of paeonol’s action on UC by conducting in-vitro and in-vivo studies using NCM460 cells and RAW264.7 cells, and the DSS-induced mice colitis model. The in vitro studies demonstrate that paeonol exerts inhibitory effects on the activation of the NF-κB signaling pathway through upregulating PPARγ expression, thereby attenuating pro-inflammatory cytokine production, reducing reactive oxygen species levels, and promoting M2 macrophage polarization. These effects are significantly abrogated upon addition of the PPARγ inhibitor GW9662. Moreover, UC mice treated with paeonol showed increased PPARγ expression, which reduced inflammation and apoptosis to maintain intestinal epithelial barrier integrity. In conclusion, our findings suggest that paeonol inhibits the NF-κB signaling pathway by activating PPARγ, reducing inflammation and oxidative stress and improving Dss-induced colitis. This study provides a new insight into the mechanism of treating UC by paeonol.
Similar content being viewed by others
Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disease characterized by inflammation and ulcers in the innermost aspect of the colon and rectum of unknown etiology. Given the high incidence of UC in developed countries and the significant increase in developing countries, it has developed into a global burden1. In addition, cases have been reported at all ages, from children to the elderly2. UC is also known to increase the risk of colorectal cancer (CRC)3. Multiple factors, including environmental factors, genetic polymorphism, and mucosal immune dysregulation, have been thought to play important roles in the pathogenesis of UC. Although the etiology of UC remains unclear, accumulating evidence suggests that the increased secretion of pro-inflammatory cytokines and dysregulation of the intestinal immune system play an important role in the disruption of the gastrointestinal mucosal barrier and the persistence of inflammation4. Studies have revealed that a large number of activated macrophages can secrete inflammatory cytokines in the colonic mucosa of patients with UC5. The levels of interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α) in the sera and colonic tissues of patients with UC were significantly higher than those of normal subjects, and their downregulation could alleviate UC6. As the central component of the cellular signaling machinery, nuclear factor-kB (NF-κB) regulates immune response and inflammation, and it is closely related to the occurrence and development of UC7,8. Many of the aforementioned inflammatory factors are highly dependent on NF-κB-associated signal transduction. NF-κB, which is expressed in almost all cell types, can be activated by a vast range of stimuli, including microbial components, chemokines, reactive oxygen species (ROS), and cytokines9. When exposed to extracellular stimulation, the NF-κB inhibitor (IκB) is rapidly phosphorylated, ubiquitinated, and degraded, which is mediated by IκB kinase. Then, NF-κB heterodimers are released from the NF-κB/IκB complex and translocated to the nucleus for phosphorylation, which leads to the expression of genes involved in inflammation10. Peroxisome proliferator-activated receptor γ (PPARγ) regulates the expression of various proinflammatory cytokines and barrier function proteins by promoting the expression of target genes and interfering with the activity of transcription factors such as NF-κB11,12. Although the gene sequence has not been changed, the expression of PPARγ in the colonic mucosal epithelia of patients with UC significantly decreases13,14. Multiple studies have indicated that drugs, including rosiglitazone and 5-aminosalicylic acid (5-ASA), are used to treat UC by acting on PPARγ. 5-ASA, a first-line drug for UC, can restore mitochondrial activity and slow down dysregulated E. coli expansion by activating PPAR-γ signaling, while rosiglitazone can act as a PPARγ agonist to inhibit the high expression of NF-κB p65 and p38 MAPK proteins in UC15,16,17. Therefore, PPARγ is an effective target for the treatment of UC.
Paeonol, the main component of the root bark of Paeonia suffruticosa, has analgesic, anti-inflammatory, antioxidant, antipyretic, and anti-allergic effects. Many traditional Chinese classic prescriptions containing Paeonia suffruticosa root bark, such as Dahuang Mudan Decoction and Huangqin Decoction, have been found to possess therapeutic effects in the treatment of colitis by regulating the function of ILC3 and so on18,19,20,21. Additionally, numerous studies have demonstrated that various extracts derived from paeonol root exhibit significant efficacy in ameliorating colitis symptoms22,23,24. To date, paeonol dosage forms for clinical use include tablets, injections, and ointments. Clinical applications of paeonol focus primarily on anti-inflammatory activity25. Paeonol has been found to inhibit the expression of proinflammatory cytokines including TNF-α, IL-1β, and IL-6 in arthritic rats via the mediation of NF-κB26. In addition, paeonol can regulate the TLR4/MyD88/NF-κB signaling pathway to reduce serum and protein expression levels of TNF-α, IL-1β, and IL-6 in Escherichia coli lipopolysaccharide (LPS)-induced acute lung injury27. Paeonol has also demonstrated significant anti-inflammatory effects in various diseases such as osteoarthritis, rheumatoid arthritis, and methotrexate-induced cardiotoxicity in rats28,29,30. Moreover, paeonol shows potential in the treatment of UC by inhibiting the secretion of proinflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-8), increasing the expression level of the anti-inflammatory cytokine IL-10 in serum and colon tissues, and using paeonol enema for the treatment of trinitrobenzene sulfonic acid-induced colitis and dextran sulfate sodium (DSS)-induced colitis in mice31,32,33. Despite extensive research on the anti-inflammatory effects of paeonol, its mechanism of action has not been well-defined, particularly in colitis. In this study, the effect of paeonol was evaluated using the LPS-induced cellular inflammation model and DSS-induced colitis model, and the underlying mechanism was explored from the perspective of PPARγ dependence.
Results
Effect of paeonol on the proliferation of RAW264.7 cells and NCM460 cells
The CCK‐8 assay was used to evaluate the effect of paeonol on cell viability. The results showed that paeonol had no cytotoxicity to RAW264.7 cells and NCM460 cells in the experimental concentration range of 20 μM, 40 μM, and 80 μM (Fig. 1a, b).
Paeonol pretreatment inhibited LPS-induced inflammatory responses in RAW264.7 cells
In order to determine the anti-inflammatory effect of paeonol, RAW264.7 cells were pretreated with different concentrations of paeonol for 2 h and then exposed to LPS for another 24 h. The level of cytokine production in the culture supernatant was measured using ELISA kits, and mRNA levels were determined by qRT-PCR. As shown in Fig. 2a–f, LPS stimulation significantly increased the production of proinflammatory cytokine (IL-6, and TNF-α) and inhibited the production of anti-inflammatory cytokine (IL-10) compared with the control. In fact, studies have shown that paeonol possesses the ability to attenuate the secretion of pro-inflammatory factors via activation of the TLR4/MyD88/NF-κB (p65) signaling pathway34 and enhance the expression of IL-10. We also prove paeonol pretreatment significantly prevented the LPS-stimulated increase in proinflammatory cytokine secretion and promoted IL-10 secretion, which indicated the anti-inflammatory effect of paeonol.
Effect of paeonol on the inflammatory response of LPS‐stimulated RAW264.7 cells. Expression levels of IL-6 (a), TNF-α (b) and IL-10 (c) were detected by enzyme-linked immunosorbent assay (ELISA). The expression of IL-6 (d), TNF-α (e), and IL-10 (f) was checked by qRT-PCR. (g) ROS was detected by ROS probe DCFH-DA on a flow cytometer. These data represent the mean ± SD (n = 3). *P < 0.5; **P < 0.01; ***P < 0.001; ****P < 0.0001 compared with the LPS group. ###P < 0.001; ####P < 0.0001, compared with the Control group.
Oxidative stress, a key factor in the development of inflammatory diseases, leads to the decline of tissue and organ systems35,36. Flow cytometry was used to analyze the production level of ROS in RAW264.7 cells treated with paeonol and LPS. It was found that LPS stimulation significantly increased the expression level of ROS compared with the control group, while paeonol treatment could effectively inhibited the LPS-induced ROS generation (Fig. 2g), with the most significant ROS-inhibiting effects observed at 80 μM.
Paeonol treatment promoted PPARγ expression and inhibited the activation of NF-κB signaling pathway
PPARγ has the ability to inhibit NF-κB activation by blocking p65 nuclear translocation, thereby preventing the transcription of pro-inflammatory factors such as TNF-α and iNOS37. To explore the effects of paeonol on the PPARγ and NF-κB signaling pathway, RAW264.7 cells were treated as described previously, and the expression levels of PPARγ and NF-ĸB pathway-related proteins in the cytoplasm were determined by WB analysis. The WB analysis revealed that paeonol upregulated the expression of PPARγ and decreased the expression levels of iNOS, COX2, p-IκBα and p-p65 in LPS-induced RAW264.7 cells (Fig. 3a). The mRNA expression levels of PPARγ were measured by qPCR (Fig. 3b). In cells treated with paeonol, the mRNA expression levels of PPARγ were significantly higher compared to those in the LPS group. Based on these findings, we speculate that paeonol may inhibit the activation of the NF-κB signaling pathway by promoting the expression of PPARγ.
Effects of paeonol on PPARγ expression and NF-κB signaling pathway activation. (a) The amount of PPARγ, IκB, p-IκB, p65, p-p65 iNOS and COX2 expression by Western blot analysis. The full blots are shown in Supplementary Information. (b) The expression of PPARγ was checked by qRT-PCR. These data represent the mean ± SD (n = 3). *P < 0.5; **P < 0.01; ***P < 0.001, ****P < 0.0001 compared with the LPS group. #P < 0.5; ##P < 0.01; ###P < 0.001; ####P < 0.0001, compared with the Control group.
PPARγ antagonist GW9662 abolished the inhibitory effects of paeonol on cellular inflammation
Pretreatment with the specific PPARγ antagonist GW9662 was performed 2 h prior to paeonol treatment and LPS stimulation. Cells and cell culture supernatants were collected to measure the secretion of inflammatory cytokines. The results demonstrated that GW9662 significantly compromised the anti-inflammatory effect of paeonol on LPS-stimulated RAW264.7 cells (Fig. 4a–c). Furthermore, the cells used for ROS detection were treated in the same manner, and the results indicated that GW9662 could suppress the inhibitory effect of paeonol on ROS production (Fig. 4d). To directly observe the inhibitory effect of GW9662 on the antioxidant effect of paeonol, intracellular ROS levels in RAW264.7 and NCM460 cells were examined using confocal microscopy. The findings revealed that paeonol at 80 μM significantly reduced ROS expression, leading to a significantly lower fluorescence intensity compared to the LPS group. However, the effects of paeonol were nearly abolished in the presence of GW9662. (Fig. 4e).
Effect of GW9662 on the inhibitory effect of paeonol on the inflammatory response. Expression levels of IL-6 (a), TNF-α (b) and IL-10 (c) were detected by enzyme-linked immunosorbent assay (ELISA). (d) ROS was detected by ROS probe DCFH-DA on a flow cytometer. (e) Then, intracellular ROS of RAW26 4.7 and NCM460 cells was detected by confocal microscopy. These data represent the mean ± SD. *P < 0.5; **P < 0.01; ***P < 0.001, ****P < 0.0001 compared with the LPS group. #P < 0.5; ##P < 0.01; ###P < 0.001; ####P < 0.0001, compared with the Control group.
Subsequently, the immunofluorescence results revealed a significant enhancement in PPARγ expression and an increase in NFκB-p65 expression, which led to the phosphorylation reduction of NFκB-p65 and subsequent inhibition of its nuclear translocation in RAW264.7 cells. However, the addition of GW9662 hindered this effect (Fig. 5). This outcome aligned with our hypothesis, indicating that paeonol inhibits the activation of the NF-κB signaling pathway by activating PPARγ. Additionally, paeonol can upregulate the expression level of PPARγ to promote the development of M2 macrophages while inhibiting the development of M1 macrophages (Fig. 6).
Paeonol reduced clinical symptoms in DSS-colitis mice model
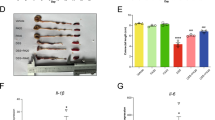
As shown in Fig. 7a, C57BL/6 mice aged 6–8 weeks were exposed distilled water or 3% DSS for 7 days to induce UC and then normal drinking water for 3 days. To evaluate the effect of paeonol on the murine DSS-induced colitis model, the mice were orally administrated the same treatment regimen with or without paeonol (200 or 400 mg/kg/day, p.o.)38 once a day, and the effect was compared to that of SASP (200 mg/kg/day, p.o.)32. During this period, the colitis mouse model exhibited symptoms such as weight loss, diarrhea, and bloody stools (Fig. 7b). Mice in the DSS group experienced greater weight loss, higher DAI scores, shorter colons, and a lower spleen weight index compared to healthy controls, indicating the successful induction of colitis by DSS therapy. In comparison to the DSS group, both the SASP-treated group (200 mg/kg) and the paeonol-treated groups (200 or 400 mg/kg) exhibited significantly reduced clinical symptoms (Fig. 7c–g). Notably, the group treated with 400 mg/kg of paeonol demonstrated the most pronounced therapeutic effect. The cohort treated with GW 9662 was excluded from the analysis due to high mortality.
Paeonol attenuates symptoms of DSS-induced colitis in mice. (a) Schematic diagram of the animal experimental design. (b) Normal mice and DSS-induced acute colitis mouse model. (c) Mouse body weights are measured daily. (d) Calculated DAI scores. (e) Spleen body weight ratio. (f, g) Intestine images and statistics for colon length in each group. Data are expressed as mean ± SEM, n = 5–6. #P < 0.5; ###P < 0.001; ####P < 0.0001, compared with the control group, *P < 0.5; ** P < 0.01; ****P < 0.0001, compared with the DSS group.
Paeonol inhibited the expression of TNF-a, COX2 and caspase3, enhancing PPARγ expression to protect the integrity of colon tissue
As depicted in Fig. 8a, treatment with SASP and paeonol significantly reduced the expression of TNF-α in the colon of mice with DSS-induced colitis. Furthermore, paeonol administration in colonic tissues enhanc ed the expression of PPARγ while decreasing the expression of COX2, iNOS and caspase 3 (Fig. 8b). To examine the protective effect of paeonol on the colon, HE staining was performed on colon tissues. The results showed that the treatment group effectively suppressed inflammation invasion and minimized the damage to colon tissue. Notably the high-dose paeonol group exhibited superior preservation of colon tissue integrity (Fig. 8c). Additionally, histochemical analysis revealed an upregulation of PPAR expression (Fig. 8d), and a significant reduction in the expression of the apoptosis protein caspase3 (Fig. 8e) in the colonic tissues of the high-dose paeonol-treated group, indicative of normal apoptotic levels.
Paeonol alleviated inflammatory invasion in DSS-induced UC mice. (a) Expression levels of TNF-α in the colon were detected by ELISA. (b) The protein levels of PPARγ, COX2, iNOS and caspase3 in the colon were tested by using Western blotting. The full blots are shown in Supplementary Information. (c) Representative HE-stained images of colon sections (scale bar, 30 µm). Immunohistochemistry staining of PPARγ. (d) and caspase3. (e) (scale bar, 30 µm). Data are expressed as the mean ± SEM, n = 5–6. ### P < 0.001 compared with the control group, *P < 0.5; **P < 0.01; ***P < 0.001, ****P < 0.0001 compared with the DSS group.
Discussion
Paeonol has demonstrated remarkable anti-inflammatory properties, which has garnered significant research interest in recent years. Although there is still ongoing research and a need for substantial data to fully elucidate the mechanism of paeonol in colitis treatment, previous studies have already indicated its effectiveness. However, the precise mechanism of paeonol in treating UC has yet to be fully explained by existing studies. In this study, we investigated the anti-inflammatory effects of paeonol using in vitro cellular inflammation models of RAW246.7 and NCM460 cells induced by LPS, with the addition of GW9662 as a control. Specifically, treatment with 200 and 400 mg/kg of paeonol has been shown to significantly reduce Disease Activity Index (DAI), colon weight/length ratio, gross and histopathological scores38. Then in vitro treatment groups were administered with 200 and 400 mg/kg of paeonol, while a positive control group was given 200 mg/kg of SASP, in order to investigate the therapeutic mechanism of paeonol in the treatment of UC. Unfortunately, due to the high mortality rate of the mice group with GW 9662 added in the in vivo experiment, our data did not yield as significant results as anticipated (The addition of GW9662 may have exacerbated the intestinal burden and mortality in colitis mice, or inhibited the anti-inflammatory effect of paeonol and promoted inflammation.).
UC is an idiopathic IBD characterized by recurring episodes of inflammation primarily affecting the colon and rectum39. The exact cause of UC is unknown, and it is believed to have a multifactorial etiology. The excessive release of inflammatory cytokines plays a pivotal role in the destruction of the intestinal barrier, which further contributes to the development of inflammation40. This inflammatory cascade leads to a continuous disruption of the intestinal barrier, fueling the chronic and progressive nature of the disease.
At the onset of UC, cytokines produced by innate immune cells, such as TNF-α, IL-1β, and IL-6, are prominently involved41,42. IL-10, a well-known anti-inflammatory cytokine, has been suggested to play a role in preventing the onset of UC during remission43. At present, IL-10 has emerged as a potential therapeutic target for UC44. Studies have demonstrated that the promoter region of the human IL-10 gene contains functional PPAR response elements (PPREs)45,46. Simultaneously, IL-10 is a distinctive product of macrophages(M2)47,48. In our in vitro experiments, we found that paeonol can inhibit the secretion of pro-inflammatory cytokines TNF-α and IL-6 while enhancing the expression of IL-10. The expression of p65, ROS, and inflammatory factors such as iNOS, IL-1β, TNF-α, IL-6, IL-17 in mice can be effectively inhibited by paeonol through various mechanisms, thereby exerting its anti-inflammatory effects. These mechanisms include the regulation of Treg/Th17 balance49, modulation of M2/M1 macrophage polarization ratio50, regulation of NLRP3 inflammasomes34 and pyroptosis and inhibition of MAPKs/NF-κB signal transduction51. However, there is limited research on the impact of paeonol on caspase-3 and COX-2 expression in mice with inflammation, particularly those with colitis. COX-2 is an immediate early response factor to inflammatory stimuli and a tumor promoter52,53. Genetic evidence showed increased susceptibility to chemically induced colitis in COX-2-deficient mice54. It is also closely related to the occurrence of colon cancer and can be regulated by various transcription factors, such as NF-κB and PPAR55. Pyroptosis plays a crucial role in the pathogenesis of colitis and its progression to colon cancer, with Caspase-3 being a key mediator in this process. Study showed that reduced caspase-3 expression is sufficient to confer apoptosis resistance56. Therefore, we chose to detect classic inflammatory factors as well as caspase-3 and COX2, which play a key role in the occurrence and development of UC. Furthermore, in our in vivo experiments using a colitis mouse model, the paeonol-treated group exhibited decreased TNF-α secretion in the serum and the expression of caspase-3 and COX2.
Oxidative stress is considered as a potential factor in the development of UC. The long-term and high-level production of ROS can cause irreversible damage or alteration to target molecules, resulting in DNA damage, lipid peroxidation, and protein oxidation, thereby promoting the development of UC57. This oxidative stress-induced damage often sets off a vicious cycle, ultimately leading to the development of CRC58. Our cell experiments demonstrated that paeonol can reduce ROS production, with the most pronounced effect observed at a concentration of 80 μM. Macrophages are polarized into two phenotypes under different conditions: M1 and M2. Macrophages play a crucial role in chronic inflammatory and can be polarized into two phenotypes under different conditions: M1 and M2. The M1 phenotype is responsible for breaking down tight junction proteins, damaging the epithelial barrier, inducing epithelial cell apoptosis, and leading to excessive inflammation. On the other hand, the M2 phenotype exhibits a strong phagocytic ability, which enables it to clear fragments and apoptotic cells, as well as promote tissue repair and angiogenesis59,60,61. Our cell experiments also demonstrated that paeonol at a concentration of 80 μM significantly elevated the percentage of M2.
PPARγ, a nuclear receptor found in various cells including colonic epithelial cells and macrophages, plays a crucial role in regulating inflammation in UC62,63. Patients with UC often exhibit low expression of PPARγ in their colonic epithelial cells64. PPARγ agonists have been shown to alleviate colitis symptoms in mice, indicating their therapeutic potential in UC13. Furthermore, PPARγ serves as an important regulator of the mucus barrier, emphasizing its role in maintaining gastrointestinal health65. Several studies have demonstrated the ability of naturally active small molecules (naringin66, honokiol67, and hyperoside68) and commonly used clinical drugs (5-ASA, rosiglitazone, and gliclazide) to regulate PPARγ, thereby treating colitis and inhibiting inflammation69,70. PPARγ has become an important target for UC treatment, and and its regulation involves the NF-κB pathway. Activation of the NF-κB signaling pathway leads to the degradation of IκBs, enabling free NFκB to translocate to the nucleus. There, it binds to DNA sites and activates the expression of inflammatory mediators such as TNF-α, IL-6, iNOS, COX271 and promote the differentiation of human monocytes into anti-inflammatory M2 macrophages. NF-κB activation can also induce apoptosis of intestinal crypt cells, contributing to severe mucosal erosion72. Our study provides evidence that paeonol effectively inhibits NF-κB activation by upregulating PPARγ expression. Consequently, the expression of downstream inflammatory factors is suppressed, leading to the preservation of colon tissue integrity (Fig. 9).
Taken together, this novel finding suggests that the mechanism of paeonol in treating UC involves the upregulation of PPARγ to inhibit NF-κB activation. By investigating the molecular interactions and signaling pathways involved, we can gain a better understanding of the precise mechanisms underlying the therapeutic effects of paeonol in UC treatment. This will contribute to the development of more targeted and effective treatments for UC patients. However, this study has certain limitations. PPAR-γ acts as a nuclear receptor and is activated upon ligand binding. Activation of receptors increases or decreases target gene transcription. We have not yet explored how paeonol up-regulates PPARγ. Does it directly enhance the expression of PPARγ through ligand-receptor interactions, or indirectly function as a regulator that facilitates ligand activation, or some other more indirect mechanism of action? Further research is needed to elucidate the specific mechanism by which paeonol regulates PPARγ.
Conclusion
Numerous studies have established the remarkable anti-inflammatory properties of paeonol. However, the specific anti-inflammatory mechanism of paeonol in the context of inflammatory bowel disease is still being investigated. In our study, we have made significant progress by being the first to demonstrate that paeonol exerts its anti-inflammatory effects, at least in part, through the regulation of PPARγ, resulting in the inhibition of NF-κB activation. This novel finding sheds light on the underlying mechanism of paeonol in the treatment of UC and provides valuable theoretical insights for the development of new therapeutic drugs.
Methods
Cell culture
RAW 264.7 murine macrophage cell lines were purchased from Procell (Wuhan, China) and NCM460 colonic cell lines were purchased from BNCC (Henan, China). RAW 264.7 cells and NCM460 cells were cultured in Dulbecco’s modified Eagle cell culture medium (Hyclone, Utah) supplemented with 10% FBS (PAN). All cells were cultured at 37 °C in a humidified atmosphere of 5% CO2. Paeonol was purchased from Sigma-Aldrich. It was first dissolved in DMSO and then diluted with the culture medium to achieve the desired concentration. Cells were pretreated with various concentrations of paeonol (20, 40, and 80 μM) for 2 h and then stimulated with LPS (1 μg/mL)73 for 24 h. The compound GW9662 is a widely utilized irreversible antagonist of PPARγ. It effectively antagonizes PPARγ in both cell-free and cell-based assays by covalently modifying a cysteine residue located within the ligand-binding site of PPARγ. Importantly, GW9662 does not induce transcription mediated by PPARγ74,75,76. In some experiments, GW9662 (10 μM)67,77 was added to the macrophage for 2 h before treatment with LPS and paeonol.
Animals
Male C57BL/6 mice (5–6 weeks old) were obtained from Xiamen University Laboratory Animal Center. Upon completion of each study, The mice were anesthetized by isoflurane gas and died of spinal dislocation.
Cell viability assay
Cell viability of RAW264.7 and NCM460 cells was assessed using a Cell Counting Kit-8 in accordance with the recommendation of the manufacturer. RAW264.7 cells were seeded into 96-well plates at a density of 3000–4000 cells/well, and NCM460 cells were seeded into 96-well plates at a density of 4000–5000 cells/well. At various time points (12, 24, and 48 h), 10 µL of CCK-8 solution (APEXBIO, USA) was added to each well plate, incubated away from light at 37 ℃ for 2 h, and the absorbance was measured at 450 nm.
Cytokine analysis by ELISA
The levels of TNF-α (DAKEWE, Beijing, China), IL-6 (ABclonal, Wuhan, China), and IL-10 (ABclonal, Wuhan, China) in cell culture supernatants were quantified using ELISA kit according to the manufacturer’s protocol. The levels of TNFα (DAKEWE, Beijing, China) in serum of the colitis mouse model were also determined.
Flow cytometry
The cells were treated and collected as described above and were then cultured in serum-free medium containing 10 mM of DCFH-DA for 30 min. The cells were subsequently treated with an ROS assay kit (50101ES01, Yeasen, China) following the manufacturer’s instructions. The fluorescence intensity was measured using flow cytometry, and the results were recorded using a fluorescence microscope. Additionally, co-incubation with CD86(105007, Biolegend) and CD206(141707, Biolegend) antibodies followed by flow cytometry analysis was performed to assess the expression of these markers associated with macrophage polarization.
Immunofluorescence assay
Cells were seeded into six-well plates and fixed in 4% paraformaldehyde at room temperature for 30 min. The cells were then permeabilized with 0.5% Triton X-100 for 15 min, blocked with 5% BSA for 1 h, and incubated overnight at 4 °C with primary antibodies against PPAR-γ and NF-κB P65. Afterward, the cells were incubated for 1 h at room temperature with secondary antibodies (Servicebio, China) and treated with Hoechst 33258 (Servicebio, China). The images were captured using a Zeiss LSM5 confocal microscope.
Western blot (WB) analysis
Total proteins were extracted using RIPA buffer with a protease inhibitor cocktail (20126ES60; Yeasen Biotech Co., Ltd.). The lysate was sonicated and then centrifuged. Protein concentration was determined using the BCA kit (Sangon Biotech, Shanghai, China), and protein expression levels were analyzed by WB. All antibodies used in this study were thoroughly validated for species specificity. Colon samples were lysed using PhosphoSafe lysis buffer (Novagen/Merck) and protein concentration was determined. Equal protein amounts were separated by SDS-PAGE and transferred to PVDF membranes (Immobilon P). The membranes were probed with primary antibodies, and immunoreactive bands were detected using chemiluminescence (Pierce). Stripping and re-staining of the membranes were performed in some experiments to measure multiple proteins in the same samples. The PVDF membranes were exposed to a stripping solution, incubated and washed, and then processed with a new primary antibody.
RNA extraction and qRT-PCR assay
RNAiso plus was used to extract total RNA (Takara, China). The Hifair® II 1st strand cDNA synthesis kit was used to create the first-strand c-DNA (11121ES60, Yeasen, China). The Hieff@ qPCR SYBR@ Green Master Mix was used for the qPCR assay (11201ES08; Yeasen). All the primers were listed as follows: IL-6 forward 5′-CCTCTGGTCTTCTGGAGTACC-3′ and reverse 5′-GTCCTTAGCCACTCCTTCTGT-3′; TNF-α forward 5′-AGCACAGAAAGCATGATCCG-3′ and reverse 5′-CTGATGAGAGGGAGGCCATT-3′; IL-10 forward 5′-AAGCTCCAAGACCAAGGTGTC-3′ and reverse 5′-TTCCGTTAGCTAAGATCCCTGG-3′; PPARγ forward 5′-GGTGAACCACTGATATTCAGGA-3′ and reverse 5′-AATGGCATCTCTGTGTCAACC-3′; GAPDH forward 5′-CCCAGCAAGGACACTGAGCAAG-3′ and reverse 5′-GGTCTGGGATGGAAATTGTGAGGG-3′.
Disease activity index (DAI)
The details of the DAI score in mice are shown in Table 1. Method: DAI = weight loss score + stool trait score + stool blood score. The degree of fecal occult blood was detected by using the urine fecal occult blood test kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Histological examination
Tissue samples from the distal colon of mice were fixed in 4% paraformaldehyde overnight, dehydrated in gradient ethanol, and embedded in paraffin. All tissue samples were cut into 3 μm slices, stained with hematoxylin and eosin, and then observed under a microscope (Microscopic digital Section Scanning System Motic VM1) (Supplementary Information 1 and 2).
Immunohistochemistry (IHC)
The 3 μm tissue slices were deparaffinized in xylene and rehydrated in a gradient of ethanol. Antigen retrieval was performed by heating the colon tissue sections in a sodium citrate solution, and endogenous peroxidase activity was blocked by incubating the sections in 3% hydrogen peroxide. The sections were then rinsed with phosphate-buffered saline (PBS) three times and nonspecific binding was blocked with 5% normal goat serum. The sections were incubated with the primary antibody overnight at 4 °C, followed by washing with PBS three times. Biotin-labeled secondary antibodies were added and incubated for 20 min at room temperature. The sections were then stained with streptavidin and horseradish peroxidase, and the colorimetric substrate diaminobenzidine (Servicebio, China) was applied. After staining, removal, and dehydration, the tissue samples were mounted and observed under a microscope (Motic VM1).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 8.0.1. All data were expressed as mean ± standard error of the mean (SEM). Statistically significant differences were evaluated by one-way analysis of variance followed by Tukey’s multiple comparison test to compare multiple groups. P < 0.05 was considered statistically significant.
Ethical approval
All animal studies were approved by the Xiamen University’s laboratory animal ethics committee and Chinese government guidelines for animal experiments. All methods used in animal experiments were carried out in accordance with relevant guidelines, and handled according to standard use protocols and animal welfare regulations, and we also confirmed that all animal methods were reported in accordance with ARRIVE guidelines (http://arriveguidelines.org) for the reporting of animal experiments.
Data availability
The data and materials presented in this study are available on request from the corresponding author.
References
Le Berre, C., Ananthakrishnan, A. N., Danese, S., Singh, S. & Peyrin-Biroulet, L. Ulcerative colitis and Crohn’s disease have similar burden and goals for treatment. Clin. Gastroenterol. Hepatol. 18, 14–23. https://doi.org/10.1016/j.cgh.2019.07.005 (2020).
Kobayashi, T. et al. Ulcerative colitis. Nat. Rev. Dis. Primers 6, 74. https://doi.org/10.1038/s41572-020-0205-x (2020).
Olén, O. et al. Colorectal cancer in ulcerative colitis: A Scandinavian population-based cohort study. Lancet 395, 123–131. https://doi.org/10.1016/S0140-6736(19)32545-0 (2020).
Yao, D., Dong, M., Dai, C. & Wu, S. Inflammation and inflammatory cytokine contribute to the initiation and development of ulcerative colitis and its associated cancer. Inflamm. Bowel Dis. 25, 1595–1602. https://doi.org/10.1093/ibd/izz149 (2019).
Mitsialis, V. et al. Single-cell analyses of colon and blood reveal distinct immune cell signatures of ulcerative colitis and Crohn’s disease. Gastroenterology https://doi.org/10.1053/j.gastro.2020.04.074 (2020).
Grabinger, T. et al. Inhibitor of apoptosis protein-1 regulates tumor necrosis factor-mediated destruction of intestinal epithelial cells. Gastroenterology 152, 867–879. https://doi.org/10.1053/j.gastro.2016.11.019 (2017).
Papoutsopoulou, S. & Campbell, B. J. Epigenetic modifications of the nuclear factor kappa B signalling pathway and its impact on inflammatory bowel disease. Curr. Pharm Des. 27, 3702–3713. https://doi.org/10.2174/1381612827666210218141847 (2021).
Nissim-Eliraz, E., Nir, E., Marsiano, N., Yagel, S. & Shpigel, N. Y. NF-kappa-B activation unveils the presence of inflammatory hotspots in human gut xenografts. PLoS One 16, e0243010. https://doi.org/10.1371/journal.pone.0243010 (2021).
Mitchell, J. P. & Carmody, R. J. NF-κB and the transcriptional control of inflammation. Int. Rev. Cell Mol. Biol. 335, 41–84. https://doi.org/10.1016/bs.ircmb.2017.07.007 (2018).
Karin, M. & Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF-[kappa]B activity. Ann. Rev. Immunol. 18, 621–663 (2000).
Venkataraman, B. et al. α-Bisabolol mitigates colon inflammation by stimulating colon PPAR-γ transcription factor: In vivo and in vitro study. PPAR Res. 2022, 5498115. https://doi.org/10.1155/2022/5498115 (2022).
Shen, P. et al. Magnolol treatment attenuates dextran sulphate sodium-induced murine experimental colitis by regulating inflammation and mucosal damage. Life Sci. 196, 69–76. https://doi.org/10.1016/j.lfs.2018.01.016 (2018).
Dubuquoy, L. et al. Impaired expression of peroxisome proliferator-activated receptor gamma in ulcerative colitis. Gastroenterology 124, 1265–1276 (2003).
Pedersen, G. & Brynskov, J. Topical rosiglitazone treatment improves ulcerative colitis by restoring peroxisome proliferator-activated receptor-gamma activity. Am. J. Gastroenterol. 105, 1595–1603. https://doi.org/10.1038/ajg.2009.749 (2010).
Arafa, E.-S.A., Mohamed, W. R., Zaher, D. M. & Omar, H. A. Gliclazide attenuates acetic acid-induced colitis via the modulation of PPARγ, NF-κB and MAPK signaling pathways. Toxicol. Appl. Pharmacol. 391, 114919. https://doi.org/10.1016/j.taap.2020.114919 (2020).
Sánchez-Hidalgo, M., Martín, A. R., Villegas, I. & de la Lastra, C. A. Rosiglitazone, a PPARgamma ligand, modulates signal transduction pathways during the development of acute TNBS-induced colitis in rats. Eur. J. Pharmacol. 562, 247–258 (2007).
Cevallos, S. A. et al. 5-Aminosalicylic acid ameliorates colitis and checks dysbiotic Escherichia coli expansion by activating PPAR-γ signaling in the intestinal epithelium. mBio https://doi.org/10.1128/mBio.03227-20 (2021).
Huang, S. et al. Dahuang Mudan decoction repairs intestinal barrier in chronic colitic mice by regulating the function of ILC3. J. Ethnopharmacol. 299, 115652. https://doi.org/10.1016/j.jep.2022.115652 (2022).
Yang, J.-Y. et al. Pharmacological effects of Huangqin Decoction prepared from roots of multi-originated peony on ulcerative colitis in mice: A comparative study. Zhongguo Zhong Yao Za Zhi 46, 6395–6402. https://doi.org/10.19540/j.cnki.cjcmm.20210508.301 (2021).
Chen, P. et al. Anti-inflammatory effects of Huangqin tang extract in mice on ulcerative colitis. J. Ethnopharmacol. 162, 207–214. https://doi.org/10.1016/j.jep.2014.12.039 (2015).
Zhang, Y., Li, X., Xu, X. & Yang, N. Mechanisms of Paeonia lactiflora in treatment of ulcerative colitis: A network pharmacological study. Med. Sci. Monit. 25, 7574–7580. https://doi.org/10.12659/MSM.917695 (2019).
Wu, K.-C. et al. Evaluations and mechanistic interrogation of natural products isolated from Paeonia suffruticosa for the treatment of inflammatory bowel disease. Front. Pharmacol. 12, 696158. https://doi.org/10.3389/fphar.2021.696158 (2021).
Chen, T.-F. et al. A systematic identification of anti-inflammatory active components derived from Mu Dan Pi and their applications in inflammatory bowel disease. Sci. Rep. 10, 17238. https://doi.org/10.1038/s41598-020-74201-x (2020).
Wang, K., Guo, J., Chang, X. & Gui, S. Painong-San extract alleviates dextran sulfate sodium-induced colitis in mice by modulating gut microbiota, restoring intestinal barrier function and attenuating TLR4/NF-κB signaling cascades. J. Pharm. Biomed. Anal. 209, 114529. https://doi.org/10.1016/j.jpba.2021.114529 (2022).
Zhang, L., Li, D.-C. & Liu, L.-F. Paeonol: Pharmacological effects and mechanisms of action. Int. Immunopharmacol. 72, 413–421. https://doi.org/10.1016/j.intimp.2019.04.033 (2019).
Chen, G. et al. Paeonol ameliorates monosodium urate-induced arthritis in rats through inhibiting nuclear factor-κB-mediated proinflammatory cytokine production. Phytother. Res. 33, 2971–2978. https://doi.org/10.1002/ptr.6472 (2019).
Wang, F. et al. Paeonol ameliorates lipopolysaccharides-induced acute lung injury by regulating TLR4/MyD88/ NF-κB signaling pathway. Pharmazie 74, 101–106. https://doi.org/10.1691/ph.2019.8860 (2019).
Al-Taher, A. Y., Morsy, M. A., Rifaai, R. A., Zenhom, N. M. & Abdel-Gaber, S. A. Paeonol attenuates methotrexate-induced cardiac toxicity in rats by inhibiting oxidative stress and suppressing TLR4-induced NF-κB inflammatory pathway. Mediators Inflamm. 2020, 8641026. https://doi.org/10.1155/2020/8641026 (2020).
Zhai, K.-F. et al. Protective effects of paeonol on inflammatory response in IL-1β-induced human fibroblast-like synoviocytes and rheumatoid arthritis progression via modulating NF-κB pathway. Inflammopharmacology https://doi.org/10.1007/s10787-017-0385-5 (2017).
Lou, Y. et al. Paeonol Inhibits IL-1β-Induced Inflammation via PI3K/Akt/NF-κB Pathways: In vivo and vitro studies. Inflammation 40, 1698–1706. https://doi.org/10.1007/s10753-017-0611-8 (2017).
Ishiguro, K. et al. Paeonol attenuates TNBS-induced colitis by inhibiting NF-kappaB and STAT1 transactivation. Toxicol. Appl. Pharmacol. 217, 35–42 (2006).
Ge, Y. et al. Paeonol alleviates dextran sodium sulfate induced colitis involving Candida albicans-associated dysbiosis. Med. Mycol. 59, 335–344. https://doi.org/10.1093/mmy/myaa053 (2021).
Jin, X. et al. Anti-inflammatory and anti-oxidative activities of paeonol and its metabolites through blocking MAPK/ERK/p38 signaling pathway. Inflammation 39, 434–446. https://doi.org/10.1007/s10753-015-0265-3 (2016).
Zhao, H., Wang, X., Liu, S. & Zhang, Q. Paeonol regulates NLRP3 inflammasomes and pyroptosis to alleviate spinal cord injury of rat. BMC Neurosci. 23, 16. https://doi.org/10.1186/s12868-022-00698-9 (2022).
Tian, T., Wang, Z. & Zhang, J. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid. Med. Cell Longev. 2017, 4535194. https://doi.org/10.1155/2017/4535194 (2017).
Yan, M. et al. ROS responsive polydopamine nanoparticles to relieve oxidative stress and inflammation for ameliorating acute inflammatory bowel. Biomater. Adv. 142, 213126. https://doi.org/10.1016/j.bioadv.2022.213126 (2022).
Wang, K. et al. Bergenin, acting as an agonist of PPARγ, ameliorates experimental colitis in mice through improving expression of SIRT1, and therefore inhibiting NF-κB-mediated macrophage activation. Front. Pharmacol. 8, 981. https://doi.org/10.3389/fphar.2017.00981 (2017).
Zong, S.-Y., Pu, Y.-Q., Xu, B.-L., Zhang, T. & Wang, B. Study on the physicochemical properties and anti-inflammatory effects of paeonol in rats with TNBS-induced ulcerative colitis. Int. Immunopharmacol. 42, 32–38. https://doi.org/10.1016/j.intimp.2016.11.010 (2017).
Bernstein, C. N., Wajda, A. & Blanchard, J. F. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology 129, 827–836 (2005).
Krugliak Cleveland, N., Torres, J. & Rubin, D. T. What does disease progression look like in ulcerative colitis, and how might it be prevented?. Gastroenterology 162, 1396–1408. https://doi.org/10.1053/j.gastro.2022.01.023 (2022).
Alex, P. et al. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm. Bowel Dis. 15, 341–352. https://doi.org/10.1002/ibd.20753 (2009).
Nakase, H., Sato, N., Mizuno, N. & Ikawa, Y. The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmun. Rev. 21, 103017. https://doi.org/10.1016/j.autrev.2021.103017 (2022).
Wang, S. et al. IL-10 enhances T cell survival and is associated with faster relapse in patients with inactive ulcerative colitis. Mol. Immunol. 121, 92–98. https://doi.org/10.1016/j.molimm.2020.03.001 (2020).
Wang, Y. et al. Long non-coding RNA MEG3 alleviated ulcerative colitis through upregulating miR-98-5p-sponged IL-10. Inflammation 44, 1049–1059. https://doi.org/10.1007/s10753-020-01400-z (2021).
Thompson, P. W., Bayliffe, A. I., Warren, A. P. & Lamb, J. R. Interleukin-10 is upregulated by nanomolar rosiglitazone treatment of mature dendritic cells and human CD4+ T cells. Cytokine 39, 184–191 (2007).
Li, Y. et al. Microglial Pdcd4 deficiency mitigates neuroinflammation-associated depression via facilitating Daxx mediated PPARγ/IL-10 signaling. J. Neuroinflamm. 21, 143. https://doi.org/10.1186/s12974-024-03142-3 (2024).
Chen, X. et al. Exosomes derived from reparative M2-like macrophages prevent bone loss in murine periodontitis models via IL-10 mRNA. J. Nanobiotechnol. 20, 110. https://doi.org/10.1186/s12951-022-01314-y (2022).
Garabuczi, É. et al. Nur77 and PPARγ regulate transcription and polarization in distinct subsets of M2-like reparative macrophages during regenerative inflammation. Front. Immunol. 14, 1139204. https://doi.org/10.3389/fimmu.2023.1139204 (2023).
Shi, X. et al. Paeonol attenuated vascular fibrosis through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Front. Pharmacol. 12, 765482. https://doi.org/10.3389/fphar.2021.765482 (2021).
Zhang, Z. et al. Paeonol accelerates skin wound healing by regulating macrophage polarization and inflammation in diabetic rats. Korean J. Physiol. Pharmacol. 27, 437–448. https://doi.org/10.4196/kjpp.2023.27.5.437 (2023).
Liu, M.-H. et al. Paeonol attenuates cigarette smoke-induced lung inflammation by inhibiting ROS-sensitive inflammatory signaling. Mediators Inflamm. 2014, 651890. https://doi.org/10.1155/2014/651890 (2014).
Dubois, R. N. et al. Cyclooxygenase in biology and disease. FASEB J. 12, 1063–1073 (1998).
Dudhgaonkar, S. P., Tandan, S. K., Kumar, D., Raviprakash, V. & Kataria, M. Influence of simultaneous inhibition of cyclooxygenase-2 and inducible nitric oxide synthase in experimental colitis in rats. Inflammopharmacology 15, 188–195 (2007).
Morteau, O. et al. Impaired mucosal defense to acute colonic injury in mice lacking cyclooxygenase-1 or cyclooxygenase-2. J. Clin. Invest. 105, 469–478 (2000).
Wang, D. & Dubois, R. N. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene 29, 781–788. https://doi.org/10.1038/onc.2009.421 (2010).
Kuo, W.-T. et al. Inflammation-induced occludin downregulation limits epithelial apoptosis by suppressing caspase-3 expression. Gastroenterology 157, 1323–1337. https://doi.org/10.1053/j.gastro.2019.07.058 (2019).
Aviello, G. & Knaus, U. G. ROS in gastrointestinal inflammation: Rescue or sabotage?. Br. J. Pharmacol. 174, 1704–1718. https://doi.org/10.1111/bph.13428 (2017).
Wang, Z. et al. Oxidative stress and carbonyl lesions in ulcerative colitis and associated colorectal cancer. Oxid. Med. Cell. Longev. 2016, 9875298. https://doi.org/10.1155/2016/9875298 (2016).
Zhang, M., Li, X., Zhang, Q., Yang, J. & Liu, G. Roles of macrophages on ulcerative colitis and colitis-associated colorectal cancer. Front. Immunol. 14, 1103617. https://doi.org/10.3389/fimmu.2023.1103617 (2023).
Yang, Z. et al. A potential therapeutic target in traditional Chinese medicine for ulcerative colitis: Macrophage polarization. Front. Pharmacol. 13, 999179. https://doi.org/10.3389/fphar.2022.999179 (2022).
Han, X., Ding, S., Jiang, H. & Liu, G. Roles of macrophages in the development and treatment of gut inflammation. Front. Cell Dev. Biol. 9, 625423. https://doi.org/10.3389/fcell.2021.625423 (2021).
Fang, J., Wang, H., Xue, Z., Cheng, Y. & Zhang, X. PPARγ: The central mucus barrier coordinator in ulcerative colitis. Inflamm. Bowel Dis. 27, 732–741. https://doi.org/10.1093/ibd/izaa273 (2021).
Bertin, B., Dubuquoy, L., Colombel, J.-F. & Desreumaux, P. PPAR-gamma in ulcerative colitis: A novel target for intervention. Curr. Drug. Targets 14, 1501–1507 (2013).
Su, C. G. et al. A novel therapy for colitis utilizing PPAR-gamma ligands to inhibit the epithelial inflammatory response. J. Clin. Invest. 104, 383–389 (1999).
Desreumaux, P. et al. Attenuation of colon inflammation through activators of the retinoid X receptor (RXR)/peroxisome proliferator-activated receptor gamma (PPARgamma) heterodimer. A basis for new therapeutic strategies. J. Exp. Med. 193, 827–838 (2001).
Dong, J. et al. Naringin exerts therapeutic effects on mice colitis: A study based on transcriptomics combined with functional experiments. Front. Pharmacol. 12, 729414. https://doi.org/10.3389/fphar.2021.729414 (2021).
Wang, N. et al. Honokiol alleviates ulcerative colitis by targeting PPAR-γ-TLR4-NF-κB signaling and suppressing gasdermin-D-mediated pyroptosis in vivo and in vitro. Int. Immunopharmacol. 111, 109058. https://doi.org/10.1016/j.intimp.2022.109058 (2022).
Cheng, C. et al. Hyperoside ameliorates DSS-induced colitis through MKRN1-mediated regulation of PPARγ signaling and Th17/Treg balance. J. Agric. Food Chem. 69, 15240–15251. https://doi.org/10.1021/acs.jafc.1c06292 (2021).
Bouhlel, M. A. et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 6, 137–143 (2007).
Zhang, O. & Zhang, J. Atorvastatin promotes human monocyte differentiation toward alternative M2 macrophages through p38 mitogen-activated protein kinase-dependent peroxisome proliferator-activated receptor γ activation. Int. Immunopharmacol. 26, 58–64. https://doi.org/10.1016/j.intimp.2015.03.005 (2015).
Mitchell, S., Vargas, J. & Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip Rev. Syst. Biol. Med. 8, 227–241. https://doi.org/10.1002/wsbm.1331 (2016).
Wong, J. et al. RIPK1 mediates TNF-induced intestinal crypt apoptosis during chronic NF-κB activation. Cell Mol. Gastroenterol. Hepatol. 9, 295–312. https://doi.org/10.1016/j.jcmgh.2019.10.002 (2020).
Lee, H.-J. et al. Anti-inflammatory effects of Canavalia gladiata in macrophage cells and DSS-induced colitis mouse model. Am. J. Chin. Med. 47, 1571–1588. https://doi.org/10.1142/S0192415X19500800 (2019).
Leesnitzer, L. M. et al. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry 41, 6640–6650 (2002).
Seargent, J. M., Yates, E. A. & Gill, J. H. GW9662, a potent antagonist of PPARgamma, inhibits growth of breast tumour cells and promotes the anticancer effects of the PPARgamma agonist rosiglitazone, independently of PPARgamma activation. Br. J. Pharmacol. 143, 933–937 (2004).
Yi, J.-H., Park, S.-W., Brooks, N., Lang, B. T. & Vemuganti, R. PPARgamma agonist rosiglitazone is neuroprotective after traumatic brain injury via anti-inflammatory and anti-oxidative mechanisms. Brain Res. 1244, 164–172. https://doi.org/10.1016/j.brainres.2008.09.074 (2008).
Wen, Q. et al. Chrysophanol demonstrates anti-inflammatory properties in LPS-primed RAW 264.7 macrophages through activating PPAR-γ. Int. Immunopharmacol. 56, 90–97. https://doi.org/10.1016/j.intimp.2018.01.023 (2018).
Acknowledgements
All the authors are thankful to the support from Xiamen University.
Funding
This work was supported by a grant from The National Natural Science Foundation of China (No. 81870388).
Author information
Authors and Affiliations
Contributions
S.Y.C. and G.Y.L. designed the “ideas”; S.Y.C., C.C.D., R.J.Z, Q.L.and Z.Z.G. to deal with the information efficiently; S.Y.C., W.J.C.and Z.Z.G. wrote the manuscript; G.Y.L. revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cheng, S., Chen, W., Guo, Z. et al. Paeonol alleviates ulcerative colitis by modulating PPAR-γ and nuclear factor-κB activation. Sci Rep 14, 18390 (2024). https://doi.org/10.1038/s41598-024-68992-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68992-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.