Abstract
Vitamin D modulates innate and adaptive immunity, the molecular mechanisms of which we aim to understand under human in vivo conditions. Therefore, we designed the study VitDHiD (NCT03537027) as a human investigation, in which 25 healthy individuals were supplemented with a single vitamin D3 bolus (80,000 IU). Transcriptome-wide differential gene expression analysis of peripheral blood mononuclear cells (PBMCs), which were isolated directly before and 24 h after supplementation, identified 452 genes significantly (FDR < 0.05) responding to vitamin D. In vitro studies using PBMCs from the same individuals confirmed 138 of these genes as targets of 1α,25-dihydroxyvitamin D3. A subset of the 91 most regulated in vivo vitamin D target genes indicated focal adhesion as the major pathway being upregulated by vitamin D3 supplementation of healthy individuals. Differences in the individual-specific responsiveness of in vivo vitamin D target genes in relation to the increase of the person’s vitamin D status allowed a segregation of the VitDHiD participants into 9 high, 12 mid and 4 low responders. The expression profile of nearly 600 genes elucidate the difference between high and low vitamin D responders, the most prominent of which is the HLA-C (major histocompatibility complex, class I, C) gene.
Similar content being viewed by others
Introduction
Vitamin D influences both innate and adaptive immunity, enhancing the body's ability to combat infectious diseases like tuberculosis1,2 while preventing immune overreactions associated with autoimmune diseases such as multiple sclerosis3,4, rheumatoid arthritis5 and type I diabetes6. The biologically inert vitamin D3 is converted in the liver to 25-hydroxyvitamin D3 (25(OH)D3), which, due to its relatively long half-life of two weeks, serves as a biomarker for vitamin D status7.
The most biologically active vitamin D metabolite is 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3), produced from 25(OH)D3 primarily in the kidneys and also in some immune cells2. This metabolite functions as a nuclear hormone that binds with high affinity to the vitamin D receptor (VDR), a transcription factor8,9. Mechanistically, vitamin D’s primary molecular effect involves the ligand-triggered binding of VDR to enhancer regions near the transcription start sites (TSSs) of primary vitamin D target genes. This binding alters chromatin accessibility10, increases H3K27ac marks at active chromatin regions like enhancers, H3K4me3 marks at TSS regions11, and facilitates DNA looping of enhancer regions to TSS regions12. Thus, vitamin D modulates the epigenome and transcriptome of VDR-expressing tissues and cell types13,14.
The genome-wide effects of vitamin D have been comprehensively investigated in the human monocytic leukemia cell line THP-115. However, these principles need validation under human in vivo conditions. PBMCs comprise B cells, CD4+ and CD8+ T cells, natural killer (NK) cells and monocytes, and can be obtained with minimal harm to human volunteers14. Studies on vitamin D have typically used these cells either in vitro, with stimulation by supraphysiological doses of 1,25(OH)2D316,17, or in vivo over months of daily vitamin D3 supplementation18,19. Contrarily, the VitDbol study (NCT02063334, ClinicalTrials.gov)20,21 isolated PBMC samples 1 and 2 days16,17 after a vitamin D3 bolus (80,000 IU) supplementation, confirming several well-known in vitro vitamin D target genes under in vivo conditions and categorizing participants into high, mid, and low responders. This led to the concept of the vitamin D response index22, based on the differential activity of vitamin D target genes in individuals23,24.
In this study, we present the molecular findings from the VitDHiD intervention trial, which follows the VitDbol study design, isolating samples one day after vitamin D3 bolus supplementation. Transcriptome analysis of PBMCs identified 452 in vivo vitamin D target genes and categorized the 25 participants into high (9), mid (12), and low (4) responders, providing insight into the molecular basis of the vitamin D response index.
Material and methods
Sample collection
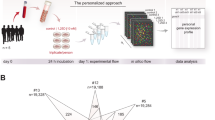
The VitDHiD intervention study had been performed in May 2018 in Kuopio, Finland. The inclusion criteria of the study were an age between 20 and 60 years and a body mass index (BMI) of 20–25 kg/m2. Exclusion criteria were a history of kidney stones, renal failure or dialysis, hypercalcemia, hypo- or hyperparathyroidism, severe liver disease (cirrhosis), sarcoidosis or other granulomatous diseases, such as active chronic tuberculosis or Wegener's granulomatosis. Moreover, further reasons for an exclusion were continuous use of anti-inflammatory medicines and regular use of supplements containing over 800 IU vitamin D3. The ethics committee of the Northern Savo Hospital District had approved the study protocol (#515/2018). In the context of the study, 25 healthy individuals (12 females and 13 males, age 21–54, BMI 21.4–25.6, Table 1) had been supplemented with a vitamin D3 bolus of in total 80,000 IU, which was given in two equal doses together with breakfast and lunch. The bolus represents a monthly dose of vitamin D3, which was applied only once to the volunteers, while other vitamin D interventions, such as ViDA25, used even 100,000 IU vitamin D3/month over a period of more than 2 years. Thus, 80.000 IU vitamin D3 used in this study represent a safe dose. Blood was drawn directly before supplementation (d0) and 24 h later (d1) for serum and PBMC isolation. All participants gave written informed consent to participate in the study. All experiments were performed in accordance with relevant guidelines and regulations.
Serum measurements
Serum total 25(OH)D (i.e., 25(OH)D2 and 25(OH)D3) levels were measured using high-performance liquid chromatography (1290 Infinity II LC System, Agilent) coupled with mass spectrometry detection (API 4000 LC–MS/MS System, SCIEX)26.
PBMC isolation and culture
Without any in vitro culture, PBMCs of the 25 VitDHiD study participants (#1–18, 20–26) were isolated within one hour after the collection of 24 ml peripheral blood at d0 and d1 using Vacutainer CPT Cell Preparation Tubes with sodium citrate (Becton Dickinson) according to manufacturer’s instructions. The PBMCs of the 25 study participants have the composition 7.4 ± 2.1% B cells, 24.5 ± 6.4% CD4+ T cells, 14.9 ± 4.1% CD8+ T cells, 13.8 ± 4.5% NK cells and 38.6 ± 6.5% monocytes, while there are only 0.8 ± 0.4% neutrophiles as determined by Cibersort27 (https://cibersortx.stanford.edu) from RNA-seq (RNA-sequencing) data. After washing with phosphate-buffered saline, PBMCs were aliquoted (4 million cells/ml) and stored in liquid nitrogen until use for either RNA isolation (d0 and d1) or in vitro cell culture (only d0 samples of the 14 study participants #1–12, 23 and 26). PBMC in vitro culture was performed in RPMI 1640 medium (0.5 million cells/ml) supplemented with 10% charcoal-stripped fetal calf serum, 2 mM L-glutamine, 0.1 mg/ml streptomycin and 100 U/ml penicillin. The cells were exposed for 24 h to either 1,25(OH)2D3 (10 nM, Sigma-Aldrich) or solvent (0.1% EtOH) at 37 °C in a humidified 95% air/5% CO2 incubator.
RNA-seq analysis
Total RNA was extracted from PBMCs using the High Pure RNA Isolation kit (Roche) following the manufacturer’s protocol. RNA quality was assessed on an Agilent Bioanalyzer and library preparation was performed after rRNA depletion applying kits and protocols from New England Biolabs. RNA-seq libraries were sequenced at 75 bp read length on a NextSeq500 system (Illumina) using standard protocols at the Gene Core of the EMBL (Heidelberg, Germany). The quality of the files was evaluated using FastQC (version 0.11.9, www.bioinformatics.babraham.ac.uk/projects/fastqc) (Table S1). Read alignment to GRCh38 reference genome (Ensembl version 111.38) was done using STAR28 (version 2.7.2a) and quantification was carried out using Featurecounts29 (version 2.0.1) with default parameters. Human genome nomenclature committee (HGNC) gene symbols of all datasets were brought up to date using the R package HGNC helper (version 0.8.1, https://CRAN.R-project.org/package=HGNChelper) and annotated with gene identifiers, description, genomic location and biotype by accessing the Ensembl database (release 100) via the R package BiomaRt30 (version 2.42.1). Entrez Gene identifiers were added with the R package org.Hs.eg.db (version 3.10.0) and incomplete mappings of target genes manually retrieved from NCBI (www.ncbi.nlm.nih.gov/home/genes). Genes missing genomic position information or being mitochondrially encoded were removed. In this study, we focused exclusively on protein coding genes.
Differential gene expression analysis
Differential gene expression analysis was computed in R (version 4.3.1) in the MacOS 13 (Ventura) operating system using the tool EdgeR31 (version 4.0.14). The analysis focused on 19,287 protein-coding genes, in order to mitigate transcriptional noise expected by non-coding genes. Read counts were normalized for differences in library size to counts per million (CPM). Genes with very low expression were filtered out by applying the function FilterByExpr(), in order to mitigate the multiple testing problem and to not interfere with the statistical approximations of the EdgeR pipeline. After filtering, library sizes were recomputed and trimmed mean of M-value normalization was applied. The transcriptome data structure was explored via the method MDS (multidimensionality scaling) (Fig. S1), which was computed via EdgeR's function plotMDS(), in which distances approximate the typical log2 fold change (FC) between the samples. This distance was calculated as the root mean square deviation (Euclidean distance) of the log2FC values of genes with significant (false discovery rate (FDR) < 0.05) changes after vitamin D3 supplementation or 1,25(OH)2D3 treatment. The gene-wise statistical test for differential expression was computed using the generalized linear model quasi-likelihood pipeline32. Trended negative binomial dispersion estimate was calculated using the method CoxReid profile-adjusted likelihood and together with empirical Bayes-moderated quasi-likelihood gene-wise dispersion estimates used for generalized linear model fitting. The empirical Bayes shrinkage was robustified against outlier dispersions as recommended32. The glmTreat approach was used to test for differential expression relative to absolute logFC > 1 for in vitro experiments. Genes with a Benjamini–Hochberg corrected p-value, i.e., FDR < 0.05, and a total trimmed mean of M value-normalized logCPM > log210 (average gene expression level for all samples) were considered as significant vitamin D target genes (Tables S2 and S3). Venn diagrams were created via the webtool jvenn33 (http://jvenn.toulouse.inrae.fr/app).
Characterization of VDR enhancers
The genomic regions of key in vivo vitamin D target genes were investigated for VDR binding enhancers by using epigenome-wide data from THP-1 cells, which had been stimulated for 24 h with 10 nM 1,25(OH)2D3 or solvent (0.1% EtOH). These were ChIP-seq (chromatin immunoprecipitation sequencing) data of (i) the histone marker of active promoter regions, H3K4me311, (ii) the histone marker of active chromatin, H3K27ac11 and (iii) VDR34 as well as FAIRE-seq (formaldehyde-assisted identification of regulatory elements followed by sequencing) data10. Comparable epigenetic data for PBMCs are not publicly available. However, a comparison of the VDR cistrome of 6 different in vitro models indicated that there is large overlap in the genome-wide VDR binding pattern of cells deriving from the same lineage35. With help of the IGV-browser36 these data sets were displayed for the genomic regions of the vitamin D target genes and VDR enhancers showing responsiveness to 1,25(OH)2D3 were marked. One Mb of the genomic region up- and downstream of the target gene’s TSS were screened but only essential regions are shown.
Relating vitamin D target genes to pathways
Gene enrichment analysis was performed via the webtool EnrichR37 (https://maayanlab.cloud/Enrichr). All genes/proteins of the indicated pathways were inspected for their function, such as type of encoded protein and its primary biological function, with the help of databases like Human Protein Atlas (www.proteinatlas.org) and GeneCards (www.genecards.org). The latter two databases were also used for the categorization of proteins encoded by vitamin D target genes. The network of interacting genes was plotted according to rules of KEGG (Kyoto Encyclopedia of Genes and Genomes)38 signaling pathway diagrams. In a pathway a set of genes (nodes) was connected, in order to reflect the functional relationships between them. Arrows connecting one node with another indicate positive downstream effects, while lines ending with a shorter perpendicular line display inhibitory effects. The nodes were colored based on the logFC in gene expression between d1 and d0 (downregulation in red, upregulation in green). White nodes indicate genes expressed in PBMCs but not being significantly regulated by vitamin D3 bolus supplementation. The arrow next to the gene name in each colored node indicates the overall net effect of vitamin D3 supplementation on the expression of the respective gene.
Classification of study participants
The classification of participants into high, mid or low responders was performed through plotting for each study participant the average absolute logFC values of all 452 in vivo vitamin D target genes (or subsets of them) (Table S2) over changes of total 25(OH)D concentrations (d1/d0) (Table 1) of the respective person. A trendline passing through 1/0 divided the plot and the orthogonal distance of each data point from the line was determined. These distances were ranked in a descending order and the algorithm K-means (KMeans function from sklearn.cluster library (https://pypi.org/project/scikit-learn/)) was used for clustering the individuals into three groups (Fig. S2A). High responders are found at the upper left of the plot (green), mid responders around the trendline (yellow) and low responder at the lower right corner (red). The distance from the trendline is proportional to the vitamin D response index, which negatively correlates very well (− 0.94 (Pearson correlation test), P-value 2.1 × 10–12 (paired T-test)) with the ratio of the vitamin D status at d1 and d0 (Fig. S2B), but less well (0.51, P-value 0.01) with the vitamin D status at d0 (Fig. S2C). In contrast, the vitamin D response index does not correlate with the BMI (0.24, P-value 0.26) of the study participants (Fig. S2D) or their age (0.04, P-value 0.84) (Fig. S2E). Please note that our segregation approach is not a linear regression analysis.
Results
The VitDHiD intervention trial
The VitDHiD intervention study had been designed as a human investigation, in order to study the effects of vitamin D3 supplementation on gene expression in human immune cells. In order to allow direct comparisons, the in vivo gene expression assessment was as similar as possible to that of in vitro cell culture experiments. In the context of the VitDHiD trial, 25 healthy individuals were supplemented with a vitamin D3 bolus (80,000 IU) and blood samples were taken directly before supplementation (d0) and 24 h later (d1). This protocol follows our previous trial VitDbol20,21. Total 25(OH)D serum levels indicated that the basal vitamin D status of the study participants ranged from 15.6 to 49.8 ng/ml and increased through vitamin D3 bolus supplementation within 24 h by 14.9–96.1% (Table 1). Thus, a single vitamin D3 dose of 80,000 IU led within one day to a significant (P-value 1.48 × 10–13, paired T-test) increase of the vitamin D status of all study participants into vitamin D sufficiency (29.5–64.1 ng/ml).
VitDHiD identified in vivo vitamin D target genes
RNA-seq analysis was executed for the 50 in vivo PBMC samples as well as for the 28 in vitro samples. The inspection of the MDS plot of the in vivo data (Fig. S1A) displayed for each of the 25 individuals in dimension 1 the d0 data point left of the respective d1 data point. The MDS plot of the in vitro data (Fig. S1B) clearly distinguished in dimension 1 the solvent samples (left) from the 1,25(OH)2D3-treated samples (right). Differential gene expression of the total set of 9374 expressed protein coding genes (average normalized expression of all 50 samples > 10) indicated 452 in vivo vitamin D target genes (FDR < 0.05 and log2CPM > log210) (Fig. S3A, Table S2), of which 181 are upregulated and 271 downregulated. For comparison, the 9628 commonly expressed protein coding genes in the 28 in vitro samples revealed 2661 target genes (FDR < 0.05) (Fig. S3B, Table S3). However, when the additional threshold of absolute logFC > 1 is applied, only 230 in vitro vitamin D target genes are remaining. From these in vitro target genes, 113 are upregulated and 117 are downregulated.
The list of 452 in vivo vitamin D target genes was compared with different in vitro datasets (all absolute logFC > 1, treatment for 24 h with 10 nM 1,25(OH)2D3), which had been obtained from the same group of 25 VitDHiD study participants. We observed an overlap with 36 of 230 target genes of the 14 participants investigated only once (Fig. S3C), with 96 of 918 target genes of the five individuals (#5, 9, 12, 13 and 14) tested in triplicate26 as well as with 65 of 466 target genes17 and with 89 of 634 target genes39 from a single person (#14) studied in triplicate (Fig. 1A). In total, 138 in vivo vitamin D target genes could be verified by in vitro data.
In vivo and in vitro vitamin D target genes. A Venn diagram was used, in order to display the overlap between the 452 in vivo vitamin D target genes identified in PBMCs isolated from 25 participants of the VitDHiD study and in vitro target genes found by different approaches from the pool of the same individuals (A). The identity of the overlapping genes can be obtained from Table S2. A schematic model indicates the location (within or outside the cell) and function (sorted into four categories) of the proteins encoded by the 16 vitamin D target genes that were overlapping between in vivo and in vitro datasets (B). Arrows indicate the direction of regulation of the respective genes/proteins.
In summary, the setup of the VitDHiD study allowed the identification of 452 genes that within 24 h respond under in vivo conditions in PBMCs to vitamin D3 supplementation. Some 30% of these in vivo vitamin D target genes could be confirmed under in vitro conditions using cells of the same cohort of participants.
In vivo function of vitamin D based on target gene subsets
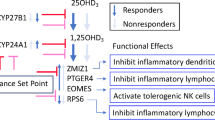
The 16 genes ALOX5 (arachidonate 5-lipoxygenase), ANPEP (alanyl aminopeptidase, membrane), AQP9 (aquaporin 9), ASAP2 (ArfGAP with SH3 domain, ankyrin repeat and PH domain 2), CD300E (CD300E molecule), CD93, CRISPLD2 (cysteine rich secretory protein LCCL domain containing 2), CXCL5 (C-X-C motif chemokine ligand 5), FPR1 (formyl peptide receptor 1), MPEG1 (macrophage expressed 1), S100A12 (S100 calcium binding protein A12), S100A9, SLC11A1 (solute carrier family 11 member 1), TGFBI (transforming growth factor beta induced), THBS1 (thrombospondin 1) and TREM1 (triggering receptor expressed on myeloid cells 1) are vitamin D targets not only under in vivo conditions but also in all four in vitro studies (Fig. 1A). The genes ASAP2 and CXCL5 are upregulated by vitamin D, while the remaining 14 genes are downregulated. A screening for VDR-bound enhancers using published ChIP-seq and FAIRE-seq datasets from THP-1 cells identified for all 16 genes one to three VDR-binding enhancers in a distance ranking from very close to the TSS (ANPEP, FPR1, S100A9 and TREM1) up to 246 kb apart (CD93) from the TSS (Fig. S4). Accordingly, all 16 genes seem to be directly regulated by ligand-triggered VDR, but they can be distinguished based on the intensity of the VDR ChIP-seq peaks into strong binders (CD93, ASAP2, CRISPLD2, TREM1 and AQP9, Fig. S4A), medium binders (ALOX5, SLC11A1, TGFBI, THBS1 and ANPEP, Fig. S4B) and weak binders (S100A9, S100A12, FPR1, CD300E, MPEG1 and CXCL5, Fig. S4C). As expected for immune cells like PBMCs, the functions of the proteins encoded by the 16 genes relates to immunity, such as “communication via cytokines”, “vesicular and secretory peptides”, “immune responsiveness” and “extracellular matrix (ECM) organization and cell motility” (Fig. 1B).
Another interesting subset of the 452 in vivo vitamin D targets are the 91 genes that in average of all 25 study participants have an absolute logFC > 0.25 (Table S2). These are 66 upregulated and 26 downregulated genes, but only the genes AQP9, ASAP2, CXCL5 and THBS1 are in common with list of 16 confirmed targets (Fig. 1A). Well-known members of the list of the 91 genes are CD14, CDKN1A (cyclin dependent kinase inhibitor 1A), GADD45A (growth arrest and DNA damage inducible alpha), HIF1A (hypoxia inducible factor 1 subunit alpha), NAMPT (nicotinamide phosphoribosyltransferase), PER1 (period circadian regulator 1) and VEGFA (vascular endothelial growth factor A). These candidates suggest an in vivo role of vitamin D in response to bacteria, cell cycle regulation, energy metabolism, circadian rhythms as well as angiogenesis. However, gene enrichment analysis via EnrichR suggested most reliable results from the pathway database Wikipathways40 (www.wikipathways.org) (Table 2). As expected, FDR ranking of the pathways identified the VDR pathway (WP2877) as most significant followed by the focal adhesion PI3K (phosphatidylinositol-4,5-bisphosphate 3-kinase)-AKT (AKT murine thymoma viral oncogene homolog)-TOR (target of rapamycin) signaling pathway (WP3932) and the regular focal adhesion pathway (WP306). Taken the latter two pathways together, 11 of the 91 genes (CDKN1A, GNG11 (G protein subunit gamma 11), HIF1A, ITGA2B (integrin subunit alpha 2B), ITGB3, ITGB5, MYL9 (myosin light chain 9), MYLK (myosin light chain kinase), PFKFB3 (6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3), THBS1 and VEGFA), are involved in the process of focal adhesion.
Taken together, the 16 in vivo vitamin D target genes that were confirmed by all in vitro PBMC datasets can be categorized into (i) communication via cytokines, (ii) vesicular and secretory peptides, (iii) immune responsiveness as well as (iv) ECM organization and cell motility. In contrast, gene enrichment analysis of the 91 most responsive in vivo vitamin D target genes indicated that the process of focal adhesion is the major pathway reflecting the in vivo function of vitamin D in immune cells of healthy individuals.
Vitamin D upregulates genes related to focal adhesion in healthy individuals
In order to visualize the effect of vitamin D3 supplementation on genes related to focal adhesion, we displayed the pathway following the rules of KEGG and color-coded the nodes representing up- and downregulated in vivo vitamin D target genes (Fig. 2A). From the 11 vitamin D-triggered genes, GNG11, ITGA2B, ITGB3, ITGB5, MYL9 and MYLK are upregulated and they promote focal adhesion. In contrast, the genes CDKN1A, HIF1A, PFKFB3, THBS1 and VEGFA are downregulated by vitamin D, but in case of CDKN1A this leads to a supportive effect on the pathway. Thus, in average of 25 study participants, the response of 7 of 11 in vivo vitamin D target genes activates focal adhesion.
Regulation of genes related to focal adhesion by vitamin D3 supplementation. Representation of the focal adhesion pathway following the design of KEGG (A). Upregulated vitamin D target genes are labeled green and downregulated red. Color intensity is proportional to logFC of gene expression between d1 and d0. A heatmap following the same color scheme indicates the regulation of 11 in vivo vitamin D target genes involved in the pathway for each of the 25 study participants (B). The individuals are ranked by sum of regulation of all 11 genes and the genes are sorted by descending intensity of upregulation.
The participant-specific regulation of the 11 target genes monitors that there is a large interindividual variation of the effect of vitamin D3 supplementation on the focal adhesion pathway (Fig. 2B). For example, in PBMCs of individuals #2, 12, 18 and 22 the genes MYL9, GNG11, ITGB3, ITGA2B, MYLK and ITGB5 (in the order of their responsiveness) are upregulated, while in other participants (#4, 5, 8 and 26) the same genes are downregulated. In contrast, the THBS1 gene is significantly downregulated in individuals #7, 12, 14 and 21 but upregulated in persons #2, 6, 11 and 18. Thus, in comparison of the study participants, in vivo vitamin D target genes can vary both in the intensity of their response as well as in the direction of their regulation.
In summary, on average of the investigated healthy subjects, genes supporting the process of focal adhesion are more upregulated through vitamin D3 supplementation than downregulated. However, the study participants differ significant in the response of their in vivo vitamin D target genes, which in part has opposite effects on pathway regulation.
Segregation of study participants into high, mid and low vitamin D responders
In order to evaluate the vitamin D responsiveness of the 25 individuals, we plotted for each person the average absolute logFC of all 452 in vivo vitamin D target genes over the ratio of the vitamin D status at d1 compared to that at d0 (Fig. 3A). K-means clustering was performed, in order to segregate the individuals into 9 high (green), 12 mid (yellow) and 4 low (red) responders (Fig. S2A). When the list of investigated genes was reduced to 91 targets, basically the same result was obtained, but individuals #7 and 26 were classified only as mid responders (Fig. 3B). Furthermore, when the gene list was restricted to the 16 in vivo vitamin D targets that were confirmed by all 4 in vitro datasets, individual #2 was graded as mid responder but participant #21 as additional high responder (Fig. 3C). We made similar observations, when we used other subsets of 452 in vivo vitamin D target genes, such as the 181 upregulated genes, the 271 downregulated genes, the 138 genes that were confirmed by at least one in vitro dataset or the 11 genes involved in focal adhesion (Fig. S5). Thus, for some individuals in the border of high to mid as well as mid to low responders the choice of the list of vitamin D target genes is critical for their classification. However, individuals #9, 12, 14 and 25 were always found as high responders, #4, 6, 8, 10, 15, 20 and 24 constantly as mid responders and #11, 16, 17 and 23 each time as low responders.
Segregation of VitDHiD study participants. The segregation of the 25 individuals into high (green), mid (yellow) and low (red) responders was based on all 452 in vivo vitamin D target genes (A), a subset of 91 genes with a logFC > 0.25 (B) and 11 genes involved in focal adhesion (C). Numbers indicate the individuals listed in Table 1.
Taken together, the responsiveness of in vivo vitamin D target genes can be used to segregate the VitDHiD study participants into high, mid and low responders. For some 40% of individuals that are at the border between high and mid or mid and low responders, the choice of target genes subset is critical for their exact classification.
Molecular distinction between high and low vitamin D responders
The observation of individual-specific responsiveness to vitamin D3 supplementation raises the question of its molecular explanation. Therefore, we compared the gene expression pattern of the 4 stable high responders (#9, 12, 14 and 25) with that of the 4 stable low responders (#11, 16, 17 and 23) at both d0 and d1. Before supplementation 588 genes were significantly (FDR < 0.05) different in their expression when comparing high with low responders (Table S4). One day of a vitamin D3 bolus reduced the total number of differently expressed genes to 265 (Table S5). Interestingly, only 107 genes of both lists are identical, i.e., vitamin D3 supplementation equalized 481 genes between high and low responders but additional 158 genes became differently expressed between the two groups (Fig. 4A). When we restrict the gene lists to an absolute logFC larger than 1, only 15 genes are differently expressed between high and low responders at d0 and even only 12 genes at d1 (Fig. 4B). Interestingly, from the five overlapping genes HLA-C, DGKK (diacylglycerol kinase kappa) and CES1 (carboxylesterase 1) were higher expressed in high responders, while EDAR (ectodysplasin A receptor) and EPPK1 (epiplakin 1) showed higher expression in low responders. The most remarkable difference in the gene expression profile of high and low responders shows the HLA-C gene, which is in high responders on an average more than 4-times higher expressed than in low responders. Interestingly, HLA-C has been reported as a vitamin D target gene17, which has a strong VDR-binding enhancer 286 kb upstream of its TSS (Fig. 4C).
Gene expression profile of high and low responders. Venn diagrams display the number (A) and name (B) of significantly (FDR < 0.05) differently expressed genes in high and low responders before (d0) and after (d1) vitamin D3 supplementation. In (B) only genes with an absolute logFC > 1 are shown. Genes being expressed higher in high responders are indicated in green, while red indicates higher expression in low responders. The IGV browser was used to visualize ChIP-seq results for H3K4me3 (purple)11, H3K27ac (green)11 and VDR (red)34 as well as FAIRE-seq data (turquois)10 obtained in THP-1 cells that had been treated for 24 h with solvent (EtOH) or 1,25(OH)2D3 (1,25D) (C). The peak tracks display merged data from three biological repeats. A VDR-binding enhancer is shaded in grey and gene structures are shown in blue.
In summary, the gene expression profile of PBMCs from high and low vitamin D responders differ in nearly 600 genes, but vitamin D3 supplementation is cutting the number of differently expressed genes to a half. The vitamin D target gene HLA-C shows the most prominent differences between high and low responders.
Discussion
The main goal of this study was to obtain a better understanding of the gene regulatory effects of vitamin D3 supplementation on immune cells of healthy individuals. With the exception of a few scenarios of non-genomic rapid signaling41, vitamin D acts via its nuclear receptor VDR. Therefore, a central question is, which genes show in response to vitamin D stimulation a statistically significant change in their expression. In average of the 25 VitDHiD participants, this resulted in 452 candidate genes, 138 of which could be confirmed by four in vitro studies with PBMCs of the same individuals and the identical treatment time (24 h). Compared with other studies16,19,42, a validation rate of 30.5% of the in vivo vitamin D target genes is a good score. However, if we had selected an even larger in vitro cohort, such as all 25 VitDHiD participants, we would probably have obtained an even higher validation rate. The in vitro studies had the clear advantage to use solvent treatment as a negative control and to apply with 10 nM 1,25(OH)2D3 a more than 100-times higher concentrated stimulus as could be reached in vivo by a bolus with 80,000 IU vitamin D323. For the same reasons in vitro experiments show far higher inducibilities of vitamin D target genes than observed in the in vivo setting.
Due to the known large interindividual variation in vitamin D target genes26, only a shortlist of 16 genes responded significantly to vitamin D both in vivo as well as in all four in vitro studies. Interestingly, all 16 genes have at least one VDR-binding enhancer in the vicinity of 0–246 kb to their TSS region, i.e., they are all primary vitamin D targets43. Although the 16 genes represent rather divergent molecular processes, such as communication via cytokines, vesicular and secretory peptides, immune responsiveness as well as ECM organization and cell motility, they all relate to immunity. A more focused gene enrichment analysis on the basis of the 91 genes with a logFC > 0.25 highlighted 11 targets being involved in the process of focal adhesion. Thus, this study concludes that a key physiological effect of vitamin D3 supplementation of healthy individuals may be the promotion of focal adhesion. The main role of focal adhesion is the communication of a cell with the ECM, i.e., with its direct environment44. Integrins, such as encoded by the upregulated in vivo vitamin D target genes ITGA2B, ITGB3 and ITGB5, form the core of large adhesion complexes that connect the cytoskeleton with proteins of the ECM. In this way, cells can adapt their shape in response to the mechanical structure of their environment45. For example, this is important for the movement of monocytes from the blood stream to inflamed tissues and their differentiation to macrophages46. Since monocytes are the most vitamin D responsive cell type of PBMCs15, the upregulation of genes involved in the process of focal adhesion may prepare healthy individuals for a possible attack by pathogenic microbes. This enhances the potency of innate immunity. Interestingly, in the context of the vitamin D-triggered differentiation of mesenchymal stem cells into osteoblasts, ITGAs and ITGBs have already been shown to be upregulated in vitro by 1,25(OH)2D347.
The individual-specific analysis of the effects of vitamin D3 supplementation on the 11 in vivo vitamin D target genes, which are involved in the pathway of focal adhesion, indicated larger variations between the study participants. In some individuals vitamin D seems to inhibit focal adhesion suggesting that these person’s immune system is less prepared to respond to a possible infection. Accordingly, the classification of the VitDHiD participants based on the responsiveness of all 452 in vivo vitamin D target genes in relation to the increase of the vitamin D status after vitamin D3 bolus supplementation allowed a distinction into 9 high, 12 mid and 4 low responders. The use of subsets of these target genes, such as the 91 genes with a logFC > 0.25 or the 16 genes being confirmed by all four in vitro studies, resulted in basically the same classification but indicated some deviations for those individuals who are at the border between high and mid or mid and low responders. Nevertheless, 4 high, 7 mid and 4 low responders turned out to be stable in their classification irrespective of the used subset of in vivo vitamin D target genes.
We described the concept of the vitamin D response index already for other Finnish cohorts and found that some 25% of the participants are low responders18,22,23. The 4 stable low responders of this study suggests a rate of only 16% in the Finnish population. However, some gene lists, such as those of the 138 in vitro confirmed candidates or the 271 downregulated targets, suggested 6 low responders, which stands for a rate of 24%, i.e., a rate very comparable to previous studies. We propose that low vitamin D responders should take special care on their vitamin D3 supplementation, in order to prevent diseases, such as musculoskeletal disorders like osteoporosis48 and sarcopenia49, autoimmune diseases like multiple sclerosis50 and different types of cancer like colon cancer51. Since a daily vitamin D3 supplementation up to 4000 IU is considered safe52, the percentage of low responders in the population should rather be overestimated than underestimated.
The comparison of the gene expression profile of stable high and low responders provided some insight into the molecular basis of the vitamin D response index. Interestingly, at basal conditions the transcriptome of PBMCs from high and low responders significantly (FDR < 0.05, abs logFC > 1 and logCPM > log210) differed in the expression strength of 588 genes, i.e., in some 5% of all expressed genes. Gene expression is primarily based on the epigenetic stage of the respective cell, since effective gene regulation requires accessible chromatin at enhancers and TSS regions53. Therefore, the difference in the gene expression between high and low responders is rather based on epigenetics and signals from the environment than on genetic variations. However, genome-wide association studies indicated that key genes of vitamin D endocrinology, such as DHCR7 (7-dehydrocholesterol reductase), GC (GC vitamin D binding protein), CYP2R1 (cytochrome P450 family 2 subfamily R member 1) and CYP24A1, significantly contribute to 25(OH)D3 serum levels54. Therefore, in analogy to the trait “vitamin D status”, it is likely that there is also a genetic contribution for the trait “vitamin D responsiveness”. This needs to be further investigated by future studies involving a significantly higher number of participants.
Interestingly, vitamin D3 bolus supplementation can be considered as a major environmental trigger, since it reduces within one day the difference in the gene expression profile of high and low responders to 265 genes, i.e., to less than half. HLA-C stands out from the differently expressed genes, since it is more than 4-times higher expressed in high responders than in low responders. Moreover, it is a vitamin D target gene in PBMCs17,55 and has a prominent VDR-binding enhancer 286 kb upstream of its TSS. Importantly, the HLA gene cluster in chromosome 6 is known as the genetically most divergent region of the human genome56. Therefore, it is possible that single nucleotide variants of the HLA-C gene as well as that of other members of the HLA gene family, most of which are also vitamin D targets17, contribute to the genetic variations that are the basis for interindividual differences in the vitamin D response index.
This study has a few limitations, the major of which is the rather low number of investigated VitDHiD participants, i.e., at present we are lacking statistical power to prove our claims concerning the molecular differences of high and low vitamin D responders. Thus, future studies, such as the presently ongoing VitDPAS trial (NCT06104111), will need to refine the results presented here. This concerns both the epigenetic as well as the genetic contribution to the vitamin D response index. Another limitation of this study is that PBMCs represent a mixture of at least five types of primary immune cells, i.e., the effects of vitamin D described here cannot be assigned to one single cell type. However, from other studies we know that monocytes are most responsive to vitamin D15.
In conclusion, in this study we present 452 in vivo vitamin D target genes that show a higher overlap with genes identified by in vitro studies than previous reports. Moreover, the process of focal adhesion, which is central for the migration and differentiation of monocytes was identified as a major pathway being upregulated by vitamin D3 supplementation in healthy individuals. This sharpens the response of the innate immune system against possible microbe infections. Furthermore, this study provides some insight into the molecular difference of high and low vitamin D responders. On one hand high responders seem to differ from low responders primarily by epigenetically and environmentally driven differences in their transcriptome. On the other hand, variations of some of the differentially expressed genes, such as HLA-C and possibly other members of this gene family, may represent the genetic contribution of the trait “vitamin D response index”.
Data availability
Fastq files of the raw data can be found at Gene Expression Omnibus (GEO, www.ncbi.nlm.nih.gov/geo) with accession numbers GSE260981 (50 in vivo samples) and GSE261699 (28 in vitro samples).
Abbreviations
- 1,25(OH)2D3 or 1,25D:
-
1α,25-Dihydroxyvitamin D3
- 25(OH)D3 :
-
25-Hydroxyvitamin D3
- AKT:
-
AKT murine thymoma viral oncogene homolog
- ALOX5:
-
Arachidonate 5-lipoxygenase
- ANPEP:
-
Alanyl aminopeptidase, membrane
- AQP9:
-
Aquaporin 9
- ASAP2:
-
ArfGAP with SH3 domain, ankyrin repeat and PH domain 2
- BMI:
-
Body mass index
- CD:
-
Cluster of differentiation
- CDKN1A:
-
Cyclin dependent kinase inhibitor 1A
- CES1:
-
Carboxylesterase 1
- ChIP-seq:
-
Chromatin immunoprecipitation sequencing
- CPM:
-
Counts per million
- CRISPLD2:
-
Cysteine rich secretory protein LCCL domain containing 2
- CXCL5:
-
C-X-C motif chemokine ligand 5
- CYP:
-
Cytochrome P450
- DGKK:
-
Diacylglycerol kinase kappa
- DHCR7:
-
7-Dehydrocholesterol reductase
- ECM:
-
Extracellular matrix
- EDAR:
-
Ectodysplasin A receptor
- EPPK1:
-
Epiplakin 1
- FAIRE-seq:
-
Formaldehyde-assisted identification of regulatory elements followed by sequencing
- FC:
-
Fold change
- FDR:
-
False discovery rate
- FPR1:
-
Formyl peptide receptor 1
- GADD45A:
-
Growth arrest and DNA damage inducible alpha
- GC:
-
GC vitamin D binding protein
- GNG11:
-
G protein subunit gamma 11
- HGNC:
-
Human genome nomenclature committee
- HIF1A:
-
Hypoxia inducible factor 1 subunit alpha
- HLA-C:
-
Major histocompatibility complex, class I, C
- ITG:
-
Integrin
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- MDS:
-
Multidimensionality scaling
- MPEG1:
-
Macrophage expressed 1
- MYL9:
-
Myosin light chain 9
- MYLK:
-
Myosin light chain kinase
- NAMPT:
-
Nicotinamide phosphoribosyltransferase
- NK:
-
Natural killer
- PBMC:
-
Peripheral blood mononuclear cell
- PER1:
-
Period circadian regulator 1
- PFKFB3:
-
6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 3
- PI3K:
-
Phosphatidylinositol-4,5-bisphosphate 3-kinase
- RNA-seq:
-
RNA sequencing
- S100:
-
S100 calcium binding protein
- SLC11A1:
-
Solute carrier family 11 member 1
- TGFBI:
-
Transforming growth factor beta induced
- THBS1:
-
Thrombospondin 1
- TOR:
-
Target of rapamycin
- TREM1:
-
Triggering receptor expressed on myeloid cells 1
- TSS:
-
Transcription start site
- VDR:
-
Vitamin D receptor
- VEGFA:
-
Vascular endothelial growth factor A
References
Huang, S. J. et al. Vitamin D deficiency and the risk of tuberculosis: A meta-analysis. Drug Des. Dev. Ther. 11, 91–102. https://doi.org/10.2147/DDDT.S79870 (2017).
Chun, R. F., Liu, P. T., Modlin, R. L., Adams, J. S. & Hewison, M. Impact of vitamin D on immune function: Lessons learned from genome-wide analysis. Front. Physiol. 5, 151. https://doi.org/10.3389/fphys.2014.00151 (2014).
Sintzel, M. B., Rametta, M. & Reder, A. T. Vitamin D and multiple sclerosis: A comprehensive review. Neurol. Ther. 7, 59–85. https://doi.org/10.1007/s40120-017-0086-4 (2018).
Lu, M., McComish, B. J., Burdon, K. P., Taylor, B. V. & Körner, H. The association between vitamin D and multiple sclerosis risk: 1,25(OH)2D3 induces super-enhancers bound by VDR. Front. Immunol. 10, 488. https://doi.org/10.3389/fimmu.2019.00488 (2019).
Jeffery, L. E., Raza, K. & Hewison, M. Vitamin D in rheumatoid arthritis-towards clinical application. Nat. Rev. Rheumatol. 12, 201–210. https://doi.org/10.1038/nrrheum.2015.140 (2016).
Infante, M. et al. Influence of vitamin D on islet autoimmunity and beta-cell function in type 1 diabetes. Nutrients 11, 2185. https://doi.org/10.3390/nu11092185 (2019).
Hollis, B. W. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: Implications for establishing a new effective dietary intake recommendation for vitamin D. J. Nutr. 135, 317–322 (2005).
Whitfield, G. K. et al. Cloning of a functional vitamin D receptor from the lamprey (Petromyzon marinus), an ancient vertebrate lacking a calcified skeleton and teeth. Endocrinology 144, 2704–2716. https://doi.org/10.1210/en.2002-221101 (2003).
Haussler, M. R. et al. Vitamin D receptor: Molecular signaling and actions of nutritional ligands in disease prevention. Nutr. Rev. 66, S98-112. https://doi.org/10.1111/j.1753-4887.2008.00093.x (2008).
Seuter, S., Neme, A. & Carlberg, C. Epigenome-wide effects of vitamin D and their impact on the transcriptome of human monocytes involve CTCF. Nucleic Acids Res. 44, 4090–4104. https://doi.org/10.1093/nar/gkv1519 (2016).
Nurminen, V., Neme, A., Seuter, S. & Carlberg, C. The impact of the vitamin D-modulated epigenome on VDR target gene regulation. Biochim. Biophys. Acta 1861, 697–905. https://doi.org/10.1016/j.bbagrm.2018.05.006 (2018).
Rivera, C. M. & Ren, B. Mapping human epigenomes. Cell 155, 39–55. https://doi.org/10.1016/j.cell.2013.09.011 (2013).
Campbell, M. J. Vitamin D and the RNA transcriptome: More than mRNA regulation. Front. Physiol. 5, 181. https://doi.org/10.3389/fphys.2014.00181 (2014).
Carlberg, C. Vitamin D genomics: From in vitro to in vivo. Front. Endocrinol. 9, 250. https://doi.org/10.3389/fendo.2018.00250 (2018).
Carlberg, C. Vitamin D signaling in the context of innate immunity: Focus on human monocytes. Front. Immunol. 10, 2211. https://doi.org/10.3389/fimmu.2019.02211 (2019).
Kariuki, S. N. et al. Mapping variation in cellular and transcriptional response to 1,25-dihydroxyvitamin D3 in peripheral blood mononuclear cells. PLoS ONE 11, e0159779. https://doi.org/10.1371/journal.pone.0159779 (2016).
Hanel, A. & Carlberg, C. Time-resolved gene expression analysis monitors the regulation of inflammatory mediators and attenuation of adaptive immune response by vitamin D. Int. J. Mol. Sci. 23, 911. https://doi.org/10.3390/ijms23020911 (2022).
Carlberg, C. et al. Primary vitamin D target genes allow a categorization of possible benefits of vitamin D3 supplementation. PLoS ONE 8, e71042. https://doi.org/10.1371/journal.pone.0071042 (2013).
Hossein-nezhad, A., Spira, A. & Holick, M. F. Influence of vitamin D status and vitamin D3 supplementation on genome wide expression of white blood cells: A randomized double-blind clinical trial. PLoS ONE 8, e58725. https://doi.org/10.1371/journal.pone.0058725 (2013).
Vukic, M. et al. Relevance of vitamin D receptor target genes for monitoring the vitamin D responsiveness of primary human cells. PLoS ONE 10, e0124339. https://doi.org/10.1371/journal.pone.0124339 (2015).
Seuter, S. et al. Molecular evaluation of vitamin D responsiveness of healthy young adults. J. Steroid Biochem. Mol. Biol. 174, 314–321 (2017).
Carlberg, C. & Haq, A. The concept of the personal vitamin D response index. J. Steroid Biochem. Mol. Biol. 175, 12–17. https://doi.org/10.1016/j.jsbmb.2016.12.011 (2018).
Seuter, S. et al. Molecular evaluation of vitamin D responsiveness of healthy young adults. J. Steroid Biochem. Mol. Biol. 174, 314–321. https://doi.org/10.1016/j.jsbmb.2016.06.003 (2017).
Gospodarska, E., Ghosh Dastidar, R. & Carlberg, C. Intervention approaches in studying the response to vitamin D3 supplementation. Nutrients 15, 3382. https://doi.org/10.3390/nu15153382 (2023).
Scragg, R. et al. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study : A randomized clinical trial. JAMA Cardiol. 2, 608–616. https://doi.org/10.1001/jamacardio.2017.0175 (2017).
Hanel, A. et al. Common and personal target genes of the micronutrient vitamin D in primary immune cells from human peripheral blood. Sci. Rep. 10, 21051. https://doi.org/10.1038/s41598-020-78288-0 (2020).
Newman, A. M. et al. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 12, 453–457. https://doi.org/10.1038/nmeth.3337 (2015).
Dobin, A. et al. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. https://doi.org/10.1093/bioinformatics/bts635 (2013).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930. https://doi.org/10.1093/bioinformatics/btt656 (2014).
Durinck, S., Spellman, P. T., Birney, E. & Huber, W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat. Protocols 4, 1184–1191. https://doi.org/10.1038/nprot.2009.97 (2009).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. https://doi.org/10.1093/bioinformatics/btp616 (2010).
Chen, Y., Lun, A. T. & Smyth, G. K. From reads to genes to pathways: differential expression analysis of RNA-Seq experiments using Rsubread and the edgeR quasi-likelihood pipeline. F1000Research 5, 1438. https://doi.org/10.12688/f1000research.8987.2 (2016).
Bardou, P., Mariette, J., Escudie, F., Djemiel, C. & Klopp, C. jvenn: An interactive Venn diagram viewer. BMC Bioinformatics 15, 293. https://doi.org/10.1186/1471-2105-15-293 (2014).
Neme, A., Seuter, S. & Carlberg, C. Selective regulation of biological processes by vitamin D based on the spatio-temporal cistrome of its receptor. Biochim. Biophys. Acta 1860, 952–962. https://doi.org/10.1016/j.bbagrm.2017.07.002 (2017).
Tuoresmäki, P., Väisänen, S., Neme, A., Heikkinen, S. & Carlberg, C. Patterns of genome-wide VDR locations. PLoS ONE 9, e96105. https://doi.org/10.1371/journal.pone.0096105 (2014).
Thorvaldsdottir, H., Robinson, J. T. & Mesirov, J. P. Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration. Brief. Bioinf. 14, 178–192. https://doi.org/10.1093/bib/bbs017 (2013).
Chen, E. Y. et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14, 128. https://doi.org/10.1186/1471-2105-14-128 (2013).
Kanehisa, M., Furumichi, M., Tanabe, M., Sato, Y. & Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 45, D353–D361. https://doi.org/10.1093/nar/gkw1092 (2017).
Malmberg, H. R., Hanel, A., Taipale, M., Heikkinen, S. & Carlberg, C. Vitamin D treatment sequence is critical for transcriptome modulation of immune challenged primary human cells. Front. Immunol. 12, 754056. https://doi.org/10.3389/fimmu.2021.754056 (2021).
Pico, A. R. et al. WikiPathways: Pathway editing for the people. PLoS Biol. 6, e184 (2008).
Zmijewski, M. A. & Carlberg, C. Vitamin D receptor(s): In the nucleus but also at membranes? Exp. Dermatol. 29, 876–884. https://doi.org/10.1111/exd.14147 (2020).
Yeh, W. Z. et al. The immune cell transcriptome is modulated by vitamin D3 supplementation in people with a first demyelinating event participating in a randomized placebo-controlled trial. Clin. Immunol. 262, 110183. https://doi.org/10.1016/j.clim.2024.110183 (2024).
Carlberg, C. Vitamin D and is target genes. Nutrients 14, 1354. https://doi.org/10.3390/nu14071354 (2022).
Geiger, B., Spatz, J. P. & Bershadsky, A. D. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 10, 21–33. https://doi.org/10.1038/nrm2593 (2009).
DuFort, C. C., Paszek, M. J. & Weaver, V. M. Balancing forces: Architectural control of mechanotransduction. Nat. Rev. Mol. Cell Biol. 12, 308–319. https://doi.org/10.1038/nrm3112 (2011).
Shi, C. & Pamer, E. G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 11, 762–774. https://doi.org/10.1038/nri3070 (2011).
Posa, F. et al. Vitamin D promotes MSC osteogenic differentiation stimulating cell adhesion and alphaVbeta3 expression. Stem Cells Int. 2018, 6958713. https://doi.org/10.1155/2018/6958713 (2018).
Sunyecz, J. A. The use of calcium and vitamin D in the management of osteoporosis. Ther. Clin. Risk Manag. 4, 827–836. https://doi.org/10.2147/tcrm.s3552 (2008).
Uchitomi, R., Oyabu, M. & Kamei, Y. Vitamin D and sarcopenia: potential of vitamin D supplementation in sarcopenia prevention and treatment. Nutrients 12, 3189. https://doi.org/10.3390/nu12103189 (2020).
Feige, J. et al. Vitamin D supplementation in multiple sclerosis: a critical analysis of potentials and threats. Nutrients 12, 783. https://doi.org/10.3390/nu12030783 (2020).
Vaughan-Shaw, P. G. et al. The effect of vitamin D supplementation on survival in patients with colorectal cancer: Systematic review and meta-analysis of randomised controlled trials. Br. J. Cancer 123, 1705–1712. https://doi.org/10.1038/s41416-020-01060-8 (2020).
Johnson, K. C. et al. Safety and tolerability of high-dose daily vitamin D3 supplementation in the vitamin D and type 2 diabetes (D2d) study-a randomized trial in persons with prediabetes. Eur. J. Clin. Nutr. 76, 1117–1124. https://doi.org/10.1038/s41430-022-01068-8 (2022).
Gibney, E. R. & Nolan, C. M. Epigenetics and gene expression. Heredity (Edinb) 105, 4–13. https://doi.org/10.1038/hdy.2010.54 (2010).
Wang, T. J. et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet 376, 180–188. https://doi.org/10.1016/S0140-6736(10)60588-0 (2010).
Neme, A. et al. In vivo transcriptome changes of human white blood cells in response to vitamin D. J. Steroid Biochem. Mol. Biol. 188, 71–76. https://doi.org/10.1016/j.jsbmb.2018.11.019 (2019).
Trowsdale, J. & Knight, J. C. Major histocompatibility complex genomics and human disease. Annu. Rev. Genom. Hum. Genet. 14, 301–323. https://doi.org/10.1146/annurev-genom-091212-153455 (2013).
Acknowledgements
The authors thank Dr. Antonio Neme and Andrea Hanel for initial data analysis as well as Emmi Hämäläinen, Ella Janzen and Retu Haikonen for PBMC isolation. Kind thanks to the Gene Core Facility at the EMBL in Heidelberg, Germany, for massively parallel sequencing services and the Centre of Informatics Tricity Academic Supercomputer and Network of the Technical University of Gdansk (https://task.gda.pl/en) for the use of their the supercomputers for sequence alignment.
Funding
This publication is part of a project that has received funding from the European Union’s Horizon2020 research and innovation program under grant agreement no. 952601, from the David and Amy Fulton Foundation, Seattle, US and from the National Science Centre (Poland), project number 2023/49/B/NZ9/00402. The dsm-firmenich AG sponsored the VitDHiD study.
Author information
Authors and Affiliations
Contributions
RGD, JJ and CC performed data analysis, while MM did RNA isolation and RNA-seq library preparation. CC wrote the manuscript, which was reviewed by all authors. The VitDHiD study had been designed and conducted by CC, JKV, TPT and IB.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ghosh Dastidar, R., Jaroslawska, J., Malinen, M. et al. In vivo vitamin D targets reveal the upregulation of focal adhesion-related genes in primary immune cells of healthy individuals. Sci Rep 14, 17552 (2024). https://doi.org/10.1038/s41598-024-68741-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68741-9
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.