Abstract
This study aimed to investigate the immediate effects of manual therapy (MT) on the respiratory functions of healthy young individuals. The study included 104 participants, consisting of university students (87 females, 17 males, mean age 20.1 ± 2.2). Participants were randomly assigned to the MT (experimental; n = 52) and sham-MT (control; n = 52) groups. The experimental group underwent thoracic manipulations and mobilizations along with diaphragm mobilization. In the control group, the hands were placed on the same regions, but no specific intervention was applied. All participants underwent respiratory function testing before and after the intervention using a portable spirometer (PEF- Peak expiratory flow; FEV 1- Forced expiratory volume in 1 s; FVC- Forced vital capacity and FEV1/FVC- Tiffeneau index). In the experimental group, there was a significant increase in the mean PEF value following MT application from 296.3 ± 110.8 to 316.1 ± 119.1 (p = 0.018). Conversely, the mean PEF value in the control group showed a slight decrease from 337.1 ± 93.3 to 324.5 ± 89.2 (p = 0.002). No significant changes were observed in FVC, FEV1, or FEV1/FVC values pre- and post-intervention in either groups. A single MT session led to a significant improvement in PEF in healthy young individuals. Further research is needed to explore the long-term effects of MT on respiratory functions and its potential implications in clinical practice.
Trial registration ClinicalTrials.gov: NCT05934240 (06/07/2023).
Similar content being viewed by others
Introduction
Manual therapy (MT) encompasses a range of treatment techniques for pain and functional disorders that target the musculoskeletal system, soft tissues, and nervous system in clinical practice. These include joint manipulation and mobilization, soft tissue mobilization, massage, neural mobilization, and stretching, as well as passive or active techniques such as muscle energy techniques1.
The importance of a holistic approach of MT, involving both the musculoskeletal and respiratory systems, could potentially have significant benefits in improving overall respiratory function and preventing future respiratory complications. Adequate respiration is essential for maintaining the body's homeostasis. The decline in respiratory function observed in adults with chronic respiratory problems often begins in childhood, frequently as a result of lingering effects from respiratory diseases. It is therefore important to investigate how MT techniques can be used to improve breathing in apparently healthy individuals, with the aim of improving respiratory health and preventing the progression of respiratory disease from an early age2. Reduced spinal mobility, particularly in the thoracic region, can lead to decreased chest cage mobility. Reduced mobility increases the stiffness of the chest wall, which reduces the space available for lung expansion and negatively affects the ventilation pumping mechanism, potentially leading to respiratory problems in later years. Increasing chest wall mobility is suggested as a way to improve respiratory function3. Manual therapy interventions can be performed for this purpose, and improvements in respiratory function can be observed4,5. Although MT is not traditionally considered a standard component in the management of respiratory diseases, it has been suggested that MT applications targeting the spinal region could positively impact lung functions. This is achived by increasing spinal and chest wall mobility, reducing respiratory muscle hypertonicity, and affecting the sympathetic nervous system via the thoracic region4,6. A number of studies have demonstrated the potential positive effects of MT interventions on the respiratory system, including improved breathing and increased vital capacity, in diseases such as chronic obstructive pulmonary disease (COPD) and asthma7,8,9. For example, one study showed that a single session of MT application reduced shortness of breath, fatigue, heart rate, and respiratory rate in COPD patients as an immediate effect, while rapidly increasing lung function, inspiratory muscle strength, and oxygen saturation7. Based on the positive effects observed in respiratory system diseases, studies examining the effects of MT on the respiratory system in healthy populations have found supportive evidence suggesting that MT improves spirometric parameters and enhances respiratory function2,10,11,12. However, there are also studies indicating no significant effects13. Furthermore, this therapeutic approach relies largely on clinical observations and hypothetical models rather than mechanical knowledge14. Mobilization or manipulation of the thoracic region improves thoracic flexibility and trunk mobility, leading to increased respiratory function2. Additionally, soft tissue techniques targeting the diaphragm promote relaxation and increased muscle length, resulting in reduced dyspnea and fatigue, as well as improved respiratory function and oxygen saturation15. Individuals who spend extended periods in a sitting position due to work or school-related activities and lead a sedentary lifestyle may experience reduced trunk mobility, leading to decreased respiratory functions, especially dynamic lung volumes such as Forced Expiratory Volume in 1 s (FEV1) and Forced Vital Capacity (FVC)16,17.
The conflicting findings in studies investigating the effect of MT on respiratory functions in healthy adults indicate the need for further research in this area. The primary objective of this study was to determine the immediate effects of MT, particularly thoracic mobilization and manipulation along with diaphragm mobilization, on respiratory functions in university students following a single session. In addition, the potential effects of prolonged sitting positions during study and educational tasks on the respiratory functions of young individuals will be evaluated. The secondary aims of the study include evaluating the effects of a history of COVID-19 (coronavirus disease 2019) and smoking habits on respiratory functions in the included students. It has been observed that differences in respiratory functions persist even 1 year after recovery from COVID-1918. Consequently, within the context of this study, we will assess the respiratory functions in young individuals with a history of COVID-19 using spirometric parameters. Furthermore we will also evaluate whether there is any difference following a single session of MT. Similarly, in smokers, respiratory function will be evaluated using spirometry before and after MT, and the results will be compared. The findings of this study are expected to contribute significantly to the understanding of the acute effects on respiratory functions in young individuals and to the evaluation of the potential use of MT.
Methods
Study design
This study is a triple-blind, randomized controlled trial registered with the identifier NCT05934240 (06/07/2023) on Clinicaltrials.gov. All procedures employed in this study were conducted in accordance with the ethical principles of the Helsinki Declaration and were approved by the Amasya University Non- Interventional Clinical Research Ethics Committee. The participants were randomly allocated to either the experimental or control groups. The study was conducted in three distinct phases. In the initial stage of the study, the participants underwent a respiratory function test, which involved spirometric measurements (PEF: Peak Expiratory Flow, FEV 1: Forced Expiratory Volume in 1 s, FVC: Forced Vital Capacity, and FEV1/FVC: Tiffeneau Index). Participants then received either MT or sham MT intervention according to their group allocation. Subsequently, spirometric measurements were repeated following the intervention. All measurements and interventions were conducted in the physiotherapy laboratory of Sabuncuoğlu Şerefeddin Health Services Vocational School of Amasya University.
Participants
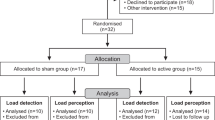
The study was announced by email to 750 students between the ages of 18 and 22 enrolled in the School of Health Sciences at Amasya University. Of the 207 students who expressed interest in participating by submitting their applications to the Student Affairs Office, 103 were excluded from the study either because they did not meet the inclusion criteria or because they expressed their unwillingness to participate after applying. Consequently, 104 male and female students were included in the study (Fig. 1). Inclusion criteria included enrollment in one of the university’s associate degree programmes, absence of chronic systemic diseases requiring medication, and voluntary participation. Furthermore, the volunteers were instructed to abstain from alcohol for a period of 24 h prior to the assessment. Exclusion criteria included any orthopedic conditions, pulmonary or cardiac problems, contraindications to MT techniques (such as osteoporosis), or a history of chest, back or abdominal surgery. Individuals who met these criteria were randomly assigned to either the experimental or control group. Both groups were provided with comprehensive information about the procedures to be performed and were required to sign informed consent forms.
Randomization and blinding
The randomization of participants into two groups was conducted by an individual not involved in the trial using the Research Randomizer web tool, which ensured allocation concealment. Furthermore, the individual responsible for administering the MT intervention remained blinded to group allocation until the start of the trial. Group 1 received the MT intervention (n = 52), while group 2 received sham MT intervention (n = 52). The participants were blinded to their group allocation. The researcher who performed the pulmonary function test and the researcher who analyzed the study data were also blinded to the group allocation of participants. However, the physiotherapist who administered the MT and sham MT interventions was not blinded to the study groups.
Potential confounding variables were identified as age, gender, body mass index (BMI), smoking status and history of COVID-19 diagnosed by polymerase chain reaction (PCR) testing. Following the randomisation of participants to the intervention and control groups, the similarity of the groups with respect to the aforementioned variables was tested.
Sample size
A sample size of 12 was calculated to be the minimum required for each control and intervention group, given a mean FEV1/FVC of 0.84 with a standard deviation of 0.0713 and 80% power and a type 1 error rate of 5%. Due to the high number of eligible students applying to participate, we decided to increase the sample size. As a result, a total of 104 individuals participated in the study, with 52 individuals in each group, thereby enhancing the robustness and generalizability of our findings.
Outcome measures
The data were collected using a quantitative method, including the results from a questionnaire and objective measurements from respiratory function tests.
Sociodemographic date, including age, educational status, body weight, height, smoking status, and a history of COVID-19 (diagnosed by PCR test), was collected via a sociodemographic questionnaire based on participants’ self-report.
The Respiratory Function Test (RFT): Spitometry was used for RFT. Spirometry is employed to evaluate the extent of an individual’s respiratory disease and response to treatment, and is regarded as the gold standard for assessing respiratory function. The parameters, including PEF, FEV1, FVC and FEV1/FVC were measured using a portable Minispir spirometer device by MIR, in accordance with the American Thoracic Society guidelines. The test was repeated three times and the most appropriate value was recorded19. RFT measurements were conducted twice, immediately prior to and within five minutes of the MT and sham MT interventions, by a blinded assessor.
Interventions
The experimental group underwent a series of thoracic mobilization and manipulation with the addition of diaphragm mobilization techniques. The control group underwent a similar procedure, although the thoracic mobilizations and manipulations were replaced with sham techniques. All techniques were performed by a qualified physiotherapist.
The experimental group (manual therapy group) underwent the following procedures:
For the purpose of thoracic manipulation, the patient was positioned in a supine position, with one hand of the therapist placed on the transverse process of the thoracic region and the other hand on the patient's elbows. Flexion and posterior gliding movements were adjusted to engage tissue barriers and allow for the fine-tuning of segmental mobility. The therapist then applies a downward force with the body until a barrier point is reached during the patient’s expiration (Fig. 2). This thrust manipulative was performed once.
For thoracic mobilization, the patient was positioned prone, with the therapist placing one hand on the edge of the pisiform and two fingers laterally on the costotransverse joint. The other hand was placed on the opposite side of the joint. The therapist applied clockwise and counterclockwise posterior-anterior intervertebral mobilization (Fig. 3). A 30-s set was performed for each costotransverse joint20.
The diaphragm was mobilized through the application of eight distinct pulling techniques. In this technique, the patient was positioned in the supine position, and the therapist grasped the patient’s lower ribs with both hands and mobilized the right lower ribs upwards and towards the midline with the left hand. Subsequently, the therapist utilised the right hand to mobilize the left lower ribs in an upwards and towards the midline direction (Fig. 4). The aforementioned mobilization was performed in a rhythmic manner for a period of 2–4 min. During the procedure, the patient was instructed to breathe normally21.
The control group (sham-manual therapy group) was subjected to the following procedure:
The participants in the sham group received the same manual contact in the same position, but no force or manoeuvre was applied. Only respiratory movements were observed, and the muscles were palpated. The total duration of the sham protocol was identical to that of the MT group.
The MT session was well tolerated by the participants, with no adverse effects observed during or after the MT session. Participants were provided with a telephone number to report any potential adverse effects within 24 h, but no reports were received.
Data analysis
The data from the study were analysed using the SPSS software package (version 22 for Windows, SPSS Inc., Chicago, IL, USA). Continuous variables are presented as mean ± standard deviation, and categorical variables as the number (%). The normality of the measures was assessed using the Kolmogorov–Smirnov test. Chi-square test was used to compare categorical variables. For within-group comparisons of continuous variables, the paired samples t-test and the Wilcoxon test were employed. The Student's t-test and the Mann–Whitney U test were employed for the purpose of making comparisons between groups. The researcher responsible for the statistical analysis was unaware of which group was the intervention and/or control group. To eliminate the potential for bias, the individuals were analysed in two groups designated as H and M, which were randomly selected. A significance level of p < 0.05 was accepted for all individuals.
Ethical approval
This study was approved by the Amasya University Non-Interventional Clinical Research Ethics Committee (Date:05/08/2022, Approval Decision Number: E-76988455–050.01.04–83,313).
Results
A total of 104 participants were included in the study, with a mean age of 20.1 ± 2.2 years, of whom 87 (83.7%) were female. Table 1 displays the socio-demographic characteristics and health behaviors of participants in both the experimental and control groups. There were no statistically significant differences between the groups in terms of age, height, body weight, BMI, and smoking status, which were considered potential confounding factors. However, the control group had a higher proportion of women and individuals diagnosed with COVID-19 compared to the intervention group (p = 0.004 and p = 0.001, respectively).
Regarding respiratory function tests, although the baseline PEF value was higher in the control group than in the experimental group (p = 0.045), no significant difference was observed between the groups after the intervention (p > 0.05). In the experimental group, PEF increased from 296.3 ± 110.8 before MT application to 316.1 ± 119.1 after the intervention (p = 0.018). Conversely, in the control group, PEF decreased from 337.1 ± 93.3 to 324.5 ± 89.2 (p = 0.002). Comparing the changes in PEF before and after the interventions between groups revealed a significantly greater increase in the experimental group (p = 0.001) (Table 2).
There were no significant differences in FVC, FEV1, and FEV1/FVC between the two groups before and after the intervention. The FEV1/FVC ratio was higher in the control group both before and after the intervention (p = 0.009 and p = 0.040, respectively), with a smaller difference observed after the intervention.
Analysis of respiratory function test results in the experimental group by smoking status showed a significant increase in PEF from 330.3 ± 115.0 to 357.0 ± 119.2 among smokers (p = 0.039). No significant changes were observed in other respiratory parameters among smokers in either group. In subjects without a history of COVID-19 within the experimental group, FVC increased from 3.5 ± 0.7 to 3.7 ± 0.9 (p = 0.041). There were no significant differences in other respiratory parameters between subjects with and without a history of COVID-19 in either group.
Discussion
The objective of this study was to investigate the acute effects of single-session manual therapy (MT) techniques (including thoracic mobilization and manipulation, diaphragm mobilization) on spirometric parameters of RFT in university students. The results demonstrated an increase in PEF values following MT in the experimental group, whereas a decrease was observed in the control group that received sham MT. Although there was an increase in both FEV1 and FVC in both groups, these increases were not statistically significant between the groups. These findings indicate the potential for a single MT session to influence respiratory function.
It is a well-established tenet of respiratory anatomy and physiology that the mobility of the thoracic spine and thorax can affect respiratory functions22. From this perspective, it is posited that MT applications facilitate respiratory function improvement by enhancing spinal and thoracic mobility and also by exerting influence on the nervous system11. The effects of MT are explained at the biomechanical, neurophysiological and psychological levels. From a biomechanical perspective, it has been proposed that MT techniques enhance the efficiency of respiratory muscles by increasing tissue elasticity, reducing joint stiffness, and improving rib cage mobility23. Neurophysiologically, it has been demonstrated that MT contributes to pain modulation and reduced excitability of alpha motor neurons through indirect effects on the peripheral and central nervous system, thus allowing respiratory muscles to work more efficiently24. With regard to autonomic responses, it has been demonstrated that MT applications elevate respiratory rate and heart rate by stimulating the sympathetic nervous system, thereby influencing respiratory functions14. From a psychological perspective, it may induce relaxation and enhance the general well-being of the patient. Nevertheless, there is a paucity of robust evidence regarding the clinical significance and magnitude of these effects6,25 Consequently, it is evident that further high-quality research is required in order to gain a comprehensive understanding of the mechanisms underlying the effects of MT applications on pulmonary function.
The effects of MT on respiratory function have been a subject of great interest to researchers, both in the short and long term. A substantial body of literature exists on the subject of age-related differences in various populations. These studies investigate the effects of various manual therapy techniques on respiratory functions in healthy participants, with different application times and techniques employed. In these studies were emplyoed, thoracic manipulation techniques5,12,26, chiropractic MT and exercise combinations2, pump manipulation techniques11,27, diaphragm techniques27,28, and high-speed, low-amplitude thrust manipulation and low-speed joint mobilization techniques13. Table 3 provides a comprehensive overview of the potential effects of MT on lung function, based on the results of the trials presented. In these trials, researchers employed a variety of techniques to assess the effectiveness of MT, measuring lung function at different times during the interventions (Table 3). One study measured at five different times after a single session of manipulation and mobilization of the thoracic region13, while another measured at baseline, immediately after the intervention, and 5 and 20 min after treatment28. In other studies, spirometric measurements were taken before and after the intervention2,5,11,26,27. In this context, spirometry measurements provided crucial data to ascertain the immediate and long-term effects of MT on respiratory function. Furthermore, a recent systematic review conducted by Stępnik et al.23, indicated that thoracic spine manipulation, diaphragm mobilization, and lymphatic techniques may enhance spirometric parameters, reduce respiratory muscle tension, and facilitate more efficient lung ventilation. A systematic review by Picchiottino et al.6 demonstrated that mobilizations resulted in a notable elevation in respiratory rate. However, the strength of this association was limited by the quality of the evidence. Furthermore, McGuiness et al.14 demonstrated that cervical mobilization techniques resulted in an increase in respiratory rate and blood pressure, which was attributed to an increase in sympathetic nervous system activity.
In this study employed an intervention comprising thoracic mobilization, manipulation and diaphragmatic relaxation techniques. Although the speed and pressure of the techniques were not measured, it was assumed that there was consistency between the techniques used, given that the therapy was performed by the same therapist. Following a single intervention session, a significant increase in PEF was observed in the experimental group, whereas a decrease was observed in the control group. This decrease in PEF for the control group could be attributed to psychological factors, lack of respiratory muscle activation, absence of neurophysiological responses, and potential effects of transitioning from a lying to a standing position without therapeutic intervention.
This finding indicates that MT may have a positive effect on respiratory functions and that specific techniques may enhance respiratory parameters. PEF, which plays an important role in the assessment of respiratory function, is defined as the maximum speed of expiratory airflow. It can decrease in conditions such as airway narrowing. Consequently, an increase in PEF may be indicative of an improvement in respiratory function and airway widening29. The observation of an increase in PEF does not prove that MT has a specific effect on respiratory function or that it is more effective in improving certain respiratory parameters than others. These findings require further confirmation with larger groups of participants and longer-term follow-up.
In this study, respiratory function was evaluated utilising a portable spirometer. Although spirometry is a widely used and valuable tool for assessing respiratory function, it does not provide a comprehensive assessment of all aspects of respiratory muscle performance. Measurements such as maximum inspiratory pressure (MIP), maximum expiratory pressure (MEP) and impulse oscillometry can provide a more comprehensive understanding of respiratory function by providing additional information on respiratory muscle strength and airway resistance30. The decision to utilise only spirometry in this study was based on a number of factors, including the accessibility and simplicity of the equipment and the primary purpose of assessing key lung function parameters. Nevertheless, the inclusion of MIP, MEP or impulse oscillometry would have enhanced the study by providing a more comprehensive assessment of respiratory muscle strength and overall respiratory mechanics. These measures may assist in elucidating the specific mechanisms by which MT affects respiratory function. It is recommended that future research considers the inclusion of these additional measures in order to provide a more comprehensive assessment of the effects of MT on respiratory function. The inclusion of a wider range of respiratory tests will not only improve our understanding of the effects of MT on respiratory health, but will also facilitate the development of more targeted and effective therapeutic interventions.
It has been proposed that diaphragm-focused MT applications may be more effective in improving lung function than other approaches28. Furthermore, significant relationships between diaphragm movements and FVC and FEV1 have been demonstrated. Thoracic mobilization and manipulation can enhance diaphragmatic mobility, particularly during deep breathing, thereby increasing FVC31. In addition, diaphragmatic stretching not only strengthens respiratory function but also has a biomechanical effect on the spine, thereby supporting postural function32. This indicates that MT is a crucial treatment modality, not only for respiratory function but also for general postural balance and support. In this context, we posit that our study demonstrated a more favourable effect on respiratory function by employing diaphragmatic mobilisation in conjunction with spinal manipulation and mobilization. However, it was not possible to evaluate the kind of postural effects that were provided. It would be beneficial for future studies to investigate the effects of these techniques on the posture and balance of the individual, in addition to respiratory function.
The results of your study are of significant importance in the assessment of the effects of potential confounders, such as smoking and history of COVID-19, on respiratory function. A significant increase in PEF was observed in smokers following MT application when compared to non-smokers. This indicates that certain mechanisms activated by MT may mitigate or reverse the adverse effects of smoking on the lungs. One such mechanism could be the promotion of airway dilation and increased lung ventilation by the application of MT to the thoracic region. This could contribute to an improvement in lung function by allowing the lungs to take in more air11. Another potential mechanism is the stimulation of the sympathetic nervous system by MT, which is thought to promote airway dilation14. Nevertheless, further research is required to gain a more comprehensive understanding of the precise nature of these mechanisms and the impact of MT on respiratory function.
A comparison of respiratory function tests between participants who had contracted COVID-19 and those who had not revealed no statistically significant difference between the two groups. Although an increase in respiratory function test results was observed in participants who underwent MT after contracting COVID-19, this increase was not statistically significant. A significant increase in FVC was observed exclusively in participants who had not contracted COVID-19. These findings provide support for the hypothesis that MT may improve respiratory parameters in healthy individuals. Studies in individuals who had contracted COVID-19 but did not receive invasive mechanical ventilation have indicated the presence of lung abnormalities in these individuals one year after disease onset18,33. In a study by Nagy et al.34 the application of the diaphragm release technique, a MT technique, to post-COVID-19 males resulted in greater improvements in conditions such as fatigue, dyspnea, and aerobic capacity. This indicates that MT may be regarded as a potential treatment option in the post-COVID-19 rehabilitation process. However, a limitation of the study is the lack of detailed information on the severity of the participants' COVID-19 infection. Furthermore, as the study only included individuals in the younger age group, further research is required to investigate the long-term effects of COVID-19 on respiratory function in different age groups. Such studies can assist in the comprehension of the impact of COVID-19 on the respiratory system, thereby facilitating the development of efficacious treatment and rehabilitation strategies.
Limitations
It should be noted that this study is subject to several limitations. Firstly, as the study was based on a single-session intervention, it was not possible to evaluate the long-term effects of MT. Further studies with longer follow-up periods are required in order to gain a deeper understanding of the long-term effects of this intervention. Secondly, the measurements in the study were based solely on spirometric parameters, including PEF, FVC, FEV1, and FEV1/FVC. In particular, the study did not include other lung function measures such as maximum inspiratory pressure (MIP), maximum expiratory pressure (MEP), or impulse oscillometry, which could provide a more comprehensive assessment of respiratory function. The inclusion of these additional measures would assist in elucidating the specific mechanisms through which MT influences respiratory function. Thirdly, the lack of detailed information on the severity of COVID-19 in the participants hinders a full understanding of its effects on respiratory function. Finally, the unequal distribution of male and female participants in both groups represents another limitation of the study.
Conclusion
The objective of this study was to investigate the acute effects of MT techniques (including thoracic mobilization and manipulation with diaphragm mobilization) on respiratory function in healthy young subjects following a single session. The results demonstrated a significant increase in PEF in the experimental group that received MT, whereas a decrease in PEF was observed in the control group. There were no significant differences in FEV1, FVC and FEV1/FVC between the two groups. However, in smokers, MT was associated with a significant increase in PEF. These findings indicate that a single session of MT may have beneficial effects on respiratory function, and that specific techniques may improve respiratory parameters. Although an increase in the respiratory function test was observed in individuals with COVID-19 after MT, this increase was not statistically significant. These findings indicate a need for further and more comprehensive research to elucidate the effects of MT on respiratory function in individuals with COVID-19. In conclusion, further research is required to investigate the potential of MT as a treatment option for improving respiratory function.
Data availability
The data that support the findings of this study are available by e-mail from the corresponding author upon reasonable request.
Abbreviations
- BMI:
-
Body mass index
- COPD:
-
Chronic obstructive pulmonary disease
- COVID-19:
-
Coronavirus disease 2019
- FEV 1:
-
Forced expiratory volume in 1 s FEV1
- FVC:
-
Forced vital capacity
- MEP:
-
Maximum expiratory pressure
- MIP:
-
Maximum inspiratory pressure
- MT:
-
Manual therapy
- PCR Tests:
-
Polymerase chain reaction tests
- PEF:
-
Peak expiratory flow
- RFT:
-
Respiratory function test
References
Bialosky, J. E. et al. Unraveling the mechanisms of manual therapy: modeling an approach. J. Orthop. Sports Phys. Ther. 48, 8–18. https://doi.org/10.2519/jospt.2018.7476 (2018).
Engel, R. M. & Vemulpad, S. The effect of combining manual therapy with exercise on the respiratory function of normal ındividuals: a randomized control trial. J. Manipulat. Physiol. Therapeut. 30, 509–513. https://doi.org/10.1016/j.jmpt.2007.07.006 (2007).
Aliverti, A., Kayser, B. & Macklem, P. T. A human model of the pathophysiology of chronic obstructive pulmonary disease. Respirology 12, 478–485. https://doi.org/10.1111/j.1440-1843.2007.01106.x (2007).
Clarke, S., Munro, P. E. & Lee, A. L. The Role of Manual Therapy in Patients with COPD. Healthcare (Basel) 7 (2019). https://doi.org/10.3390/healthcare7010021
Shin, D. C. & Lee, Y. W. The immediate effects of spinal thoracic manipulation on respiratory functions. J. Phys. Therapy Sci. 28, 2547–2549. https://doi.org/10.1589/jpts.28.2547 (2016).
Picchiottino, M., Leboeuf-Yde, C., Gagey, O. & Hallman, D. M. The acute effects of joint manipulative techniques on markers of autonomic nervous system activity: a systematic review and meta-analysis of randomized sham-controlled trials. Chiropr. Man Therap. 27, 17. https://doi.org/10.1186/s12998-019-0235-1 (2019).
Yilmaz Yelvar, G. D., Çirak, Y., Demir, Y. P., Dalkilinç, M. & Bozkurt, B. Immediate effect of manual therapy on respiratory functions and inspiratory muscle strength in patients with COPD. Int. J. Chron. Obstruct. Pulmon Dis. 11, 1353–1357. https://doi.org/10.2147/copd.S107408 (2016).
Hondras, M. A., Linde, K. & Jones, A. P. Manual therapy for asthma. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD001002.pub2 (2005).
Parnell Prevost, C. et al. Manual therapy for the pediatric population: a systematic review. BMC Complement. Alternative Med. https://doi.org/10.1186/s12906-019-2447-2 (2019).
Vicenzino, B., Cartwright, T., Collins, D. & Wright, A. Cardiovascular and respiratory changes produced by lateral glide mobilization of the cervical spine. Manual Therapy 3, 67–71. https://doi.org/10.1016/S1356-689X(98)80020-9 (1998).
Lima, I. S. et al. Chest and neck mobilization effects on spirometric responses in healthy subjects. J. Manipulative Physiol. Ther. 34, 622–626. https://doi.org/10.1016/j.jmpt.2011.08.004 (2011).
Jonely, H. et al. Changes in pulmonary function following thoracic spine manipulation in a healthy inactive older adult population-a pilot study. J. Phys. Ther. Sci. 35, 492–496. https://doi.org/10.1589/jpts.35.492 (2023).
Wall, B. A., Peiffer, J. J., Losco, B. & Hebert, J. J. The effect of manual therapy on pulmonary function in healthy adults. Sci. Rep. 6, 33244. https://doi.org/10.1038/srep33244 (2016).
McGuiness, J., Vicenzino, B. & Wright, A. Influence of a cervical mobilization technique on respiratory and cardiovascular function. Man. Ther. 2, 216–220 (1997).
Braga, D. K. A. P. et al. Manual therapy in diaphragm muscle: effect on respiratory muscle strength and chest mobility. Manual Ther. Posturol. Amp. Rehabilit. J. 14, 1–5. https://doi.org/10.17784/mtprehabjournal.2016.14.302 (2020).
Lorbergs, A. L. et al. Severity of Kyphosis and decline in lung function: the framingham study. J. Gerontol. A Biol. Sci. Med. Sci. 72, 689–694. https://doi.org/10.1093/gerona/glw124 (2017).
Almeida, V. P. et al. Correlation between pulmonary function, posture, and body composition in patients with asthma. Rev. Port Pneumol. 19, 204–210. https://doi.org/10.1016/j.rppneu.2013.03.004 (2013).
Rajotiya, S. et al. Post-COVID-19 cardio-pulmonary manifestations after 1-year of SARS-CoV-2 infection among Indian population: a single centre, case-control study (OneCoV2 study). J. Infect Public Health 17, 145–151. https://doi.org/10.1016/j.jiph.2023.11.013 (2024).
Crapo, R. O. & Jensen, R. L. Standards and interpretive issues in lung function testing. Respir. Care 48, 764–772 (2003).
Bergmann, T. F. & Peterson, D. H. Chiropractic Technique - E-Book: Principles and Procedures. (Elsevier Health Sciences, 2010).
Hebgen, E. Complementary medicine (Thieme (Firm)) (Thieme, 2011).
Guyton AC, H. J. Textbook of medical physiology. 471 (Elsevier, 2006).
Stępnik, J., Czaprowski, D. & Kędra, A. Effect of manual osteopathic techniques on the autonomic nervous system, respiratory system function and head-cervical-shoulder complex-a systematic review. Front. Med. (Lausanne) 11, 1358529. https://doi.org/10.3389/fmed.2024.1358529 (2024).
Lascurain-Aguirrebeña, I., Newham, D. & Critchley, D. J. Mechanism of action of spinal mobilizations: a systematic review. Spine (Phila Pa 1976) 41, 159–172. https://doi.org/10.1097/brs.0000000000001151 (2016).
Jones, L. M. et al. Effect of osteopathic manipulative treatment on pulmonary function testing in children with asthma. J. Osteopath. Med. 121, 589–596. https://doi.org/10.1515/jom-2020-0040 (2021).
Mustafaoğlu, R., Birinci, T., Mutlu, E. K. & Özdincler, A. R. Torakal manipülasyonun torakal mobilite, solunum fonksiyonları ve fonksiyonel kapasite üzerine etkisi: pilot çalışma. J. Exercise Ther. Rehabilit. 6, 93–103 (2019).
Stępnik, J., Kędra, A. & Czaprowski, D. Short-term effect of osteopathic manual techniques (OMT) on respiratory function in healthy individuals. PLOS ONE 15, e0235308. https://doi.org/10.1371/journal.pone.0235308 (2020).
González-Álvarez, F. J., Valenza, M. C., Cabrera-Martos, I., Torres-Sánchez, I. & Valenza-Demet, G. Effects of a diaphragm stretching technique on pulmonary function in healthy participants: a randomized-controlled trial. Int. J. Osteopathic Med. 18, 5–12. https://doi.org/10.1016/j.ijosm.2014.08.001 (2015).
Koegelenberg, C. F. Guideline for office spirometry in adults. South African Med. J. 103, 52–62 (2013).
Maitland, G. Maitland’s vertebral manipulation, Management of Neuromusculoskeletal Disorders. 8th Edition edn, Vol. Volume 1 (Butterworth-Heinemann, 2013).
Jung, S. H., Hwang, U. J., Ahn, S. H., Kim, J. H. & Kwon, O. Y. Does mobilisation of the thoracic spine using mechanical massage affect diaphragmatic excursion in individuals with thoracic hyperkyphosis?. J. Back Musculoskelet. Rehabil. 35, 517–523. https://doi.org/10.3233/bmr-210143 (2022).
Nair, A. et al. Comparison of diaphragmatic stretch technique and manual diaphragm release technique on diaphragmatic excursion in chronic obstructive pulmonary disease: a randomized crossover trial. Pulm Med. 2019, 6364376. https://doi.org/10.1155/2019/6364376 (2019).
Zarei, M. et al. Long-term side effects and lingering symptoms post COVID-19 recovery. Rev. Med. Virol. 32, e2289. https://doi.org/10.1002/rmv.2289 (2022).
Nagy, E. N., Elimy, D. A., Ali, A. Y., Ezzelregal, H. G. & Elsayed, M. M. Influence of manual diaphragm release technique combined with ınspiratory muscle training on selected persistent symptoms in men with post-covid-19 syndrome: a randomized controlled trial. J. Rehabil. Med. 54, jrm00330. https://doi.org/10.2340/jrm.v54.3972 (2022).
Acknowledgements
We sincerely thank all participants for their valuable contributions to this trial. We are also grateful to the student who provided written consent for their image to be captured and used for publication.
Funding
The authors report that there is no funding related to the work presented in this article.
Author information
Authors and Affiliations
Contributions
EK contributed to data collection, analysis, and interpretation and was responsible for writing the manuscript. DT contributed to data collection, design of the study, analysis and interpretation of the data, and critical revision of the manuscript. BT contributed to the design of the study, analysis and interpretation of the data, and critical revision of the manuscript. GC contributed to the conception and design of the study, interpretation of the data, and critical revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Küçük, E., Tozcu, D., Topaktaş, B. et al. Immediate effects of manual therapy on respiratory functions in healthy young individuals: a randomized controlled trial. Sci Rep 14, 17419 (2024). https://doi.org/10.1038/s41598-024-68654-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68654-7
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.