Abstract
Endophytic fungi are widely known as fungi that infect internal tissues of host plants for all or part of their life cycles, without causing visible symptoms of disease. The present study was carried out to identify and investigate the pathogenicity of endophytic fungi residing in husks, silks, and kernels of corn. Endophytic fungi were isolated from surface-sterilised silks, kernels, and husks of healthy corn plants and identified using sequencing of multiple markers comprising TEF-1α, β-tubulin, calmodulin, ITS, LSU, and ACT. A total of 56 isolates of endophytic fungi belonging to 17 species, namely Fusarium pseudocircinatum (n = 8), F. verticillioides (n = 2), F. andiyazi (n = 4), F. sacchari (n = 1), F. mangiferae (n = 1), F. fujikuroi (n = 1), F. proliferatum (n = 3), F. incarnatum (n = 2), Penicillium oxalicum (n = 2), P. polonicum (n = 2), P. citrinum (n = 11), Aspergillus flavus (n = 10), A. tubingensis (n = 1), Cladosporium tenuissimum (n = 3), Aureobasidium pullulans (n = 3), Curvularia lunata (n = 1), and Epicoccum sorghinum (n = 1) were identified. Pathogenicity test showed that all endophytic fungi induced varying severities of disease symptoms on corn plants such as leaf chlorosis and necrosis, stem malformation, wilt, and stunted growth with F. verticillioides being the most virulent. The study revealed that corn tissues harbour diverse genera of endophytic fungi that can infect corn plants and may cause harmful effects to the host plants.
Similar content being viewed by others
Introduction
Endophytes are microorganisms that infect and colonise the internal tissues of healthy host plants without causing any clear manifestations of disease1. These microbes have been found in all plant families and their biological diversity is enormous between species and within different plant parts of same species, across both tropic and temperate regions1,2,3,4,5.
Within the corn plant, endophytes show extensive functional diversity, ranging from pathogenesis to mutualism, depending on the fungal strain, host genotype, and growth conditions6,7. Although studies have identified endophytic fungi from different parts of the corn plant8,9,10,11, knowledge of the extent of fungal endophytism is still relatively new, and much is yet to be understood concerning the interactions between fungal endophytes and their corn hosts12. Furthermore, although recent studies have reported the identification and functional roles of endophytic fungi from corn plants13,14,15,16, there is still a paucity of information on the pathogenic potentials of endophytic fungi on corn plants.
To enable accurate identification of endophytic fungi, molecular identification is recommended. Molecular identification of endophytic fungi often relies on sequence and phylogenetic analyses of DNA, amplified using specific genetic markers17,18. Due to advantages such as ease of amplification, vast usage, and significantly wide barcode gap between inter- and intraspecific variation, the internal transcribed spacer (ITS) region was proposed as the barcode for fungal identification by the International Fungal Barcoding Consortium19. However, since the ITS region shows non-uniformity in variability in a number of fungal groups, the use of protein-coding genes such as actin (ACT), translation elongation factor 1α (TEF-1α), β-tubulin, and calmodulin has been recommended. These protein-coding genes are able to distinguish between closely related species, cryptic species, and also reveal the phylogenetic relationships among different fungal species within the same genus20.
The symptomless relationship between endophytic fungi and host plants is maintained by the balance between biotic factors such as host genotype, and abiotic factors such as temperature, and relative humidity. Hence, a disturbance in this balanced relationship often results in plant stress, leading to the occurrence of disease21. Fusarium verticillioides is a known example of an endophytic fungus that is able to switch from endophyte to pathogen of corn plants, resulting in disease symptoms such as stem and ear rots of infected corn plants22. A number of studies have investigated the pathogenicity of endophytic fungi in different host plants such as wild banana (Musa acuminata)23, tropical almond (Terminalia mantaly and Terminalia catappa)24, Japanese knotweed (Fallopia japonica)25, black cottonwood of the Pacific Northwest (Populus trichocarpa)26, tomato (Solanum lycopersicum)13, lawn grass (Axonopus compressus)18, and Beruwas laut (Scaevola taccada)27. These studies demonstrated switching of endophytes to pathogen. Nevertheless, such studies are yet to be reported on corn plants in Malaysia.
The endophytic fungi isolated from healthy corn tissues have the possibility to become pathogenic at a later time. To address the paucity of knowledge regarding the occurrence and pathogenicity of endophytic fungi residing in corn husks, silks, and kernels, the fungal endophytes were isolated and identified using molecular methods and tested for pathogenicity through artificial inoculation of healthy corn plants. The findings of this study will provide additional understanding of the functional roles of endophytic fungi residing in corn tissues.
Results
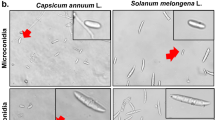
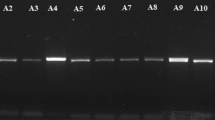
Morphological characterization and Molecular identification. Fifty-six isolates of endophytic fungi were recovered from corn ears and grouped into seven genera and 17 species (Table 1). The morphological characteristics of the endophytic fungi are shown in Supplementary Fig. 1. The majority of the isolates were recovered from husk tissues (n = 30), followed by kernels (n = 21), and silks (n = 5). The isolates were confirmed as endophytes by the absence of mycelial growths on imprints of surface sterilized tissues on Potato Dextrose Agar (PDA) as described by Schulz et al.28. Gel electrophoresis with the PCR products amplified using the selected markers are shown in Supplementary Fig. 2.
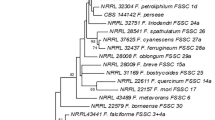
Eight endophytic Fusarium spp., F. pseudocircinatum, F. verticillioides, F. sacchari, F. mangiferae, F. fujikuroi, F. proliferatum and F. incarnatum were molecularly identified based on TEF-1α and β-tubulin sequences. Phylogenetic analysis showed that the same species were clustered with their epitype strains (Fig. 1).
Endophytic Penicillium and Aureobasidium were identified based on combined ITS and β-tubulin sequences. Three species of Penicillium, P. polonicum, P. oxalicum and P. citrinum (n = 11) were identified, while only one species of Aureobasidium, A. pullulans was identified (Fig. 2).
Based on phylogenetic analysis of combined ITS, β-tubulin, and CaM sequences, two endophytic Aspergillus species, A. tubingensis and A. flavus were identified (Fig. 3). Both endophytic Aspergillus species were recovered from different tissues of corn ear.
Three isolates of endophytic Cladosporium were identified as C. tenuissimum based on ITS and ACT sequences (Fig. 4). Endophytic isolates of Curvularia and Epicoccum were identified based on ITS and LSU regions sequences. The isolate of Curvularia was identified as C. lunata, while the isolate of Epicoccum as E. sorghinum (Fig. 5).
Pathogenicity of endophytic fungi. Endophytic fungi recovered from corn tissues initiated disease symptoms on the inoculated plants, ranging in severity from low to severe (Table 2). Disease symptoms observed on endophyte-infected plants were leaf chlorosis, leaf necrosis, stem rot, stem malformation, and plant wilt. While leaf chloroses and necroses were induced in all endophyte-infected corn plants, stem rot and plant wilt were exclusive to corn plants infected by endophytic F. verticillioides, and stem malformation by F. sacchari and F. fujikuroi. Severities of stem rot induced by F. verticillioides (DSI = 50%), and stem malformation by F. fujikuroi (DSI = 45%) were significantly different from other endophyte infections and the uninfected control (p ≤ 0.05) (Table 2).
Corn plants infected with endophytic fungi showed variations in growth characteristics (Supplementary Table S1). Plant height (17.13 cm), stem girth (0.31 cm), and leaf length (12.00 cm) were significantly reduced in corn plants infected with endophytic F. verticillioides compared to the uninoculated control (p ≤ 0.05). Significant reductions in leaf width were observed in corn plants infected with F. sacchari, F. fujikuroi, and C. tenuissimum, which had mean leaf widths of 1.02 cm, 1.00 cm, and 1.06 cm, respectively, compared to the uninoculated control (1.56 cm) (p ≤ 0.05). Figure 6 shows disease symptoms observed on corn plants after 2 weeks of inoculation with endophytic Fusarium spp.
Disease symptoms on corn plants after 2 weeks of inoculation with endophytic Fusarium spp. Leaf chlorosis (Lc), leaf necrosis (Ln), stem rot (Sr), and stem malformation (Sm) on corn plants inoculated with (a) F. fujikuroi; (b) F. andiyazi; (c) F. mangiferae; (d) F. proliferatum; (e) F. verticillioides; (f) F. sacchari; (g) F. pseudocircinatum; (h) F. incarnatum; (i) uninoculated control.
Infection of corn by endophytic fungi affected biomass accumulation (Supplementary Table S2). The total dry weight of the uninoculated control plants was significantly higher that total dry weight of all corn plants infected with endophytic fungi (p ≤ 0.05) (Supplementary Table S2).
Discussion
Molecular identification and phylogenetic analysis of endophytic Fusarium isolates from corn tissues identified eight species, namely F. pseudocircinatum, F. verticillioides, F. andiyazi, F. mangiferae, F. sacchari, F. fujikuroi, F. proliferatum and F. incarnatum. Of the eight identified Fusarium spp., only F. incarnatum did not belong to the Fusarium fujikuroi Species Complex (FFSC). Members of the FFSC are the most common Fusarium spp. associated with corn plants as pathogens as well as endophytes, particularly F. proliferatum and F. verticillioides. Species of Fusarium that have been recovered as endophytes of corn plants include F. verticillioides, F. andiyazi, F. proliferatum and F. incarnatum9,29,30,31.
Pathogenicity tests of the endophytic Fusarium species showed varying degrees of leaf chlorosis, leaf necrosis, and growth alterations in the infected plants, with F. verticillioides being the most virulent, followed by F. fujikuroi, F. andiyazi, F. sacchari, F. proliferatum, F. mangiferae, F. pseudocircinatum and F. incarnatum. Some of these endophytic Fusarium spp. have been reported to be associated with corn diseases. For instance, while F. verticillioides and F. proliferatum are known as common corn ear rot pathogens, F. fujikuroi, F. andiyazi, and F. sacchari are less frequently reported as causal pathogens of corn ear rot32. Fusarium incarnatum is a causal agent of corn stalk rot33. Fusarium mangiferae and F. pseudocircinatum are often associated with mango malformation34 but not corn. Therefore, species of Fusarium are often found to be endophytes and pathogen associated with corn plant as well as in other plants.
The switch of endophytic Fusarium species to pathogens has only been reported between endophytic F. verticillioides and corn hosts. Yates et al.9 reported that corn seedlings inoculated with endophytic F. verticillioides showed suppressed growth of shoot diameter, plant height, leaf length, and plant dry weight, after 7 days of inoculation. Pinto et al.35 also reported that healthy corn leaves inoculated with endophytic F. verticillioides, showed 50% reduction in chlorophyll content. Bacon et al.36 linked the shift from the behavior of F. verticillioides as endophyte of corn to its occurrence as pathogen, to changes in biotic factors such as host genotype, and abiotic factors such as temperature and relative humidity, which alter the required balanced relationship by weakening the host plant’s immunity, thereby paving way for the onset of disease.
Three endophytic Penicillium species, namely P. citrinum, P. oxalicum, and P. polonicum were identified from corn plants in the present study. Although endophytic infection of corn plants by P. citrinum, P. oxalicum, and P. polonicum is yet to be reported, their occurrence as endophytes of other plants has been documented in sunflower (Helianthus annuus), the Asian flowering plant, Ixeris repenes, Malacca ginger (Alpinia malaccensis), coffee (Coffea arabica), lemon (Citrus limon), Malabar nutmeg (Myristica malabarica), winter cherry (Withania somnifera), toothed clubmoss (Huperzia serrata), and yew (Taxus fauna)37,38,39.
Corn plants infected with endophytic P. citrinum, P. oxalicum and P. polonicum showed low to moderate severities of leaf necrosis and chlorosis. These findings indicated that endophytic Penicillium spp. from corn are also able to switch to pathogenic lifestyles. The three Penicillium spp. have been found to be associated with corn diseases, of which P. citrinum and P. oxalicum caused corn ear rot40, while P. oxalicum caused leaf blight41. Penicillium citrinum, P. oxalicum and P. polonicum are also responsible for various diseases of crops, such as fruit rot of oranges, grapes and strawberry42,43,44, stem rot of tomato45 and blue mold of onion46.
Two endophytic Aspergillus spp. recovered from corn plants were identified as A. tubingensis and A. flavus. Similarly, Orole and Adejumo11 identified A. flavus as an endophytic colonizer of corn kernels and was also isolated from corn leaves by El-Lebody et al.47. Other than corn plant, endophytic A. flavus was also reported from Thymelaea hirsute root and leaves48 and Ephedra pachyclada leaves49. Colonisation of corn root tissues by A. tubingensis has been reported30,50 and the fungal endophyte has also been found residing in apple tree branches51, leaves of Andrographis paniculata52 and foliar parts of Debregeasia salicifolia53.
Endophytic A. flavus and A. tubingensis recovered from corn plants were moderately pathogenic. Aspergillus flavus is a well-known causative agent of corn ear and kernel rots, and a producer of aflatoxin in infected corn kernels54, while A. tubingensis has been associated with poor seed germination, reduction of root weight, shoot weight, and shoot length of infected corn plants50. The pathogenicity test demonstrated that endophytic A. flavus and A. tubingensis also shifted into pathogens when inoculated in corn plants as earlier observed for endophytic Fusarium spp. and Penicillium spp. Although the pathogenicity of endophytic A. flavus and A. tubingensis is yet to be reported, both A. flavus and A. tubingensis have been reported as pathogens in a number of crops. For instance, A. flavus is a pathogen of cotton boll55, and causes fruit rot of apple and kiwi56,57, while pathogenic A. tubingensis infects grape berries58, cotton leaves59 and pomegranate fruits60.
Endophytic A. pullulans from corn is yet to be reported. However, A. pullulans is a well-known endophyte of a vast array of plant hosts including drupes of sweet cherry (Prunus avium)61, petioles of Indian acalypha (Acalypha indica)62, and olive leaves63. In the present study, high severity of leaf chlorosis, and moderate severity of leaf necrosis, were observed in corn plants inoculated with endophytic A. pullulans. This is thought to be the first report of pathogenic effects of endophytic colonisation of any plant by A. pullulans. Findings of the study suggest that endophytic A. pullulans are also capable of pathogenic behaviour in infected plant hosts.
Endophytic C. tenuissimum from corn is yet to be documented but has been reported from several plants including Livistona chinensis64, Pterocarpus santalinus65, coffee plants66 and Salvia miltiorrhiza67. Corn plants infected with endophytic C. tenuissimum showed low severities of leaf chlorosis and necrosis. Although the effects of plant infection and colonisation by endophytic C. tenuissimum have not been reported, pathogenic strains of C. tenuissimum have been found to cause a number of disease symptoms in infected plants. For instance, fruit swelling of cucumber, as a result of fruit infection by C. tenuissimum, was reported in Palestine68.
Endophytic C. lunata have been recovered from corn plants in Peninsular Malaysia as well as from corn plants in India10. In addition to corn, endophytic C. lunata have also been recovered from sugarcane69, Azadirachta indica leaves70, oil palm71, and lawn grass14. Corn plants inoculated with endophytic C. lunata showed significant reduction in plant biomass, accompanied by high severity of leaf chlorosis. Although C. lunata is a well-known leaf spot pathogen causing huge yield losses of corn crops annually72, disease initiation by endophytic C. lunata has also been reported on wounded leaves of lawn grass18.
Endophytic E. sorghinum has been reported from corn10 and other plants such as mangrove plant73 and sorghum74. Corn plants infected with endophytic E. sorghinum produced moderate severity on the inoculated plants. Although disease initiation by endophytic E. sorghinum, is yet to be reported, plant infections by pathogenic E. sorghinum have been reported to cause leaf spot of corn75, and as one of the most important fungi in the grain-mold complex, causing significant yield loss due to reduced seed viability, and kernel weight76.
The symptomless relationship between fungal endophytes and host plants is maintained by a balance in biotic and abiotic factors influencing the internal and external environments of host plants, whereby a disruption of the balance of antagonism in favour of fungal virulence weakens the host plant’s immunity, paving way for the onset of disease21. The pathogenicity of endophytic fungi recovered from healthy corn tissues in the present study is indicative of the behavioral switch from endophyte to pathogen, which might have been stimulated by changes in nutritional and environmental conditions, which affect the physiology and increase stress of plant hosts77. Among the environmental conditions that are detrimental to plant growth and development include severe changes in moisture content and temperature, high salinity and heavy metal contamination21,78,79,80. Under these circumstances, imbalanced antagonism occurs between the endophyte and the host plant that resulted in switching of endophyte to pathogen with visible disease symptoms81. Consequently, Redman et al.82 suggested that since the status of an endophyte may change due to a change in host defense in response to the environment, the plant is the main trigger for the endophytes’ change in status. It is also likely that the significant decline in biomass of plants infected by the endophytic fungi could be a result of a growth-defense trade-off, in which infected corn plants increased their physiological allocations to defense against fungal infections at the expense of increased growth rate and biomass production83.
The findings of this study suggest that endophytic colonisation of plants might pose a threat to crop health and productivity. Therefore, integrated plant disease management involving the use of resistant varieties, cultural practices, biological control, and use of fungicides are recommended to protect crop health and preserve yield of sweet corn. Resistant varieties involve using cultivars that have the capacity to tolerate or resist disease infection which is considered as one of effective methods to control corn diseases84. For the application of biocontrol agent, it is more effective with combination of other methods such as cultural practices and fungicide applications. Cultural practices such as crop rotation, management of tillage and crop debris help to reduce inoculum levels in the soil and in the debris which can also reduce risk of disease development85. Several types of fungicides are recommended to control corn diseases and the information on the fungicide effectiveness was developed by Corn Disease Working Group86. Also, to further our understanding of the mechanisms and dynamics of endophyte pathology on corn and other plants, future research could consider co-infection of corn plants with two or more endophytic fungi to evaluate their synergistic effect on plant growth and overall health. Cross-pathogenicity tests of endophytic fungi recovered from corn on other plants will also provide useful information on the host-range of the pathogenic endophytes from corn. Transcriptome analysis can be utilized to identify possible sets of fungal and plant genes that correspond to endophytic and pathogenic lifestyles which give an insight into molecular mechanisms of lifestyle switching.
Conclusion
Fifty-six endophytic fungal isolates from corn ears were identified, belonging to seven genera and 17 species. Pathogenicity test indicated that endophytic fungi residing in tissues of corn are able to switch to pathogens, causing disease symptoms under certain conditions within the host plants and their growth environments that encourage disease development. Although the factors responsible for the probable switch from endophyte to pathogen were not investigated in the present study, it is suggested that the tropical conditions of high humidity and elevated temperatures prevalent in Malaysia, may have provided a favourable climate for such transitions to occur. Hence, future research focused on the specific effects of varying growth and environmental conditions on the pathogenicity of endophytic fungi could provide a better understanding of the triggers for virulence in endophytic fungi, as they play important roles in determining plant health and productivity. Disease management practices that are targeted at eliminating latent pathogens masking as endophytes in host tissues, are required to enhance crop health and preserve yield of corn.
Materials and methods
Sample collection
Mature and disease-free corn ears of approximately 70–80 days were randomly hand-plucked from corn plants in nine locations within six states of Peninsular Malaysia, namely Penang (5° 21′ 01.9″ N, 100° 12′ 58.6″ E), Titi Gantung I, Perak (4° 21′ 4.137″ N, 100° 50′ 58.145″ E), Titi Gantung II, Perak (4° 21′ 5.171″ N, 100° 50′ 55.446″ E), Titi Gantung III, Perak (4° 21′ 3.605″ N, 100° 50′ 52.835″ E), Tanjung Karang, Selangor (3° 32′ 48″ N, 101° 16′ 19″ E), Kampung Sungai Dua, Negeri Sembilan (2° 34′ 44.535″ N, 102°29′5.568″ E), Machang, Marang, Terengganu (5° 0′ 17.673″ N, 103° 18′ 47.056″ E), Kampung Pak Da Malik, Terengganu (5° 44′ 21.646″ N, 102° 32′ 20.659″ E), and Perupok, Kelantan (6° 5′ 15.74″ N, 102° 22′ 56.454″ E). Corn samples were transported in paper bags to the Plant Pathology Laboratory, School of Biological Sciences, Universiti Sains Malaysia, Penang, for further processing. Permission to collect the samples was obtained from the farmers and from the state Department of Agriculture.
Isolation of endophytic fungi from corn tissues
The protocol described by Latiffah and Ning87 was adopted with few modifications. Husk tissue segments measuring about 1 cm2, and 1 cm length of silk strands were cut using a sterile scalpel and washed in running water to remove dirt. Washed tissues were surface sterilised by immersion for 2 min in 5% sodium hypochlorite solution, followed by immersion in 70% ethanol for 2 min. Surface disinfected corn tissues were further rinsed three times in sterile distilled water for 1 min each time, and blotted dry on sterilized Whatman No.1 filter paper to remove excess water.
For isolation of endophytic fungi from corn kernels, about 50 g of corn kernels removed by gently pulling out from husked corn cobs, were washed in running tap water to remove dirt and other plant debris. Washed kernels were surface-sterilized by immersing in 75% ethanol for 30 s, followed by 1% sodium hypochlorite solution for 1 min, and lastly 95% ethanol for 3 min. Surface-sterilized kernels were rinsed thrice in sterile distilled water and blotted dry on sterile filter paper for 5 min.
After surface sterilisation, corn tissues were aseptically transferred onto sterile PDA plates at the rate of five tissues per plate, spaced about 5 cm apart, and incubated for a period of 4–7 d, for the emergence of mycelia. A total of 60 tissues each of husks, silks, and kernels were cultured from samples collected from every location. Pure cultures of endophytic fungi isolated from cultured corn tissues were maintained on sterile ½ strength PDA in 1.5 mL Eppendorf microcentrifuge tubes at 28 °C, until required.
In order to confirm the efficacy of the surface sterilisation, the imprint technique reported by Schulz et al.25 were adopted. Surfaces of sterilized plant tissue segments were gently pressed onto the surface of Potato Dextrose Agar (PDA), thereafter, imprinted PDA plates were incubated at 28 °C, and observed for any fungal growths for a period of 4 d. The absence of fungal growths on imprinted plates was taken as proof of effective surface sterilisation.
Morphological characterisation
The endophytic fungi isolated were categorised into genera according to their morphological characteristics before molecular identification was performed. A combination of macroscopic and microscopic characteristics was used for morphological characterisation. Macroscopic characteristics observed were colony features such as colony colour, texture, elevation, shape, and pigmentation. Microscopic features observed include shape and size of conidia, orientation of conidia on conidiogenous cells, and hyphal structure.
DNA extraction and PCR amplification
Approximately 10 mm diameter mycelial plugs obtained from 4 to 7 d old cultures of the fungal isolates were cultured in 10 mL of Potato Dextrose Broth in 25 mL volume Bijou bottles and incubated at ambient temperature for 24–48 h for Penicillium and Aspergillus species, and up to 7 d for members of other fungal genera. Mycelial mats were harvested, dried on sterilized filter paper, and ground to powder (homogenized) in liquid nitrogen using a sterile mortar and pestle. Approximately 60 mg of ground mycelia were dispensed into 1.5 mL microcentrifuge tubes and stored at 4 °C until required for DNA extraction. DNA was extracted using the Invisorb Spin Plant Mini Kit (STRATEC Molecular GmbH, Berlin, Germany) according to the manufacturer’s instructions, and stored 1.5 mL receiver tubes at 4 °C.
Selected markers were amplified using different sets of primers, depending on the fungal genera (Supplementary Table S3). The PCR reaction mixture was prepared in 50 μL total volume comprising 5 × Green Buffer (8 μL) (Promega, Wisconsin, USA), MgCl2 (8 μL) (Promega, Wisconsin, USA), 1.0 μL of dNTP mix (Promega, Wisconsin, USA), 8 μL of each primer (5.0 μM for ITS, LSU, and calmodulin; 2.5 μM for β-tubulin, and 1.0 μM for ACT), 0.3 μL of 5 U/μM GoTaq® DNA polymerase (Promega, Wisconsin, USA), and 0.6 μL of DNA template. The reaction mixture was brought to a total volume of 50 μL with sterile distilled water.
PCR amplifications were performed in a Bio-Rad MyCycler™ Thermal Cycler. For amplification of ITS, ACT and LSU sequences, initial denaturation was performed at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 56 °C for 30 s, and extension at 72 °C for 1 min. Final extension was performed at 72 °C for 5 min.
PCR reactions for β-tubulin gene commenced with an initial denaturation at 94 °C for 1 min, followed by 39 cycles of denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s and extension at 72 °C for 1 min. Final extension of β-tubulin gene was performed at 72 °C for 5 min.
For calmodulin gene, an initial denaturation at 95 °C for 5 min was followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for 30 s, and extension for 72 °C for 30 s. Final extension of calmodulin gene was performed at 72 °C for 5 min.
Amplification of TEF-1α gene commenced with initial denaturation at 94 °C for 2 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 59 °C for 30 s, and extension at 72 °C for 1 min. Final extension was carried out at 72 °C for 10 min.
Gel electrophoresis
PCR products were detected by electrophoresis in 1% agarose gel prepared in 1 × Tris Borate-EDTA (TBE) buffer, stained with 4 µL of Florosafe DNA Stain (1st BASE, Singapore), and run for 90 min at 80 V and 400 mA. After running, gels were visualized under UV light in the Bio-RAD Molecular Imager® Gel Doc™ XR + Systems (Hercules, California, USA), and photographed using the Discovery Series™ Quantity One® 1-D Analysis software. The sizes of the amplified fragments were estimated by matching them with a 100 bp DNA ladder (Thermo Scientific, USA).
Molecular identification and phylogenetic analysis
Molecular identification was carried out to confirm the morphologically assigned identities of endophytic fungal isolates recovered from corn tissues. PCR products were sent to a service provider for sequencing. Forward and reverse sequences obtained were aligned and edited to produce consensus sequences using the ClustalW in the Molecular Evolution and Genetic Analysis version 7 (MEGA7) software88. Aligned sequences were further compared with deposited sequences in the GenBank (http://www.ncbi.nlm.nih.gov) using the Basic Local Alignment Search Tool (BLAST). Identification of the isolates was based on highest similarity of BLAST search. Multiple sequence alignments of endophytic fungi recovered from corn plants, and epitype strains sourced from GenBank were generated and deployed for the construction of phylogenetic trees, using the MEGA7 software88.
Prior to the construction of phylogenetic trees, multiple sequence alignments were subjected to model tests to determine the best suited models for tree construction. Models with the lowest Bayesian Information Criterion, and Akaike Information Criterion (AIC) scores were designated for tree construction using the Maximum Likelihood (ML) method. The bootstrap method at 1000 replications was used to estimate the reliability of the ML trees, and all positions containing gaps and missing data were eliminated by choosing the complete-deletion option.
Pathogenicity of endophytic fungi
Seeds of sweet corn Leckat 592 variety, purchased from the Leckat Corporation, Malaysia, were subjected to external and internal sterilisation, using the heat shock procedure reported by Palencia89, and pre-germinated in water agar for three days. Pre-germinated corn seedlings at the rate of 10 seedlings per plate, were inoculated by incubating overnight in 10 mL conidial suspensions (106 conidia/ml) of endophytic fungi and dried on sterile Whatman filter paper for 6 h at room temperature, in a laminar flow chamber.
Pre-germinated seedlings inoculated with fungal spores were aseptically transferred into sterile sandy-clay soils in sterile plastic containers, measuring 17 × 7 cm (width × height). Planting depth was standardized by placing each seed in a 2.5 cm deep depressions in the growth soil prior to covering the seed9. Each treatment was replicated four times, with the control group consisting of seeds treated with sterilized distilled water without fungal inoculum. All plants were grown for 14 d in the Plant House, School of Biological Sciences, Universiti Sains Malaysia, Penang, and watered every 24 h prior to germination, and twice daily (morning and evening) after germination. The experiment was repeated twice.
Height of plant from the base of the stem to the top of the longest leaf, width of plant at the widest portion of leaf, length of leaf, and stem girth, were measured 14 d after inoculation, to assess growth responses of inoculated plants to artificial inoculation of fungal endophytes. After 14 d, the roots of the corn plants were removed from experimental pots and washed in running water to remove planting soils. Soil-free plant materials were separated into root and aerial tissues by cutting immediately above the first crown root with the aid of a scalpel. Root and aerial plant tissues were packed in paper bags and oven-dried at 60 °C to a constant weight, to determine plant biomass90. Disease symptoms appearing on plant tissues within the growth period were assessed using a modification of the disease rating scale of Mehta and Mondal90.
Data analysis
Data obtained from pathogenicity tests were subjected to Analysis of Variance (ANOVA), using IBM SPSS statistical software version 27. Means were separated using Tukey’s Honestly Significant Difference test (HSD) at 5% level of probability.
Data availability
The sequence data of this study have been deposited in the GenBank and the accession numbers are presented in Table 1.
References
Carroll, G. C. The biology of endophytism in plants with particular reference to woody perennials. In Microbiology of the Phyllosphere (eds Fokkema, N. J. & Van den Heuve, J.) 205–222 (Cambridge University Press, 1986).
Lu, Y., Chen, C., Chen, H., Zhang, J. & Chen, W. Isolation and identification of endophytic fungi from Actinidia macrosperma and investigation of their bioactivities. Evid. Based Complement. Alternat. Med. https://doi.org/10.1155/2012/382742 (2012).
Nair, D. N. & Padmavathy, S. Impact of endophytic microorganisms on plants, environment and humans. Sci. World J. https://doi.org/10.1155/2014/250693 (2014).
Maadon, S. N., Wakid, S. A., Zainudin, I. I., Rusli, L. S. & Mohd, M. S. Isolation and identification of endophytic fungi from UiTM reserve forest. Negeri Sembilan. Sains Malaysiana. 47, 3025–3030 (2018).
Sun, D. et al. Identification and application of an endophytic fungus Arcopilus aureus from Panax notoginseng against crop fungal disease. Front. Plant Sci. https://doi.org/10.3389/fpls.2024.1305376 (2024).
Eaton, C. J., Cox, M. P. & Scott, B. What triggers grass endophytes to switch from mutualism to pathogenism?. Plant Sci. 180, 190–195 (2011).
Zhao, C., Onyino, J. & Gao, X. Current advances in the functional diversity and mechanisms underlying endophyte-plant interactions. Microorganisms 12, 779 (2024).
Fisher, P. J., Petrini, O. & Scott, H. L. The distribution of some fungal and bacterial endophytes in maize (Zea mays L.). New Phytol. 122, 299–305 (1992).
Yates, I. E., Bacon, C. W. & Hinton, D. M. Effects of endophytic infection by Fusarium moniliforme on corn growth and cellular morphology. Plant Dis. 81, 723–728 (1997).
Renuka, S. & Ramanujam, B. Fungal endophytes from maize (Zea mays L.): isolation, identification and screening against maize stem borer, Chilo prtellus (Swinhoe). J. Pure Appl. Microbiol. 10, 523–528 (2016).
Orole, O. O., Adejumo, T. O., Link, T. & Voegele, R. T. Molecular identification of endophytes from maize roots and their biocontrol potential against toxigenic fungi a Nigerian maize. Sci Prog. https://doi.org/10.1177/00368504231186514 (2023).
Torres, M. S., Tadych, M., White, J. F. & Bills, G. F. Isolation and Identification of Fungal Endophytes in Prospects and Application for Plant-associated Microbes, a Laboratory Manual Part b: Fungi. (ed. Pirttila, A. M., Sorvari, S.) 153-164 (Finland, 2011).
Manzotti, A. et al. Insights into the community structure and lifestyle of the fungal root endophytes of tomato by combining amplicon sequencing and isolation approaches with phytohormone profiling. FEMS Microbiol. Ecol. https://doi.org/10.1093/femsec/fiaa052 (2020).
Akram, S. et al. Uniting the role of endophytic fungi against plant pathogens and their interaction. J. Fungi https://doi.org/10.3390/jof9010072 (2023).
Martínez-Soto, D., Yu, H., Allen, K. S. & Ma, L. J. Differential colonization of the plant vasculature between endophytic versus pathogenic Fusarium oxysporum strains. Mol. Plant-Microbe Interact. 36(1), 4–13 (2023).
Tao, F. et al. Insight into the composition and differentiation of endophytic microbial communities in kernels via 368 maize transcriptomes. J. Adv. Res. https://doi.org/10.1016/j.jare.2024.05.018 (2024).
Huang, W. Y. et al. Molecular phylogenetic identification of endophytic fungi isolated from three Artemisia species. Fungal Divers. 36, 69–88 (2009).
Azuddin, N. F., Azmy, Mohamad Noor & M. S., & Zakaria, L.,. Molecular identification of endophytic fungi in lawn grass (Axonopus compressus) and their pathogenic ability. Sci. rep. 13, 4239 (2023).
Schoch, C. L. et al. Fungal barcoding consortium, Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc. Natil. Acad. Sci. 109, 6241–6246 (2012).
Tekpinar, A. D. & Kalmer, A. Utility of various molecular markers in fungal identification and phylogeny. Nova Hedwigia. 109, 187–224 (2019).
Schulz, B. & Boyle, C. The endophytic continuum. Mycol Res. 109, 661–686 (2005).
Kuldau, G. A. & Yates, I. E. Evidence for Fusarium endophytes in cultivated and wild plants. In Microbial Endophytes (eds Bacon, C. W. & White, J. F.) 85–120 (Taylor Francis, 2010).
Photita, W., Lumyong, S., Lumyong, P., McKenzie, E. H. C. & Hyde, K. Are some endophytes of Musa acuminata latent pathogens?. Fungal Divers. 16, 131–140 (2004).
Begoude, B. A. D., Slippers, B., Wingfield, M. J. & Roux, J. The pathogenic potential of endophytic Botryosphaeriaceous fungi on Terminalia species in Cameroon. For. Pathol. 41, 281–292 (2011).
Kurose, D., Furuya, N., Tsuchiya, K., Tsushima, S. & Evans, H. C. Endophytic fungi associated with Fallopia japonica (Polygonaceae) in Japan and their interactions with Puccinia polygoniamphibii var, tovariae, a candidate for classical biological control. Fungal Biol. 116, 785–791 (2012).
Raghavendra, A. K. & Newcombe, G. The contribution of foliar endophytes to quantitative resistance to Melampsora rust. New Phytol. 197, 909–918 (2013).
Haq, N. et al. Molecular characterization of endophytic fungi from the leaves of Beruwas Laut (Scaevola taccada) as antibacterial producer. Trop. J. Nat. Prod. Res. https://doi.org/10.26538/tjnpr/v8i1.20 (2024).
Schulz, B., Wanke, U., Draeger, S. & Aust, H. J. Endophytes from herbaceous plants and shrubs: Effectiveness of surface sterilisation methods. Mycol. Res. 97, 1447–1450 (1993).
Aiyaz, M. et al. Molecular diversity of seed-borne Fusarium species associated with maize in India. Curr. Genom. 17, 132–144 (2016).
Potshangbam, M., Devi, S. I., Sahoo, D. & Strobel, G. A. Functional characterisation of endophytic fungal community associated with Oryza sativa L. and Zea mays L.. Front. Microbiol. 8, 1–15 (2017).
Brookes, J. J. Endophytes in Maize (Zea mays) in New Zealand. Preprint at https://researcharchive.lincoln.ac.nz/handle/10182/8444 (2022).
Zhang, H. et al. First report of Fusarium ear rot of maize caused by Fusarium andiyazi in China. Plant Dis. 98, 1428 (2014).
Gai, X. T. et al. First report of Fusarium incarnatum causing stalk rot on maize in China. Plant Dis. 100, 1010 (2016).
Freeman, S. et al. First report of mango malformation disease caused by Fusarium pseudocircinatum in Mexico. Plant Dis. 98, 1583 (2014).
Pinto, L. S., Azevedo, J. L., Pereira, J. O., Vieira, M. L. & Labate, C. A. Symptomless infection of banana and maize by endophytic fungi impairs photosynthentic efficiency. New Phytol. 147, 609–615 (2000).
Bacon, C. W., Glenn, A. E. & Yates, I. E. Fusarium verticillioides: managing the endophytic association with maize for reduced fumonisins accumulation. Toxin Rev. 27, 411–446 (2008).
Waqas, M. et al. Endophytic fungi promote plant growth and mitigate the adverse effects of stem rot: an example of Penicillium citrinum and Aspergillus terreus. J. Plant Interact. 10, 280–287 (2015).
Vega, F. E., Posada, F., Peterson, S. W., Gianfagna, T. J. & Chaves, F. Penicillium species endophytic in coffee plants and ochratoxin A production. Mycologia 98, 31–42 (2017).
Fatima, N. et al. Bioactive constituents from an endophytic fungus, Penicillium polonicum NFW9, associated with Taxus fauna. Med. Chem. 13, 689–697 (2017).
Munkvold, G. P., Arias, S., Taschl, I. & Gruber-Dorninger, C. Mycotoxins in corn: Occurrence, impacts, and management in Corn 235–287 (Elsevier, 2019).
Han, J. X. et al. First Report of Penicillium oxalicum Causing Leaf Blight on Maize in China. Plant Dis. 107, 2554 (2023).
Coutinho, T. C., Ferreira, M. C., Rosa, L. H., de Oliveira, A. M. & de Oliveira Junior, E. N. Penicillium citrinum and Penicillium mallochii: New phytopathogens of orange fruit and their control using chitosan. Carbohydr. Polym. 234, 115918 (2020).
Hussein, M. A., El-Said, A. H. & Yassein, A. S. Mycobiota associated with strawberry fruits, their mycotoxin potential and pectinase activity. Mycology 11, 158–166 (2020).
Felšöciová, S., Rybárik, Ľ, Tančinová, D., Mašková, Z. & Kačániová, M. Microfungi and mycotoxins of grapes from Tokaj wine region. J. Microbiol. Biotechnol. Food Sci. 2021, 16–18 (2021).
Picos-Munoz, P. A., Garcia-Estrada, R. S., Carrillo-Fasio, J. A., Leon-Felix, J. & Allende-Molar, R. First report of blue mold caused by Penicillium oxalicum in tomato (Solanum lycopersicum) in Mexico. Plant Dis. 95, 1195–1195 (2011).
Duduk, N., Vasić, M. & Vico, I. First report of Penicillium polonicum causing blue mold on stored onion (Allium cepa) in Serbia. Plant Dis. 98, 1440 (2014).
El-Lebody, K., Soliman, M. S., EL Metwally, E. M., Elaziz, M. & Moustafa, H. Z. Isolation and pathogenicity of endophytic fungi associated with some maize hybrids against certain Lepidoptera pests. Egypt. J. Agric. Res. 99, 49–60 (2021).
Rashad, Y. M., Abdalla, S. A. & Shehata, A. S. Aspergillus flavus YRB2 from Thymelaea hirsuta (L.) Endl., a non-aflatoxigenic endophyte with ability to overexpress defense-related genes against Fusarium root rot of maize. BMC Microbiol. 22, 229 (2022).
Khalil, A. M. A. et al. Isolation and characterization of fungal endophytes isolated from medicinal plant Ephedra pachyclada as plant growth-promoting. Biomolecules 11, 140 (2021).
Weieneth, L. K. Seedborne black Aspergillus species as maize seedling pathogens: role of fumonisin production and interaction with soilborne Pythium species (Master of Science dissertation). Retrieved from https://lib.dr.iastate.edu/etd/14429/. (2015).
Mohamed, H. A., Ebrahim, W., Peterson, A. M., Özkaya, F. C. & Proksch, P. Tensidols A and B from Aspergillus tubingensis strain and their biological activity. Mикoлoгия и фитoпaтoлoгия 53, 223–228 (2019).
Puri, S. K., Habbu, P. V., Kulkarni, P. V. & Kulkarni, V. H. Isolation and characterization of secondary metabolites from endophytic fungi, Aspergillus tubingensis strain CS/7/2 from leaves of Andrographis paniculata (Burm. F.) Nees. Int. J. Pharm. Res. 13(2), 09752366 (2021).
Nisa, S. et al. Identification and bioactivities of two endophytic fungi Fusarium fujikuroi and Aspergillus tubingensis from foliar parts of Debregeasia salicifolia. Arab. J. Sci. Eng. 45, 4477–4487 (2020).
Jackson-Ziems, T. A. et al. Corn disease profile III: ear rot diseases and grain molds. Retrieved from http://extensionpublications.unl.edu/assets/pdf/ec1901.pdf (2012).
Lutfunnessa, R. J. F. & Shamsi, S. Fungal diseases of cotton plant Gossypium hirsutum L. Bangladesh. Dhaka Univ. J. Bio. Sci. 20, 139–146 (2011).
Hasan, H. A. H. Patulin and aflatoxin in brown rot lesion of apple fruits and their regulation. World J. Microbiol. Biotechnol. 16, 607–612 (2000).
Zhu, G. Y. et al. First report of Aspergillus flavus causing fruit rot on kiwifruit in China. Plant Dis. 106, 1990 (2022).
Lim, Y. S., Hassan, O., Kim, M. K. & Chang, T. First report of bunch rot caused by Aspergillus tubingensis of shine muscat grape in Korea. Plant Dis. 103, 2953 (2019).
Khizar, M. et al. Aspergillus tubingensis causes leaf spot of cotton (Gossypium hirsutum L.) in Pakistan. Phyton. 89, 103 (2020).
Guo, M. J., Wang, Q. T., Cheng, Y. H. & Hou, C. L. Identification of Aspergillus tubingensis causing pomegranate fruit rot in China. Australas. Plant Pathol. 50, 233–240 (2021).
Schena, L., Nigro, F., Pentimone, I., Ligorio, A. & Ippolito, A. Control of postharvest rots of sweet cherries and table grapes with endophytic isolates of Aureobasidium pullulans. Postharvest Biol. Technol. 30, 209–220 (2003).
Kurandawad, J. M. & Lakshman, H. C. Diversity of the endophytic fungi isolated from Acalypha Indica Linn–A promising medicinal plant. Int. J. Sci. Res. Pub. 4, 2250–3153 (2014).
Costa, H., Ramos, V., Pereira, J. A. & Baptista, P. Understanding fungal communities of olive tree leaves for application to climate change adaptation. Biol. Life Sci. Forum. 4, 13 (2021).
Guo, L. D., Hyde, K. D. & Liew, E. C. Identification of endophytic fungi from Livistona chinensis (Palmae) using morphological and molecular techniques. New Phytol. 147, 617–630 (2000).
Venkateswarulu, N. et al. Isolation and characterization of plumbagin (5-hydroxyl-2-methylnaptalene-1, 4-dione) producing endophytic fungi Cladosporium delicatulum from endemic medicinal plants. Biotechnol Rep. 20, e00282 (2018).
Oliveira, R., Souza, R., Lima, T. & Cavalcanti, M. Endophytic fungal diversity in coffee leaves (Coffea arabica) cultivated using organic and conventional crop management systems. Mycosphere 5, 523–530 (2014).
Chen, H. et al. Endophytic fungus Cladosporium tenuissimum DF11, an efficient inducer of tanshinone biosynthesis in Salvia miltiorrhiza roots. Phytochemistry 194, 113021 (2022).
Batta, Y. A. Cladosporium tenuissimum Cooke (Deuteromycotina: Hyphomycetes) as a causal organism of new disease on cucumber fruits. Eur. J. Plant Pathol. 110, 1003–1009 (2004).
Fors, R. O. et al. Dark septate endophytic fungi associated with sugarcane plants cultivated in São Paulo, Brazil. Diversity 12, 1–21 (2020).
Chukwuemerie, O. L. et al. Antimicrobial properties and characterization of secondary metabolites obtained from Curvularia lunata, an endophyte of Azadirachta indica. J. Drug Deliv. Ther. 12, 110–119 (2022).
Parlindo, F., Wiyono, S. & Tondok, E. T. Endophytic fungi and their potential in controlling white root disease of cashew. Plant Prot. Sc. 59(1), 73–91 (2023).
Assunção, I. P., Limaz, G. S. D. A., Amorim, E. P. D. R., Muniz, M. D. F. S. & Endres, L. Occurrence of Curvularia lunata on ″Jurubeba″ in Alagoas State, Brazil. Summa Phytopathol. 32, 386–387 (2006).
Feng, N. N. et al. Metabolites and antifungal activities of an endophytic fungus Epicoccum sorghinum from mangrove. J. South China Agric. Univ. 43, 77–81 (2022).
Silva, R. M. et al. Effect of climate and phenological stage on fungal endophytes community in Sorghum bicolor leaves. Mycol. Prog. 22, 19 (2023).
Amaral, A. L., Carli, M. L., Neto, J. F. B. & Soglio, F. K. Phoma sorghina, a new pathogen associated with Phaeosphaeria leaf spot on maize in Brazil. Plant Pathol. 53, 259–259 (2004).
Navi, S. S., Bandyopadhyay, R., Reddy, R. K., Thakur, R. P. & Yang, X. B. Effects of wetness duration and grain development stages on sorghum grain mold infection. Plant Dis. 89, 872–878 (2005).
Verhoeff, K. Latent infections by fungi. Ann Rev Phytopathol. 12, 99–110 (1974).
Clay, K. & Schardl, C. L. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am. Nat. 160, 99–127 (2002).
Kado, C. I. Asymptomatic and latent infections. In Plant Bacteriology (ed. St Paul, M. N.) (American Phytopathological Society, 2016).
Cui, J. et al. A review on plant endophytes in response to abiotic stress. Environ. Pollut. Bioavailab. 36(1), 2323123 (2024).
Schulz, B., Rommert, A.-K., Dammann, U., Aust, H.-J. & Strack, D. The endophyte-host interaction: A balanced antagonism?. Mycol. Res. 103, 1275–1283 (1999).
Redman, R. S., Dunigan, D. D. & Rodriguez, R. J. Fungal symbiosis from mutualism to parasitism: Who controls the outcome, host or invader?. New Phytol. 151, 705–716 (2001).
Lind, E. M. et al. Life-history constraints in grassland plant species: A growth-defence trade-off is the norm. Ecol. Lett. 16, 513–521 (2013).
Legreve, A. & Duveiller, E. Preventing potential diseases and pest epidemics under a changing climate. In Climate Change and Crop Production (ed. Reynolds, M. P.) 50–70 (CABI Publishing, 2010).
Singh, V. K. & Chawla, S. Cultural Practices: An Ecofriendly Innovative Approach in Plant Disease Management 01–20 (International Book Publishers and Distributors, 2012).
Wise, K. Fungicide efficacy for control of corn diseases. Crop Prot Network, (2018).
Latiffah, Z. & Ning, C. H. Endophytic Fusarium spp. from roots of lawn grass (Axonopus compressus). Trop. Life Sci. Res. 24, 85–90 (2013).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 33, 1870–1874 (2016).
Palencia, E. R. Endophytic associations of species in the Aspergillus section Nigri with maize (Zea mays) and peanut (Arachis hypogea) hosts, and their mycotoxins. Preprint at http://getd.libs.uga.edu/pdfs/palencia_edwin_r_201205_phd.pdf (2012).
Mehta, P. P. & Mondal, K. K. Field screening of groundnut cultivars against rust of tikka. Indian Phytopathol. 31, 259–260 (1978).
Author information
Authors and Affiliations
Contributions
L.Z. conceptualized the project. L.Z., N.M.N. and P.T.T. designed the experiment. L.Z. and N.M.N. supervised the project. P.T.T. carried out the experiments and analyzed the data. P.T.T. prepared the first draft of the manuscript. N.F.A. analyzed the data and prepared the figures. L.Z. for funding acquisition and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Terna, P.T., Mohamed Nor, N.M.I., Azuddin, N.F. et al. Molecular identification and pathogenicity of endophytic fungi from corn ears. Sci Rep 14, 17146 (2024). https://doi.org/10.1038/s41598-024-68428-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68428-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.