Abstract
Human-based modelling and simulation offer an ideal testbed for novel medical therapies to guide experimental and clinical studies. Myocardial infarction (MI) is a common cause of heart failure and mortality, for which novel therapies are urgently needed. Although cell therapy offers promise, electrophysiological heterogeneity raises pro-arrhythmic safety concerns, where underlying complex spatio-temporal dynamics cannot be investigated experimentally. Here, after demonstrating credibility of the modelling and simulation framework, we investigate cell therapy in acute versus chronic MI and the role of cell heterogeneity, scar size and the Purkinje system. Simulations agreed with experimental and clinical recordings from ionic to ECG dynamics in acute and chronic infarction. Following cell delivery, spontaneous beats were facilitated by heterogeneity in cell populations, chronic MI due to tissue depolarisation and slow sinus rhythm. Subsequent re-entrant arrhythmias occurred, in some instances with Purkinje involvement and their susceptibility was enhanced by impaired Purkinje-myocardium coupling, large scars and acute infarction. We conclude that homogeneity in injected ventricular-like cell populations minimises their spontaneous beating, which is enhanced by chronic MI, whereas a healthy Purkinje-myocardium coupling is key to prevent subsequent re-entrant arrhythmias, particularly for large scars.
Similar content being viewed by others
Introduction
Heart failure, commonly the result of myocardial infarction (MI), remains a leading cause of mortality worldwide with novel treatments needed to reduce the clinical burden. During MI, an insufficient blood and nutrient supply causes the affected tissue and bordering regions to undergo electrophysiological as well as structural remodelling. As a result, the heart’s contractile force decreases and adverse remodelling occurs also in the remote myocardium1,2. Although regenerative cell therapy has been shown as a promising treatment in pre-clinical studies3,4,5, safety concerns remain over the cells’ immaturities facilitating ventricular arrhythmias, as observed experimentally6,7. While animal models present a valuable tool to evaluate new therapies, they are costly, time-consuming, exhibit limited control over experimental conditions and raise ethical and translational concerns. This limits progress in optimising therapies for MI.
Human modelling and simulation of cardiac electrophysiology is a mature and well-established technology enabling multiscale investigations into disease mechanisms8,9,10, therapeutic interventions such as ablation11, diagnostics12 and pharmacological treatment13. While several studies have provided insights into specific pro-arrhythmic mechanisms following cell delivery14,15,16,17,18, they so far did not include thorough multi-scale validation from ionic to ECG level, nor did they address crucial factors such as disease progression, scar size and cell heterogeneity caused by differences in differentiation and purification protocols19,20.
The goal of this study is twofold: (1) to develop and establish the credibility of a state-of-the-art human modelling and simulation framework for personalised electrophysiological investigations including the Purkinje system, through multiscale validation with experimental and clinical data; (2) to evaluate key modulators of spontaneous beating and re-entrant arrhythmias following cell delivery in three infarct stages (acute, healing and chronic), considering variability in cell heterogeneity, scar size and the role of the Purkinje system.
Results
Credibility of human modelling and simulation from ionic to ECG dynamics
The anatomically accurate human biventricular electrophysiology models post-MI incorporate state-of-the-art human cellular models of Purkinje and ventricular electrophysiology, with credibility for healthy and MI conditions supported by data in Supplementary Table S1 and previous studies10,21,22,23. Simulated ECGs in sinus rhythm following activation of the Purkinje system were independently evaluated against clinical recordings of anteroseptal MI progression24,25,26, as illustrated in Fig. 1 and Supplementary Figs. S1 and S2. Both simulated and clinical ECGs in acute and healing MI exhibited fragmented QRS-complexes and inverted T-waves (Fig. 1A,B). Chronic MI ECGs exhibited increased QRS-complex duration, persistent ST-segment elevation and normalised T-waves. Comparison with three further clinical post-MI cases is shown in Supplementary Fig. S3C, lending credibility to the simulations.
Comparison of simulated (A) and clinical ECGs (B) for acute, healing and chronic infarction, and following cell therapy (C). Clinical ECGs from the PTB Diagnostic ECG Database, https://physionet.org/content/ptbdb/1.0.0/26,75, of patient 033 at 1, 10 and 89 days after anteroseptal infarction. Blue arrows highlight key changes compared to the healthy control. In (C), simulated ECGs before (purple solid lines) and after (black dotted lines) cell delivery are compared. Shown simulations are in the medium scar and high injected cell heterogeneity with 50% ventricular-like, 25% atrial and 25% nodal-like phenotypes.
ECGs before and after cell delivery during acute and healing MI were almost identical, as expected due to similar conduction velocity (CV) and repolarisation times of human stem cell-derived cardiomyocytes (hPSC-CMs) and the infarct (Fig. 1C). In chronic MI, hPSC-CM delivery caused a small increase in ST-segment elevation in leads V1 to V3 and T-wave depression in leads V4 and V5. This was caused by hPSC-CMs improving conduction in the electrically non-active infarct. hPSC-CM population heterogeneity had no effect on ECG signals under sinus rhythm across all scar sizes and infarct stages (see Supplementary Fig. S4).
Spontaneous beating is favoured by heterogeneous injected cell populations, depolarised infarcts and slow sinus beating
Figure 2 reports spontaneous activity in homogeneous versus heterogeneous stem cell populations injected in acute, healing and chronic infarctions at three scar sizes. Heterogeneous hPSC-CM populations with atrial and nodal-like phenotypes favoured ectopic activity, whereas homogeneous ventricular-like hPSC-CM populations did not beat spontaneously for any scenario (see Fig. 2A). Spontaneous hPSC-CM activity arose from infarct centres, where injections were simulated (Fig. 2B, Supplementary Fig. S5).
Modulators of spontaneous hPSC-CM beats. (A) Spontaneous hPSC-CM beating intervals after the last sinus beat for increasing hPSC-CM population heterogeneity across 3 infarct stages and 3 scar sizes (27 cases in total); dark shades indicate faster beating. (B) Action potentials of infarcted cells from dense (solid lines) and sparse (dotted lines) hPSC-CM areas with the most heterogeneous hPSC-CM population in medium-sized acute (green) and chronic (purple) MI; left (LV) and right (RV) ventricle indicated. (C) Effect of sinus heart rate on spontaneous hPSC-CM beats. Left: Single cell action potential and sarcoplasmic reticulum calcium deposit of ventricular-like hPSC-CM. Right: hPSC-CM beating intervals in single cell ventricular, atrial and nodal-like hPSC-CM phenotypes (0D) and coupled in the medium scar biventricular model with the most heterogeneous population in acute MI (3D). Spontaneous single cell atrial and nodal-like hPSC-CMs beating exceeded pacing below 60 and 100 bpm, respectively.
The moderately heterogeneous cell populations (with 80% ventricular-like hPSC-CMs) beat spontaneously only in chronic MI, being fastest in the medium scar and considerably slower in the large scar (Fig. 2A, middle column). Chronic MI promoted spontaneous beating through depolarisation of hPSC-CM diastolic membrane potential by electrotonic coupling with the infarct. Hence, an increase in scar size from small to medium led to faster spontaneous rates due to more depolarised membrane potentials (Fig. 2A, Supplementary Fig. S6). However, being electrically inactive, large scars in chronic MI delayed the propagation of the spontaneous beat (Fig. 2B, low action potential amplitude in the infarct in chronic MI compared to acute MI). This resulted in large current sinks dampening the spontaneous activity, as highlighted by a reduced upstroke velocity of hPSC-CMs action potentials for larger scars (1.2, 0.8, and 0.4 mV/ms in the small, medium and large chronic scar for the moderately heterogeneous population).
The most heterogeneous population (50% ventricular-like hPSC-CMs) beat spontaneously in all post-MI scenarios (Fig. 2A, last column and Supplementary Video S1) and increasing scar size had very small effects on the beating interval (± 10 ms, Fig. 2A, last column). These much smaller differences in beating times can be explained by a faster spontaneous upstroke velocity (2.9, 2.6, and 2.2 mV/ms in the small, medium and large chronic scar), highlighting the reduced current sink effects of the surrounding tissue. This increase in upstroke velocity with higher heterogeneity may appear counterintuitive as, in single cell, the ventricular-like phenotype had the fastest upstroke velocity (20.6 versus 8.9 and 4.3 mV/ms in the atrial and nodal-like phenotypes as shown in Fig. 4). However, in tissue, the quicker beating of atrial and nodal-like phenotypes drove the spontaneous depolarisation, having to excite not only the native ventricular tissue but also the slower beating ventricular-like hPSC-CMs. Hence, our results imply that higher numbers of atrial-like and nodal-like hPSC-CMs in the injected cell population not only increase the rate of the spontaneous beating but also its strength.
Furthermore, as illustrated in Fig. 2C, spontaneous beating slowed down with increasing heart rate (from 1950 to 2250 ms for 50 to 100 bpm for the ventricular-like phenotype). Mechanistic investigation showed that this is caused by a depletion of the sarcoplasmic reticulum (SR) calcium deposit (Fig. 2C, left, and more details in Supplementary Fig. S7). Accordingly, the peak of the SR calcium release during the following spontaneous beat decreased from 0.15 to 0.08 mM/s. Faster spontaneous beats after slower heart rates were also observed in the biventricular simulations, with the medium scar during the acute stage shown as an example in the right panel (green circles) of Fig. 2C.
Impaired Purkinje propagation increases arrhythmia susceptibility following spontaneous hPSC-CM beats
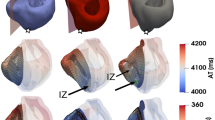
Re-entrant arrhythmia formation after spontaneous hPSC-CM activity under bradycardic heart rates was explored in conjunction with healthy versus impaired retrograde Purkinje propagation (Fig. 3). Spontaneous hPSC-CM beating did not lead to the establishment of re-entry since the native tissue was already completely repolarised as shown in Fig. 3A, and propagation engulfed the ventricles without the occurrence of unidirectional block (the first condition for re-entrant arrhythmias). However, spontaneous beating altered repolarisation patterns and provided the substrate for re-entrant arrhythmias following bradycardic beats through the bundle of His (between 250 and 450 ms after the spontaneous beat, Fig. 3B). As shown in Fig. 3C, the bradycardic beat met conduction block in the infarcted area still refractory from the spontaneous beat. For healthy Purkinje propagation, two re-entrant arrhythmias occurred in the large scar during the acute and chronic stage (Fig. 3B), enabled by the wavefront retrogradely propagating through a Purkinje-myocyte junction (PMJ). In both cases, this PMJ was located within one of the scar cores, once in the left ventricular septo-apical wall and once on the antero-septal wall (see Supplementary Video S2). The re-entry observed in the large scar during the acute stage was sustained for 3000 ms, with wavefronts re-entering also through the antero-basal and through the epicardial antero-apical wall (see left column Fig. 3C).
Depolarisation of the ventricles through spontaneous hPSC-CM beats and subsequent arrhythmia inducibility and sustainability. (A) Emergence of spontaneous hPSC-CM beats in the large scar during the acute stage with (left) and without (right) retrograde Purkinje propagation. (B) Vulnerable windows for the small, medium and large scar across the acute, healing and chronic stages with and without retrograde propagation through the Purkinje network. (C) Propagation of electrical excitation following a proceeding bradycardic beat in the large scar during the acute stage post-MI with and without retrograde Purkinje propagation. The dark spots in the scenarios without retrograde Purkinje propagation indicate the PMJs, showing that current was not able to flow from the myocardial tissue into the PMJs.
Impaired retrograde Purkinje propagation (Fig. 3, right column) promoted re-entry induction as shown by a widened vulnerable window for re-entry, which combining all infarct stages and scar sizes was 900 ms versus 20 ms with Purkinje retrograde propagation. This was because spontaneous beats could not propagate through the Purkinje system to remote regions, thereby increasing activation and repolarisation heterogeneity - the substrate for re-entry establishment. The vulnerable window increased with larger scar size (130 ms versus 420 ms for small versus large scar). Overall, acute MI was the most pro-arrhythmic in comparison with healing and chronic MI, and accounted for 54, 54 and 48% of re-entrant arrhythmias in the small, medium and large scar, respectively. The chronic stage was the least pro-arrhythmic in all scar sizes, accounting for 0, 6 and 23% of re-entries in the small, medium and large scar, respectively. The proportion of sustained arrhythmias continuing for at least two consecutive re-entry cycles increased from 31 over 69 to 81% in the small, medium and large scar, respectively (Fig. 3B). Re-entries were sustained for over 5000 ms, as shown in the ECG in the right column of Fig. 3C. We identified different re-entrant circuit locations in the septum, anterior wall and apex, which varied across the different scar sizes, as illustrated in Fig. 3C left and right column. No preference of re-entrant circuit location for any infarct size or stage was apparent. However, re-entry complexity increased with increasing scar size (see Supplementary Fig. S8). In the small scar, we observed at most two re-entrant circuit locations per re-entrant simulation. In the medium and large scar, 25% of re-entries occurred through three or more sites simultaneously. The number of re-entrant circuit sites was similar during the acute and healing stages across all scar sizes. Re-entries during the chronic stage showed the least complexity; for example, in the large scar most re-entries occurred through a single circuit in the septal wall.
To summarise, while healthy Purkinje propagation enabled the spontaneous hPSC-CM beat to activate remote regions quickly and uniformly, impaired retrograde propagation caused a heterogeneous refractory substrate, which can be seen when comparing the left and right column of Fig. 3A at 1500 ms. Furthermore, when retrogradely activated, the increased refractoriness of Purkinje cells compared to the ventricular tissue prevented re-excitation of the bundle of His for bradycardic intervals below 1780 ms when most of the ventricles had already repolarised, reducing the substrate for conduction block and re-entry. Overall, our results show how impaired retrograde Purkinje propagation can cause dyssynchronous propagation of the spontaneous beat and accordingly larger repolarisation gradients in the ventricles. In our study, these resulted in a multitude of re-entrant pathways and an overall increased re-entry inducibility, sustainability and complexity.
Discussion
The first contribution of this study is the human multiscale modelling and simulation framework to investigate post-MI mechanisms and treatments, such as regenerative cell therapy. The credibility of the framework is supported by extensive consistency of simulation results in the present and previous studies21,27 with experimental and clinical data from ionic, cellular and importantly ECG dynamics from acute to chronic MI. The precise control in modelling and simulation studies allowed us to break down the key factors and mechanisms modulating the therapy’s safety. Specifically, here we investigated the interplay between infarct stage and size, Purkinje coupling and immature stem cell heterogeneity in modulating the pro-arrhythmic substrate following cell therapy.
Our key findings are as follows. Firstly, homogeneity in the injected cell population is crucial to avoid spontaneous beating activity, which is enhanced by depolarised infarcts such as in chronic MI, and slow beating rates. Secondly, subsequent arrhythmic burden due to re-entrant arrhythmias was highest in acute MI with large scars and with impaired retrograde Purkinje coupling. Taken together, our simulations suggest that ventricular-like only cell populations in healing scars are less likely to produce pro-arrhythmic spontaneous beats and that healthy Purkinje-myocardium coupling is also crucial.
Our simulations indicate that a substantial proportion of atrial and nodal-like phenotypes (> 20%) is necessary for spontaneous beats to emerge in the ventricles. Heterogeneity within hPSC-CM cultures has been shown experimentally resulting from imperfect purification protocols and depending on maturation and differentiation protocols19,20. Heterogeneities include the action potential duration (APD), the diastolic membrane potential (DMP) and the spontaneous beating interval and may be even larger than investigated here28,29,30. Such heterogeneities may have crucial arrhythmic implications, as suggested by a recent experimental study31, which need to be assessed before therapy implementation, especially as longitudinal data of the cells after delivery are scarce. As injection of non-ventricular cells to repair the ventricles is unlikely, our homogeneous population in this study was comprised of ventricular-like cells. However, as variability between cultured hPSC-CMs is large, some ventricular hPSC-CMs may be better represented with our nodal or atrial-like phenotype. This may warrant future investigations to explore the arrhythmic risk of fully atrial or nodal-like populations.
Our results further show that fast pacing slows following spontaneous beating, due to a depletion of the SR calcium deposit, and reduces the amplitude of the intracellular calcium. In contrast, intracellular calcium is known to accumulate in adult human cardiomyocytes under fast pacing32. Supporting our findings of a reverse effect in injected cells, experiments have shown that fast pacing reduces hPSC-CMs’ contractile amplitude33.
A previous in silico study showed that the risk of spontaneous stem cell beats increases with higher stem cell density, clustering and injection proximal rather than distal to the infarct17. In our study, instead of varying these parameters, we modelled the spatial distribution of injected cells based on experimental observations from34. Therefore, although the number of delivered cells relative to the scar size was maintained in our study, absolute cell number, density and clustering varied between the different scar geometries. For example, our medium scar contained an infarct core at the antero-septal wall towards the apex where the heart wall is very thick causing more cells to cluster there. According to Yu et al.17, this might influence the spontaneous beating rate, but as shown in our results, the spontaneous beats emerged from similar sites across all scars, suggesting this effect to be secondary here.
In our study, we focus on quantifying the pro-arrhythmic consequences of variability in infarct size and stage, and we identify infarct stage as more crucial than infarct size in determining the occurrence of spontaneous beats. Although cell delivery is aimed at patients with chronic infarction, exploring the safety of cell injection across earlier infarct stages is crucial, since delivery protocols remain to be optimised and re-infarction cannot be ruled out. In our study, chronic infarction, modelled as conductive but non-excitable, promoted spontaneous beating but large scars slowed its upstroke and propagation. According to another in silico study, slow-conducting passive scars may reduce the occurrence of spontaneous beats compared to non-conducting scars16. Gibbs et al. also showed how increasing electrical coupling as the injected cells mature modulates the burden of ectopic activity, which is highest for existing but incomplete coupling16. This is supported in results by Fassina et al., which suggest that cells with a CV 20% or less than that of healthy myocardium, like chosen here, may promote arrhythmic events14,15.
While some experimental studies have suggested that the stem cells’ rapid automaticity directly induces ventricular tachycardia35, our study showed how the spontaneous beats may facilitate re-entrant arrhythmias, also involving the Purkinje system. Our simulations confirm that large scars may be more pro-arrhythmic than small scars and further suggest that the increased repolarisation time of the Purkinje network and its ability to uniformly distribute the spontaneous beat across the rest of the ventricles, may be protective against some arrhythmias. Increased repolarisation times in Purkinje cells compared to ventricular cardiomyocytes have been shown experimentally36, replicated in the cellular models used here. Rapid Purkinje conduction eliminated some re-entrant pathways while enabling new ones, which has been highlighted in other in silico studies37,38. Since in our study we only examined completely enabled or completely disabled retrograde Purkinje propagation, future studies may investigate impaired conductivity in specific PMJs relative to infarcted areas. Overall, our investigation of the Purkinje network highlights its importance as a key modulator of arrhythmic risk, so far rarely included in computational studies.
Based on the mechanisms for spontaneous beating and re-entry we observed in this study, we speculate that the heart size would not have a direct effect on spontaneous beating but could impact re-entry pathways. Given the lack of micro-re-entries in our simulations, we don’t expect changes in wall thickness to have a major effect on the arrhythmic profile. Nevertheless, future studies could evaluate the importance of additional factors such as anatomical and electrophysiological variability for further translation to large patient populations.
Methods
Human multiscale modelling and simulation framework
A human multiscale modelling and simulation framework (shown in Fig. 4) was developed and evaluated using experimental and clinical data from ionic to ECG level, building on previous studies9,10,21,39. Our framework allows for personalisation of anatomical, structural and electrophysiological properties to clinical data. The healthy biventricular anatomy depicted in Fig. 4A was obtained from clinical MRI40. Ventricular fibre orientation was incorporated using the rule-based method41 following anatomical observations42. The anatomical description of the Purkinje network was incorporated using an established algorithm43,44 to achieve consistency between simulated and clinical QRS complexes, as shown in Fig. 4A. CV in fibre, sheet and normal directions were 6545, 38 and 47 cm/s (see Supplementary Fig. S9). Propagation across the PMJs occurred with a delay of 2 ms in the anterograde direction, similar to experimental values of 3–7 ms46, and 1 ms in the retrograde direction.
(A) Patient heart and torso geometries with electrode locations40 were combined with a personalised Purkinje conduction network to reproduce the patient’s ECG43,44; ECGs were normalised. (B) Small, medium and large scar created in the healthy anatomic model57,58. (C) Action potential traces of the endocardial human adult ventricular ToR-ORd cell model during the acute, healing and chronic stages of myocardial infarction. (D) Contact points representing the anatomy of the left anterior descending artery; left (LV) and right (RV) ventricle indicated. (E) Stem cell delivery locations in the left ventricle and delivered hPSC-CMs; basal, anterior and transmural view. (F) Spontaneous action potential traces of the ventricular-like, atrial-like and nodal-like hPSC-CM Paci2020 model.
Electrical propagation was simulated with the monodomain equation in the GPU-enabled open source MonoAlg3D simulator47. Cellular membrane kinetics were represented by the ToR-ORd model for human ventricular cells22,48, the Trovato model for Purkinje cells23 and the Paci2020 model for hPSC-CMs49. Electrophysiological heterogeneity across the ventricles was modelled including APD gradients of 35 ms transmurally based on50,51 with 70% endocardial and 30% epicardial cells and in the apicobasal direction of 10 ms by scaling GKs (Supplementary Figs. S9, S10).
Modelling stages of myocardial infarction
Three models of anteroseptal MI were produced corresponding to acute (first days), healing (first weeks) and chronic (several weeks) infarction (Fig. 4C). Electrophysiological alterations were considered in the core of the infarct, the border zone and the remote myocardium (distant from the scar but affected over time). These were introduced by scaling conductances and time constants of key ionic currents, as reported experimentally and described previously10,21 (summarised in Supplementary Table S1). The infarct zone during the chronic stage was modelled as non-electrically active but conducting52 by setting the starting membrane potential to − 60 mV53, accounting for the elevated DMP of fibroblasts which make up large proportions of myocardial scars54. Thus, the membrane potential in the chronic infarct changed only through diffusivity. Chronically infarcted elements produced AP-like changes in membrane voltage when coupled in tissue, which resembled experimentally and computationally observed behaviour of fibroblasts electrically coupled to cardiomyocytes55,56.
Using the algorithms described in57,58, three physiological scar geometries were produced in the healthy patient-derived anatomy and referred to as small, medium and large (Fig. 4B). For this, six landmarks representing contact points of the left anterior descending artery and the myocardium59 were considered. As shown in Fig. 4D, two contact points in the septum, three on the anterior wall and one in the apex were selected; apical involvement in anterior scars was highlighted in clinical studies such as60. To create the three scar sizes, the number of scar cores was set to 75, 200 and 275; further algorithm parameters are listed in Supplementary Table S2. Scars covered 14, 23 and 27% of the ventricles (composed of 5, 10 and 14% infarct core and 9, 12 and 13% border zone, respectively), in line with experimentally and clinically reported ranges61,62,63.
Diffusivity in the infarct and border zone was decreased to match a 70% reduced CV in the healing stage, based on clinical observations64,65. The calibrated diffusivities were maintained in the acute and chronic stages, resulting in about 75 and 90% reduced CV in the infarct centre due to further cellular remodelling (see Supplementary Table S3 for a full list of CVs).
Modelling cell therapy
Virtual cell delivery was introduced at six injection locations across the infarct, as shown in Fig. 4E, to cover all scar sizes. Existing adult ventricular cells (ToR-ORd model) were randomly replaced by the hPSC-CMs (Paci model), with probabilities depending on infarct versus border zone, transmurality and distance to the nearest delivery location (see Supplementary Table S4 for further detail). Probabilities were calibrated using experimental insights, where in non-human primate tissue slabs, more injected cells settled in the infarct core compared to the border zone34. Informed by Poch et al.’s in vivo porcine models34, in our simulations, 28.4, 22.1 and 23.4% (different due to scar morphology) of the core infarcted area was replaced by hPSC-CMs in the small, medium and large scar, respectively. In the border zone, 9.3, 9.3 and 8.8% of cells were replaced. Poch et al. also showed injected cells to migrate through the transmural wall34; we incorporated this finding by modelling hPSC-CMs to be most likely to locate in the subendocardium, as shown in Fig. 4E. Overall, with decreasing scar size, our algorithm resulted in similar proportions but lower absolute numbers of infarct being replaced with hPSC-CMs.
To investigate the effect of hPSC-CM heterogeneity, as experimentally observed in19,20, three hPSC-CM populations were considered for each scar size with varying degrees of heterogeneity: a homogeneous population consisting of ventricular-like hPSC-CMs only, a moderately heterogeneous population (made up of 80% ventricular-like and 10% atrial and nodal-like hPSC-CMs each) and a severely heterogeneous population (containing 50% ventricular-like and 25% nodal and atrial-like hPSC-CMs each). hPSC-CM conductivities were calibrated to achieve an isotropic CV of 10 cm/s66 in the ventricular-like hPSC-CMs. Due to electrophysiological differences (Supplementary Table S6), this resulted in a CV of 8 and 6 cm/s for atrial-like and nodal-like hPSC-CMs, respectively. As we simulated cell injection, we did not assume hPSC-CMs to have a stronger coupling to each other than to the adult tissue, as one may expect in a pre-grown hPSC-CM tissue patch. Hence, at the interface between hPSC-CM and adult volumes, the conductivity was calculated as the harmonic mean between the hPSC-CM and adult conductivities47. This led to a higher conductivity between adult cardiomyocytes and hPSC-CMs than amongst hPSC-CMs, which is motivated by hPSC-CMs expressing lower connexin 4366.
Cell heterogeneity was reproduced by creating a population of 10,000 single cell hPSC-CM models using the Paci2020 model as baseline and scaling key ionic current conductances between 0 and 5. From this population, an atrial-like and a nodal-like cell type was selected by calibrating against experimental data30. The AP traces, biomarkers (after 1000 spontaneous beats to reach steady state) and corresponding current conductance scaling factors of the three resulting models can be seen in Fig. 4F and Supplementary Tables S5 and S6, respectively.
Spontaneous beats in the human ventricles were defined as beats originating from the hPSC-CMs and depolarising both ventricles. The onset of spontaneous beats was quantified as the time when hPSC-CMs depolarised above − 45 mV (75% of the nodal-like, i.e., most depolarised, DMP). DMP in this work was defined as the most negative membrane potential between two upstrokes.
Verification, validation and uncertainty quantification
Human modelling and simulation is a mature and well-established technology8,10,11,67, shown to accurately replicate disease conditions and make precise predictions into underlying mechanisms and optimal treatment. To demonstrate the credibility of our framework, we considered verification, validation and uncertainty quantification principles68,69.
Correct single cell model transfer across solvers is depicted in Supplementary Fig. S11. Verification of the monodomain model solver used in this work was ensured through benchmark tests47 and CV convergence analysis (Supplementary Figs. S12, S13).
The single cell models used here were constructed using data obtained in human preparations, clinically supporting their biological relevance. Their credibility is further shown through extensive consistency with experimental data in control conditions and under drug block22,49,67,70. The three biventricular models from acute to chronic infarction demonstrate agreement with experimental ionic remodelling10,21 and clinical ECG data (Supplementary Fig. S3). The Purkinje system was introduced using experimental data and personalisation of the activation sequences yielding ECG biomarkers consistent with the clinical ECG for the corresponding anatomy44 (Supplementary Fig. S10).
Regarding parameter uncertainty, sensitivity analyses were conducted for single cell model parameters in their original publications and further studies71,72. The sensitivity of the biventricular model to disabled retrograde Purkinje propagation was investigated and as shown in Supplementary Fig. S14, ECG traces under sinus pacing were almost identical. We further investigated the sensitivity to hPSC-CM conductivity by varying the hPSC-CM CV by ± 20% (resulting in a CV of 12 and 8 cm/s in the ventricular-like phenotype, respectively). As shown in Supplementary Fig. S15, this had a small effect on the timing of the spontaneous beat, which occurred 2 ms faster for slowed conductivity and 2 ms slower for increased conductivity. To allow for accurate comparisons throughout our simulations, parameters were varied in a controlled manner to test specific hypotheses and only where necessary. Specifically, the biventricular geometry, activation pattern, occluded areas and hPSC-CM delivery locations were kept constant but could be personalised to individual patients in future studies. To maintain their spatial distribution, hPSC-CM heterogeneity scenarios were applied on top of the randomly created hPSC-CM locations. Uncertainty of our random hPSC-CM distribution algorithm was assessed by creating an additional two models of heterogeneous hPSC-CM delivery in the medium-sized chronic infarct (considering the same general injection locations as shown in Fig. 4E). As shown in Supplementary Fig. S16, the effects on the ECG were very small and only slightly affected the QRS amplitude of the spontaneous beat.
Simulation software and protocol
In total, we conducted 550 simulations for precise investigation of arrhythmia mechanisms in human hearts. These considered 27 scenarios, composed of three scar geometries across three infarct stages for three different hPSC-CM population heterogeneities. Initial conditions in the biventricular geometry were taken from the single cell models after reaching steady state (500 and 1000 paced beats at 1 Hz in the ToR-ORd and Trovato model, respectively, and 1000 spontaneous beats in the Paci model). Each biventricular scenario was paced for 3 beats under 1 Hz at the bundle of His, reaching consistency between beats. To quantify arrhythmic risk, we observed whether hPSC-CMs beat spontaneously after the third beat, followed by a fourth beat applied at varying intervals ranging from 1620 to 1880 ms after the last sinus beat. This fourth beat is representative of slow heart rates between 32 and 37 beats per minute (bpm). If a re-entrant wavefront occurred following the bradycardic beat, the simulations were observed for a further two seconds to evaluate arrhythmia sustainability. To investigate the arrhythmogenic role of Purkinje coupling, which may be affected by MI73, we ran our simulation set-up once with and once without retrograde Purkinje propagation by disabling current flow from the tissue into the Purkinje network at PMJ sites.
We also studied the effect of heart rate on spontaneous hPSC-CM activity. In the single cell models, 1000 s spontaneous activity followed by 1000 paced beats (at 50 to 100 bpm) were simulated with the proceeding spontaneous beat analysed. Increasing pacing was explored also in the biventricular model with the medium scar and the most heterogeneous hPSC-CM population across all infarct stages.
Simulations were run using the finite volume method monodomain GPU model solver MonoAlg3D47, see configuration settings in Supplementary Table S7. ECG signals were simulated with MonoAlg3D, based on the pseudobidomain approach from74. Through CV convergence analysis, the human geometry was refined to 300 μm resolution to give a CV error of 12% (see Supplementary Fig. S12). With this setup, one in silico second took 8 h to simulate using the Piz Daint Platform at the CSCS Swiss National Supercomputing Centre.
Data availability
Code to reproduce this work is available at https://github.com/LLRiebel/MonoAlg3D_C-2023.
References
Holmes, J. W., Borg, T. K. & Covell, J. W. Structure and mechanics of healing myocardial infarcts. Annu. Rev. Biomed. Eng. 7(1), 223–253. https://doi.org/10.1146/annurev.bioeng.7.060804.100453 (2005).
Sutton, M. G. S. J. & Sharpe, N. Left ventricular remodeling after myocardial infarction. Circulation 101(25), 2981. https://doi.org/10.1161/01.CIR.101.25.2981 (2000).
Foo, K. S. et al. Human ISL1+ ventricular progenitors self-assemble into an in vivo functional heart patch and preserve cardiac function post infarction. Mol. Ther. 26(7), 1644–1659. https://doi.org/10.1016/j.ymthe.2018.02.012 (2018).
Querdel, E. et al. Human engineered heart tissue patches remuscularize the injured heart in a dose-dependent manner. Circulation 143(20), 1991–2006. https://doi.org/10.1161/CIRCULATIONAHA.120.047904 (2021).
Zimmermann, W.-H. et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat. Med. 12(4), 1394. https://doi.org/10.1038/nm1394 (2006).
Chong, J. J. H. et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510(7504), 13233. https://doi.org/10.1038/nature13233 (2014).
Romagnuolo, R. et al. Human embryonic stem cell-derived cardiomyocytes regenerate the infarcted pig heart but induce ventricular tachyarrhythmias. Stem Cell Rep. 12(5), 967–981. https://doi.org/10.1016/j.stemcr.2019.04.005 (2019).
Arevalo, H., Plank, G., Helm, P., Halperin, H. & Trayanova, N. Tachycardia in post-infarction hearts: Insights from 3D image-based ventricular models. PLoS ONE 8(7), 872. https://doi.org/10.1371/journal.pone.0068872 (2013).
Martinez-Navarro, H., Mincholé, A., Bueno-Orovio, A. & Rodriguez, B. High arrhythmic risk in antero-septal acute myocardial ischemia is explained by increased transmural reentry occurrence. Sci. Rep. 9(1), 2. https://doi.org/10.1038/s41598-019-53221-2 (2019).
Wang, Z. J. et al. Human biventricular electromechanical simulations on the progression of electrocardiographic and mechanical abnormalities in post-myocardial infarction. EP Europace 23, 405. https://doi.org/10.1093/europace/euaa405 (2021).
Roney, C. H. et al. In silico comparison of left atrial ablation techniques that target the anatomical, structural, and electrical substrates of atrial fibrillation. Front. Physiol. 11, 874. https://doi.org/10.3389/fphys.2020.572874 (2020).
O’Hara, R. P. et al. Personalized computational heart models with T1-mapped fibrotic remodeling predict sudden death risk in patients with hypertrophic cardiomyopathy. Elife 11, 73325. https://doi.org/10.7554/eLife.73325 (2022).
Dasí, A. et al. In-silico drug trials for precision medicine in atrial fibrillation: From ionic mechanisms to electrocardiogram-based predictions in structurally-healthy human atria. Front. Physiol. 13, 46. https://doi.org/10.3389/fphys.2022.966046 (2022).
Fassina, D. et al. Assessing the arrhythmogenic risk of engineered heart tissue patches through in silico application on infarcted ventricle models. Comput. Biol. Med. 154, 106550. https://doi.org/10.1016/j.compbiomed.2023.106550 (2023).
Fassina, D. et al. Modelling the interaction between stem cells derived cardiomyocytes patches and host myocardium to aid non-arrhythmic engineered heart tissue design. PLoS Comput. Biol. 18(4), e1010030. https://doi.org/10.1371/journal.pcbi.1010030 (2022).
Gibbs, C. E. et al. Graft-host coupling changes can lead to engraftment arrhythmia: A computational study. J. Physiol. https://doi.org/10.1113/JP284244 (2023).
Yu, J. K. et al. A comprehensive, multiscale framework for evaluation of arrhythmias arising from cell therapy in the whole post-myocardial infarcted heart. Sci. Rep. 9(1), 1. https://doi.org/10.1038/s41598-019-45684-0 (2019).
Yu, J. K. et al. Assessment of arrhythmia mechanism and burden of the infarcted ventricles following remuscularization with pluripotent stem cell-derived cardiomyocyte patches using patient-derived models. Cardiovasc. Res. https://doi.org/10.1093/cvr/cvab140 (2021).
Ban, K., Bae, S. & Yoon, Y. Current strategies and challenges for purification of cardiomyocytes derived from human pluripotent stem cells. Theranostics 7(7), 19427. https://doi.org/10.7150/thno.19427 (2017).
Jiang, B., Yan, L., Shamul, J. G., Hakun, M. & He, X. Stem cell therapy of myocardial infarction: A promising opportunity in bioengineering. Adv. Ther. (Weinh.) 3(3), 182. https://doi.org/10.1002/adtp.201900182 (2020).
Zhou, X. et al. Clinical phenotypes in acute and chronic infarction explained through human ventricular electromechanical modelling and simulations. eLife. https://doi.org/10.7554/eLife.93002.1 (2024).
Tomek, J. et al. Development, calibration, and validation of a novel human ventricular myocyte model in health, disease, and drug block. Elife 8, 890. https://doi.org/10.7554/eLife.48890 (2019).
Trovato, C. et al. Human Purkinje in silico model enables mechanistic investigations into automaticity and pro-arrhythmic abnormalities. J. Mol. Cell Cardiol. 142, 1. https://doi.org/10.1016/j.yjmcc.2020.04.001 (2020).
Chew, D. S. et al. Fragmented QRS complexes after acute myocardial infarction are independently associated with unfavorable left ventricular remodeling. J. Electrocardiol. 51(4), 607–612. https://doi.org/10.1016/j.jelectrocard.2018.04.004 (2018).
Nable, J. V. & Brady, W. The evolution of electrocardiographic changes in ST-segment elevation myocardial infarction. Am. J. Emerg. Med. 27(6), 734–746. https://doi.org/10.1016/j.ajem.2008.05.025 (2009).
Bousseljot, R., Kreiseler, D. & Schnabel, A. Nutzung der EKG-Signaldatenbank CARDIODAT der PTB über das Internet. Biomed. Tech. 1, 317–318. https://doi.org/10.1515/bmte.1995.40.s1.317 (2009).
Zhou, X., Wang, Z., Tomek, J., Wang, L. & Rodriguez, B. Post myocardial infarction ionic remodelling promotes repolarisation dispersions and abnormalities in acute and chronic stages. EP Europace 23, 573. https://doi.org/10.1093/europace/euab116.573 (2021).
Doss, M. X. et al. Maximum diastolic potential of human induced pluripotent stem cell-derived cardiomyocytes depends critically on IKr. PLoS ONE 7(7), 40288. https://doi.org/10.1371/journal.pone.0040288 (2012).
He, J.-Q., Ma, Y., Lee, Y., Thomson, J. A. & Kamp, T. J. Human embryonic stem cells develop into multiple types of cardiac myocytes. Circ. Res. 93(1), 99. https://doi.org/10.1161/01.RES.0000080317.92718.99 (2003).
Ma, J. et al. High purity human-induced pluripotent stem cell-derived cardiomyocytes: Electrophysiological properties of action potentials and ionic currents. Am. J. Physiol. Heart Circ. Physiol. 301(5), 1. https://doi.org/10.1152/ajpheart.00694.2011 (2011).
Selvakumar, D. et al. Cellular heterogeneity of pluripotent stem cell derived cardiomyocyte grafts is mechanistically linked to treatable arrhythmias. Heart Lung Circ. 31, S37–S38 (2022).
O’Hara, T., Virág, L., Varró, A. & Rudy, Y. Simulation of the undiseased human cardiac ventricular action potential: Model formulation and experimental validation. PLoS Comput. Biol. 7(5), 61. https://doi.org/10.1371/journal.pcbi.1002061 (2011).
Hinata, Y. et al. Importance of beating rate control for the analysis of drug effects on contractility in human induced pluripotent stem cell-derived cardiomyocytes. J. Pharmacol. Toxicol. Methods 118, 107228. https://doi.org/10.1016/j.vascn.2022.107228 (2022).
Poch, C. M. et al. Migratory and anti-fibrotic programmes define the regenerative potential of human cardiac progenitors. Nat. Cell Biol. 24(5), 659–671. https://doi.org/10.1038/s41556-022-00899-8 (2022).
Marchiano, S. et al. Gene editing to prevent ventricular arrhythmias associated with cardiomyocyte cell therapy. Cell Stem Cell 30(4), 396–414. https://doi.org/10.1016/j.stem.2023.03.010 (2023).
Boyden, P. A., Hirose, M. & Dun, W. Cardiac Purkinje cells. Heart Rhythm 7(1), 127–135. https://doi.org/10.1016/j.hrthm.2009.09.017 (2010).
Deo, M., Boyle, P., Plank, G. & Vigmond, E. Arrhythmogenic mechanisms of the Purkinje system during electric shocks: A modeling study. Heart Rhythm 6(12), 1782–1789. https://doi.org/10.1016/j.hrthm.2009.08.023 (2009).
Jian, K., Li, C., Hancox, J. C. & Zhang, H. Pro-arrhythmic effects of discontinuous conduction at the Purkinje fiber-ventricle junction arising from heart failure-induced ionic remodelling—Insights from computational modelling. Front. Physiol. 13, 428. https://doi.org/10.3389/fphys.2022.877428 (2022).
Riebel, L. L. et al. Modelling and simulation reveals density-dependent re-entry risk in the infarcted ventricles after stem cell-derived cardiomyocyte delivery. In 2022 Computing in Cardiology (CinC). https://doi.org/10.22489/CinC.2022.392 (2022).
Mincholé, A., Zacur, E., Ariga, R., Grau, V. & Rodriguez, B. MRI-based computational torso/biventricular multiscale models to investigate the impact of anatomical variability on the ECG QRS complex. Front. Physiol. 10, 1103. https://doi.org/10.3389/fphys.2019.01103 (2019).
Doste, R. et al. A rule-based method to model myocardial fiber orientation in cardiac biventricular geometries with outflow tracts. Int. J. Numer. Method Biomed. Eng. 35(4), e3185. https://doi.org/10.1002/cnm.3185 (2019).
Streeter, D. D. et al. Stress distribution in the canine left ventricle during diastole and systole. Biophys. J. 10(4), 345–363. https://doi.org/10.1016/S0006-3495(70)86306-8 (1970).
Berg, L. A. et al. Enhanced optimization-based method for the generation of patient-specific models of Purkinje networks. Sci. Rep. 13(1), 11788. https://doi.org/10.1038/s41598-023-38653-1 (2023).
Camps, J. et al. Digital twinning of the human ventricular activation sequence to clinical 12-lead ECGs and magnetic resonance imaging using realistic Purkinje networks for in silico clinical trials. arXiv e-prints (2023).
Taggart, P. et al. Inhomogeneous transmural conduction during early ischaemia in patients with coronary artery disease. J. Mol. Cell Cardiol. 32(4), 105. https://doi.org/10.1006/jmcc.2000.1105 (2000).
Joyner, R. W. & Overholt, E. D. Effects of octanol on canine subendocardial Purkinje-to-ventricular transmission. Am. J. Physiol. Heart Circ. Physiol. 249(6), H1228–H1231. https://doi.org/10.1152/ajpheart.1985.249.6.H1228 (1985).
Sachetto Oliveira, R. et al. Performance evaluation of GPU parallelization, space-time adaptive algorithms, and their combination for simulating cardiac electrophysiology. Int. J. Numer. Method Biomed. Eng. 34(2), 2913. https://doi.org/10.1002/cnm.2913 (2018).
Tomek, J., Bueno-Orovio, A. & Rodriguez, B. ToR-ORd-dynCl: An update of the ToR-ORd model of human ventricular cardiomyocyte with dynamic intracellular chloride. BioRxiv. https://doi.org/10.1101/2020.06.01.127043 (2020).
Paci, M. et al. All-optical electrophysiology refines populations of in silico human iPSC-CMs for drug evaluation. Biophys. J. 118(10), 18. https://doi.org/10.1016/j.bpj.2020.03.018 (2020).
Boukens, B. J. et al. Transmural APD gradient synchronizes repolarization in the human left ventricular wall. Cardiovasc. Res. 108(1), 188–196. https://doi.org/10.1093/cvr/cvv202 (2015).
Franz, M. R., Bargheer, K., Rafflenbeul, W., Haverich, A. & Lichtlen, P. R. Monophasic action potential mapping in human subjects with normal electrocardiograms: Direct evidence for the genesis of the T wave. Circulation 75(2), 379–386. https://doi.org/10.1161/01.CIR.75.2.379 (1987).
Rog-Zielinska, E. A., Norris, R. A., Kohl, P. & Markwald, R. The living scar—Cardiac fibroblasts and the injured heart. Trends Mol. Med. 22(2), 99–114. https://doi.org/10.1016/j.molmed.2015.12.006 (2016).
Ringenberg, J. et al. Effects of fibrosis morphology on reentrant ventricular tachycardia inducibility and simulation fidelity in patient-derived models. Clin. Med. Insights Cardiol. 8, 15712. https://doi.org/10.4137/CMC.S15712 (2014).
Kohl, P. Heterogeneous cell coupling in the heart. Circ. Res. 93(5), 381–383. https://doi.org/10.1161/01.RES.0000091364.90121.0C (2003).
Klesen, A. et al. Cardiac fibroblasts. Herzschrittmacherther. Elektrophysiol. 29(1), 62–69. https://doi.org/10.1007/s00399-018-0553-3 (2018).
Yue, L., Xie, J. & Nattel, S. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc. Res. 89(4), 744–753. https://doi.org/10.1093/cvr/cvq329 (2011).
Cardone-Noott, L. et al. A computational investigation into the effect of infarction on clinical human electrophysiology biomarkers. Comput. Cardiol. 2014, 673–676 (2014).
Hill, Y. R. et al. Investigating a novel activation-repolarisation time metric to predict localised vulnerability to reentry using computational modelling. PLoS ONE 11(3), e0149342. https://doi.org/10.1371/journal.pone.0149342 (2016).
Brinkman, A. M., Baker, P. B., Newman, W. P., Vigorito, R. & Friedman, M. H. Variability of human coronary artery geometry: An angiographic study of the left anterior descending arteries of 30 autopsy hearts. Ann. Biomed. Eng. 22(1), 34–44. https://doi.org/10.1007/BF02368220 (1994).
Ørn, S. et al. Effect of left ventricular scar size, location, and transmurality on left ventricular remodeling with healed myocardial infarction. Am. J. Cardiol. 99(8), 1109–1114. https://doi.org/10.1016/j.amjcard.2006.11.059 (2007).
Reindl, M. et al. Impact of infarct location and size on clinical outcome after ST-elevation myocardial infarction treated by primary percutaneous coronary intervention. Int. J. Cardiol. 301, 14–20. https://doi.org/10.1016/j.ijcard.2019.11.123 (2020).
Spath, N. B. et al. Assessment of stunned and viable myocardium using manganese-enhanced MRI. Open Heart 8(1), e001646. https://doi.org/10.1136/openhrt-2021-001646 (2021).
Tülümen, E. et al. Extent of peri-infarct scar on late gadolinium enhancement cardiac magnetic resonance imaging and outcome in patients with ischemic cardiomyopathy. Heart Rhythm 18(6), 954–961. https://doi.org/10.1016/j.hrthm.2021.01.023 (2021).
Aronis, K. N. et al. Accurate conduction velocity maps and their association with scar distribution on magnetic resonance imaging in patients with postinfarction ventricular tachycardias. Circ. Arrhythm. Electrophysiol. 13(4), 792. https://doi.org/10.1161/CIRCEP.119.007792 (2020).
Jamil-Copley, S. et al. Application of ripple mapping to visualize slow conduction channels within the infarct-related left ventricular scar. Circ. Arrhythm. Electrophysiol. 8(1), 76–86. https://doi.org/10.1161/CIRCEP.114.001827 (2015).
Hansen, K. J., Laflamme, M. A. & Gaudette, G. R. Development of a contractile cardiac fiber from pluripotent stem cell derived cardiomyocytes. Front. Cardiovasc. Med. 5, 52. https://doi.org/10.3389/fcvm.2018.00052 (2018).
Passini, E. et al. Human in silico drug trials demonstrate higher accuracy than animal models in predicting clinical pro-arrhythmic cardiotoxicity. Front. Physiol. 8, 668. https://doi.org/10.3389/fphys.2017.00668 (2017).
Musuamba, F. T. et al. Scientific and regulatory evaluation of mechanistic in silico drug and disease models in drug development: Building model credibility. CPT Pharmacometr. Syst. Pharmacol. 10(8), 804–825. https://doi.org/10.1002/psp4.12669 (2021).
Viceconti, M. et al. In silico trials: Verification, validation and uncertainty quantification of predictive models used in the regulatory evaluation of biomedical products. Methods 185, 120–127. https://doi.org/10.1016/j.ymeth.2020.01.011 (2021).
Trovato, C., Mohr, M., Schmidt, F., Passini, E. & Rodriguez, B. Cross clinical-experimental-computational qualification of in silico drug trials on human cardiac Purkinje cells for proarrhythmia risk prediction. Front. Toxicol. 4, 650. https://doi.org/10.3389/ftox.2022.992650 (2022).
Riebel, L. L. et al. In silico identification of the key ionic currents modulating human pluripotent stem cell-derived cardiomyocytes towards an adult phenotype. In 2021 Computing in Cardiology (CinC) 1–4. https://doi.org/10.23919/CinC53138.2021.9662683 (IEEE, 2021).
Paci, M. et al. Comparison of the simulated response of three in silico human stem cell-derived cardiomyocytes models and in vitro data under 15 drug actions. Front. Pharmacol. 12, 713. https://doi.org/10.3389/fphar.2021.604713 (2021).
Kienzle, M. G., Tan, R. C., Ramza, B. M., Young, M. L. & Joyner, R. W. Alterations in endocardial activation of the canine papillary muscle early and late after myocardial infarction. Circulation 76(4), 860–874. https://doi.org/10.1161/01.CIR.76.4.860 (1987).
Bishop, M. J. & Plank, G. Bidomain ECG simulations using an augmented monodomain model for the cardiac source. IEEE Trans. Biomed. Eng. 58(8), 2297–2307. https://doi.org/10.1109/TBME.2011.2148718 (2011).
Goldberger, A. L. et al. PhysioBank, PhysioToolkit, and PhysioNet. Circulation 101(23), 215. https://doi.org/10.1161/01.CIR.101.23.e215 (2000).
Acknowledgements
This project was funded by: a BBSRC PhD iCASE (BB/V509395/1) and Russell Studentship Agreement with AstraZeneca (R67719/CN001) for L.L.R. awarded to B.R., an Engineering and Physical Sciences Research Council doctoral award to J.C., an Oxford-Bristol Myers Squibb Fellowship to X.Z. (R39207/CN063), a Wellcome Trust Senior Research Fellowship in Basic Biomedical Sciences to B.R. (214290/Z/18/Z), the Oxford BHF Centre of Research Excellence (RE/13/1/30181), an NC3Rs Infrastructure for Impact Award (NC/P001076/1), the EPSRC project CompBioMed X (EP/X019446/1), the CompBioMed project (European Commission Horizon 2020 research and innovation programme, Grant Agreements No. 675451 and No. 823712) and by the Brazilian Government via CAPES, CNPq, FAPEMIG, UFSJ and UFJF. The Paci2020 model was provided by Dr. Michelangelo Paci and the Computational Biophysics and Imaging Group (CBIG) at Tampere University Foundation. Simulations were enabled through PRACE-ICEI Grants icp013 and 019 and carried out at the Piz Daint Platform of the Swiss National Supercomputing Centre. For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) public copyright licence to any Author Accepted Manuscript version arising from this submission.
Author information
Authors and Affiliations
Contributions
L.L.R.: Study design, methodology, study execution, results analysis and original draft preparation. Z.J.W., H.M. and C.T.: Study design, methodology, reviewing and editing. J.C., L.A.B., X.Z., R.D., R.S. and R.W.S.: Methodology (including tools and software), reviewing and editing. J.B.: Study design. B.R.: Study design, reviewing and editing.
Corresponding authors
Ethics declarations
Competing interests
C. T. and J. B. are employees of AstraZeneca and may hold shares and/or stock options in the company.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Riebel, L.L., Wang, Z.J., Martinez-Navarro, H. et al. In silico evaluation of cell therapy in acute versus chronic infarction: role of automaticity, heterogeneity and Purkinje in human. Sci Rep 14, 21584 (2024). https://doi.org/10.1038/s41598-024-67951-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67951-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.