Abstract
The aim of this study was to evaluate whether a constant rate infusion of dexmedetomidine could prolong the analgesic effect of peripheral nerve blocks. Twenty client-owned dogs were enrolled and randomly divided into 2 groups. The DEX group received dexmedetomidine infusion at 1 mcg kg−1 h−1, and the NaCl group received an equivalent volume infusion of saline. Infusions were started after securing vascular access and continued for 10 min, after which intravenous (IV) methadone at 0.2 mg kg−1 and propofol to effect were administered. All animals were maintained with isoflurane in 70% oxygen. Sciatic, saphenous and obturator nerve blocks were performed using 0.1 mL kg−1 0.5% ropivacaine/block. Intraoperative fentanyl was administered if the heart rate and/or mean arterial pressure (MAP) increased > 15% from the previous measurement, and vasopressors were administered if MAP was ≤ 70 mmHg. Postoperative pain was assessed every hour using the Glasgow Composite Pain Scale (GCPS) until the first rescue analgesia administration. Postoperative rescue analgesia (methadone (0.2 mg kg−1 IV) and carprofen (2 mg kg−1 IV)) was administered if the pain score was higher than 6/24 or 5/20. Duration of analgesia was defined as the time between the nerve block procedure and initial postoperative rescue analgesia. Ambulation, proprioception, and skin sensitivity were evaluated to assess the duration of the motor and sensory block. A Student T and chi-square test were used to compare groups for duration of postoperative analgesia and intraoperative fentanyl and vasopressor use, respectively (p values ≤ 0.5 considered significant). A greater number of dogs in the NaCl group required fentanyl (5/10 p = 0.03) and vasopressors (8/10, p = 0.02) than did those in the DEX group (0/10 and 2/10, respectively). The duration of postoperative analgesia was significantly longer (604 ± 130 min) in the DEX group than in the NaCl group (400 ± 81 min, p = 0.0005).
Dexmedetomidine infusion at 1 mcg kg−1 h−1 delays the time to first administration of rescue analgesia and reduces intraoperative analgesic and vasopressor requirements during Tibial Tuberosity Advancement surgery.
Similar content being viewed by others
Introduction
The use of locoregional techniques in clinical practice has improved intra- and postoperative analgesia and decreased the dose of analgesics and anesthetics required during surgery1. Local anesthetics are widely used in veterinary medicine because they are effective and safe in most settings. To extend the duration of peripheral nerve blockade (PNB), several adjuvants, including opioids, alpha-2-agonists, epinephrine, phenylephrine, ketamine, and magnesium sulfate, have been added to local anesthetics2,3,4,5.
Dexmedetomidine is an alpha-2-adrenergic agonist commonly used in veterinary medicine for its sedative, analgesic, and anesthetic-sparing effects6,7.
Its mechanism of action involves binding to alpha-2 receptors in the central and peripheral nervous systems, leading to sedation, analgesia, and other physiological effects. Studies in human medicine have reported a significantly shorter onset time of sensory and motor blockade when dexmedetomidine is administered perineurally8,9,10,11,12. This effect has only been partially and recently studied in veterinary medicine4,5,13,14. In 2017, Trein and colleagues studied for the first time the administration of dexmedetomidine, perineurally or intramuscularly combined with perineural ropivacaine 0.75% (0.1 mL kg−1), for femoral and sciatic nerve blocks and the results of their study showed that dexmedetomidine administration did not significantly change the onset time of sensory or motor blockade nor prolonged the duration of motor blockade in dogs4.
A recent study revealed that intravenous infusion of dexmedetomidine at 1 mcg kg−1 h−1 increased the duration of sensory sciatic and femoral nerve blockade performed with lidocaine and reduced the need for additional analgesia during the immediate postoperative period in dogs undergoing Tibial Tuberosity Advancement (TTA)15, an orthopedic surgery for cranial cruciate ligament rupture. Although the exact underlying mechanisms are not fully understood, there is evidence suggesting that dexmedetomidine, when administered intravenously, can potentially prolong the duration of peripheral sensory block, and reduce the need for additional analgesics during the postoperative period in dogs undergoing TTA15.
The primary aim of the present study was to evaluate whether a continuous rate infusion (CRI) of dexmedetomidine at a dose of 1 mcg kg−1 h−1 can prolong the duration of peripheral sensory blockade and analgesic effect during elective surgical orthopedic procedures in dogs. The secondary aim of the study was to evaluate whether dexmedetomidine infusion can reduce the administration of intraoperative opioids and vasopressors. Our hypothesis is that a CRI of dexmedetomidine can prolong the duration of peripheral sensory blockade and reduce the need for systemic analgesic and vasoactive drugs.
Materials and methods
Study design
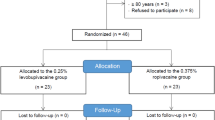
This prospective randomized blinded superiority study was conducted at the Veterinary Teaching Hospital of the University of Pisa. The study received approval by the Ethical Committee for animal welfare of University of Pisa (n° 22/2020), and the owners sign an informed consent to enroll their animals in the study. Dogs undergoing TTA orthopedic surgery, performed by the same orthopedic surgeon, were included. All methods were carried out in accordance with relevant guidelines and regulations for studies involving animals and following ARRIVE guidelines. The study did not involve humans.
Animals
To identify a difference of at least 120 min between the 2 groups, considering a mean nerve block duration of 480 minutes16, an α error of 0.05 and a β error of 0.2 (80% power), the minimum number of dogs necessary for each group was 7. To account for higher than expected values, standard deviations and data loss of up to 30%, the number of dogs in each group was increased to 10.
All dogs had a complete physical examination and full bloodwork (complete blood count, plasma biochemistry panel, and coagulation test) at hospital admission.
Exclusion criteria included a C-reactive protein (CRP) level > 0.3 mg dL−1, albumin concentration < 2.00 g dL−1, dogs > 13 years of age, diagnosis of malignancy, aggressive disposition, skin infections, or inability to secure vascular access before premedication.
Study protocol
All dogs were randomly divided into two groups according to the use of an online software program (random.org): the dexmedetomidine group (DEX) and the saline group (NaCl). The dexmedetomidine group received a CRI of dexmedetomidine at 1 mcg kg−1 h−1, while the saline group received an equivalent volume infusion of 0.9% NaCl; for the dexmedetomidine group, 1 mL of dexmedetomidine (Dextomitor, Vétoquinol, Italy S.r.l.) in 49 mL of NaCl was used, with a final concentration of 10 mcg mL-1. The infusion syringes, only labeled with the patient identification number, were prepared by an operator not directly involved in the study. On the day of surgery, a peripheral venous catheter was placed (cephalic or saphenous) to facilitate CRI administration, and Ringer's lactate at 2 mL kg−1 h−1 was administered for the entire duration of anesthesia. The CRIs (dexmedetomidine or saline) were started 10 min prior to anesthetic induction and discontinued at the end of anesthesia. Ten minutes after the start of the CRI, all dogs received 0.2 mg kg−1 IV methadone (Semfortan, 10 mg mL−1; Dechra, Turin, Italy) and were induced with propofol (Proposure, Boehringer Ingelheim Animal Health, Italy S.p.A.) to achieve orotracheal intubation. Patients were connected to an anesthetic machine (Avance CS2 Pro, GE, Milan, Italy) by a rebreathing system and maintained on isoflurane (Vetflurane, Virbac S.r.l., Milan, Italy) in oxygen at an inspired fraction of 70%. A dorsal pedal arterial catheter (22 or 20 Gauge) was placed to measure arterial blood pressure. All patients received prophylactic ampicillin (Vetamplius, Fatro Spa, Milan, Italy) at a dosage of 22 mg kg−1 IV prior to induction. To keep the investigators blinded to the study, an independent clinician, also blinded and external to the study, monitored dogs from initiation of the CRI to the time of induction.
All patients were ventilated using volume-controlled settings with a tidal volume of 10 mL kg−1 and a variable respiratory rate targeted to maintain an end-expiratory carbon dioxide (PE’CO2) value between 35 and 45 mmHg. To combat intraoperative atelectasis, positive end-expiratory pressure (PEEP) was set at 5 cm H2O.
Patients were positioned in lateral recumbency with the target leg to block in the nongravity-dependent position. Nerve blockade was then performed using a sterile technique. Ultrasound-guided sciatic, saphenous and obturator nerve blocks were performed as previously described17,18,19 by two operators with similar experience of nerve blockade, using 0.1 mL kg−1 0.5% ropivacaine (Naropina; AstraZeneca, Verona, Italy). All blocks were performed using an in-plane along the visual axis technique20 with a dedicated echogenic needle (Visioplex, Vygon Italia S.r.l., Padua, Italy) and a linear transducer (HFL50, 15–6 MHz Linear Transducer). The time to execute the blocks (from the first anesthetic injection to the last) was recorded. Operators performing blocks and clinicians monitoring patients throughout the intra- and postoperative periods were blinded to the study CRI administered.
In the event nociception was suspected based on an increase in heart rate and/or mean arterial pressure (MAP) greater than 15% in comparison with the previous value21, an IV bolus of fentanyl at 1 mcg kg−1 (Fentadon, 50 mcg mL−1, Eurovet Animal Health B.V., Bladel, The Netherlands) was administered. If parameters were not restored following the fentanyl bolus, a variable CRI of fentanyl was initiated at 2 mcg kg−1 h−1. If dogs experienced hypotension (MAP < 70 mmHg), dopamine (Dopamina Hospira, 40 mg mL−1; Hospira Italia, Naples, Italy) was administered at a starting dose of 5 mcg kg−1 min−1 increasing to a maximum of 9 mcg kg−1 min−1 as needed. If dopamine failed to correct hypotension within 5 min it was discontinued and norepinephrine was started at a dose of 0.01 mcg kg−1 min−1 (Noradrenalina tartrato 2 mg mL−1; S.A.L.F. S.p.A. Bergamo, Italy).
During surgery, heart rate (HR), arterial blood pressure, capillary refill time (CRT), ECG rhythm, end-tidal CO2 (PE’CO2), temperature (T), oxygen saturation (SpO2), fraction of expired isoflurane (Fe’Iso) and spirometry were monitored every five minutes (Avance CS2 Pro, GE, Milan, Italy) and recorded at specific surgical time points (Table 1).
For invasive blood pressure, a catheter (22 or 20 Gauge) was placed, after the peripheral nerve blocks, in the dorsal pedal artery. A pre-calibrated transducer (Transpac IV Disposable Pressure Transducer; ICU Medical Europe, Rome, Italy), placed at the level of right atrium and zeroed to atmospheric pressure, was used.
Anesthetic depth and Fe’Iso were adjusted based on the clinical evaluation of eyelid reflex, eye position and jaw tone. A bear hugger (3 M Health Care, New York, USA) was used to combat hypothermia as defined by rectal temperature. The need and dose of intraoperative fentanyl and vasoactive drugs (dopamine, norepinephrine) administered were also recorded. Bradycardia (HR < 40 bpm) was treated in case of concomitant hypotension with atropine at a dosage of 0.02 mg kg−1 IV. Infusion of dexmedetomidine or NaCl was discontinued when inhalant anesthesia was stopped. Postoperative pain was assessed every hour from the time of extubation until the first rescue analgesia administration using the Glasgow Composite Pain Scale (GCPS); when the pain score was greater than 6 out of 24 or 5 out of 20, methadone at 0.2 mg kg−1 IM (Semfortan, 10 mg mL−1; Dechra, Turin, Italy), and carprofen were administered at 2 mg kg−1 IV (Rimadyl, 50 mg mL−1; Zoetis Italia S.r.l., Rome, Italy). Duration of anesthesia was defined as the time between CRI initiation and discontinuation of isoflurane, while duration of analgesia was defined as the time from the nerve block until the first rescue analgesia administration.
Ambulation, proprioception, and skin sensitivity were evaluated to assess the duration of the motor and sensory block, respectively, every hour until the first postoperative rescue analgesia was necessary, as described by Portela et al.22. To test the sensory branch of the femoral and saphenous nerves, Halsted-mosquito clamps were applied at the level of the medial aspect of the thigh; the sensory branch of the sciatic nerve was also evaluated at the level of the skin over the caudal aspect of the metatarsus. Responses to these stimulations were graded as follows: 1, no effect (normal response to stimulus, with vigorous or rapid withdrawal of the limb and/or vocalization); 2, partial block (attenuated response to stimulus, with slower withdrawal of the limb without vocalization); 3, complete block (absence of response to stimulus without limb movement or head movement toward the stimulated area).
The postoperative analgesic requirements, possible side effects, complications during hospitalization, and at the surgical follow-up were recorded.
Statistical analysis
The data were analyzed for normality using D'Agostino and Pearson tests and a commercial software program (Prism 9; GraphPad Prism, Inc.). Parametric data are expressed as the mean and standard deviation, while nonparametric data are expressed as the median and range. Student's T test and Mann‒Whitney test were used to compare parameters between groups. One-way ANOVA with Dunnett's post hoc test was used to compare values within groups over time. A chi-square test was used to compare groups requiring rescue analgesia and vasopressors. P values < 0.05 were considered to indicate statistical significance.
Results
Twenty dogs were enrolled and completed the study uneventfully.
The mean body weight and age did not differ between the groups: 30 ± 12 kg (NaCl) and 32 ± 13 kg (DEX), 6.3 ± 2.5 years (NaCl) and 7.8 ± 2.7 years (DEX). Mean time for the blocks execution was 7 ± 2.3 min (NaCl) and 6 ± 2.9 min (DEX), mean time from the block execution to the starting of the surgery (skin incision) was 23 ± 5.7 min (NaCl) and 25 ± 4.8 min (DEX). There was no statistical difference in duration of anesthesia (194 ± 22 min DEX and 204 ± 24 min NaCl group) duration of surgery (77 ± 10 min DEX, and 72 ± 9 min NaCl) and time to initiation of the dexmedetomidine infusion and skin incision (121 ± 15 min DEX and 128 ± 17 min NaCl) between the 2 groups.
Intraoperative administration of fentanyl was required in 5/10 dogs in the NaCl group, median dose 0.5 mcg kg−1 (range 0–4 mcg kg−1) and 0/10 dogs in the DEX group (p = 0.03).
A significantly greater number of dogs in the NaCl group (8/10) required vasopressors (p = 0.02) than the DEX group (2/10); the median dose of total dopamine administered was significantly higher (p = 0.02) in the NaCl group, 79 mcg kg−1 (0–848 mcg kg−1) in comparison to DEX group 0 mcg kg−1 (0–235 mcg kg−1). The median dose of norepinephrine was not significantly different between the 2 groups (p = 0.07): 1.5 mcg kg−1 (0–9 mcg kg−1) for the NaCl group and 0 mcg kg−1 (0–3 mcg kg−1) for the DEX group. Heart rate was significantly lower in DEX group than in NaCl group (p < 0.0001) throughout the entire study period (Fig. 1). There were no significant differences in systolic or mean arterial pressure (SAP or MAP), Fe’Iso (Figs. 2 and 3), temperature, saturation of peripheral oxygen (SpO2), or PE’CO2 between the groups. Bradycardia was treated with atropine in one DEX group dog and two NaCl group dogs, which was not statistically different.
The duration of postoperative analgesia was significantly longer (604 ± 130 min) in the DEX group than in the NaCl group (400 ± 81 min) (p = 0.0005). Assessment of skin sensitivity, ambulation, and proprioception are summarized in Figs. 4 and 5. Sensory blockade score was significantly higher (p < 0.001) in the DEX group for both the saphenous and sciatic nerve in comparison to group NaCl (Fig. 4), while no differences were detected between the 2 groups regarding ambulation and proprioception (motor blockade assessment, Fig. 5).
Sensory blockade score (median and range) for saphenous and sciatic nerves; 1, no effect (normal response to stimulus, with vigorous or rapid withdrawal of the limb and/or vocalization); 2, partial block (attenuated response to stimulus, with slower withdrawal of the limb without vocalization); 3, complete block (absence of response to stimulus without limb movement or head movement toward the stimulated area)22; * significantly different from NaCl group.
Motor blockade scores (median and range) for saphenous and sciatic nerves; for ambulation (1 normal gait, 2 ataxia) and proprioception; 1, no effect (normal motor response); 2, partial loss (partial motor response); 3, complete loss (absence motor response)22; * significantly different from NaCl group.
All the dogs recovered uneventfully, with no complications noted during follow-up evaluation at 24 h, 7 days, 14 days, and 30 days after surgery. During follow-up, patients received a complete clinical and orthopedic examination.
Discussion
This study confirmed that the administration of a 1 mcg kg−1 h−1 dexmedetomidine CRI in dogs undergoing TTA surgery prolongs the duration of postoperative analgesia and reduces the requirement for intraoperative analgesics and vasopressors.
The mechanism through which dexmedetomidine enhances the efficacy of peripheral nerve blockade is uncertain and may differ if given intravenously or locally. Brummett and colleagues evaluated the activity of alpha-2-adrenergic agonists administered perineurally in rats in combination with local anesthetics; they hypothesized that the synergistic effects of dexmedetomidine are likely due to the increased blockade of the hyperpolarization-activated cation current and not to agonism of the alpha-2 adrenoceptor. In that study, dexmedetomidine was administered locally (perineural), and the authors concluded that the effects were peripheral and not due to centrally mediated or systemic analgesia23. It has also been proposed that dexmedetomidine-induced vasoconstriction may decrease the plasma absorption of locally administered local anesthetics, subsequently leading to a prolonged and increased concentration of local anesthetic available to nerve fibers. This hypothesis is supported by the findings of a study in which the plasma concentration of bupivacaine administered intraperitoneally was similar between groups when the local anesthetic was administered with either dexmedetomidine or epinephrine24. Another option is the supraspinal action of dexmedetomidine; the alpha-2 receptor binds to the locus ceruleus in the brainstem, decreases the release of norepinephrine, inhibits sympathetic activity and action at the dorsal horn, and alters the modulation of nociceptive signals23.
Our study confirms the results of the study of Stabile et al. in which IV infusion of dexmedetomidine prolonged the duration of perineural lidocaine sensory blockade without impacting motor function15. It also aligns with a veterinary study which demonstrated increased sensory blockade without motor function impairment or bradycardia during bupivacaine-induced spinal anesthesia when dexmedetomidine was given as a CRI25. That study showed that dexmedetomidine infusions increased the analgesic duration of bupivacaine-induced spinal anesthesia by approximately 50 minutes25. These findings suggest that dexmedetomidine can selectively prolong sensory blockade over motor blockade. By contrast, human studies report conflicting results26,27,28. One study, which administered a 45 min IV infusion of 0.5 mcg kg−1 of dexmedetomidine, showed prolonged sensory but not motor blockade26. Another study in which a bolus of 1 mcg kg−1 of dexmedetomidine was followed by CRI at 0.4 mcg kg−1 h−1 showed a shorter onset of sensory block, a longer duration of sensory and motor block, and longer duration of analgesia in comparison to the group in which dexmedetomidine was administered perineurally27. Finally, results of a meta-analysis in human patients indicate that IV dexmedetomidine has no impact on sensory and motor blockade and it is less efficacious than dexamethasone28.
One factor that may account for some of the discrepancy between studies is the dosage and duration of dexmedetomidine used. The current study used a dexmedetomidine CRI at a dose of 1 mcg kg−1 h−1 for 240 min, which is longer than some previously reported veterinary studies which used a 1 mcg kg−1 h−1 dexmedetomidine CRI for about 7025 and 12013 min. Further studies are needed to determine the effect of CRI dexmedetomidine dosage and duration on regional blockade.
In our study, dogs that received a dexmedetomidine CRI did not require rescue analgesia during surgery, suggesting that the dose used, in combination with the nerve blocks, is helpful for covering intraoperative analgesia during TTA. In fact, it is possible that the dose of 1 mcg kg−1 h−1 dexmedetomidine CRI fills any “analgesic gaps” not covered by regional blockade, thus contributing to a multimodal analgesic approach. Alternatively, it is possible that the anesthetic plane in the DEX group was deeper than the NaCl group as a result of the sedative effect of dexmedetomidine infusion. Although this was not specifically assessed, if there was a difference in the anesthetic plane between groups it would likely be a result of the dexmedetomidine CRI, as the isoflurane dosage was the same for both groups. There are several reasons that may explain the reduced need for intraoperative analgesia in dogs receiving a dexmedetomidine CRI, which are widely discussed in human and veterinary medicine. The purely analgesic effect mediated by the dorsal horn of the spinal cord and the supraspinal structures, as locus ceruleus and periaqueductal gray (PAG), may be involved in dexmedetomidine mediated analgesia29,30,31, nevertheless the dosage used for the present study (1 mcg kg−1 h−1) was lower than the dosage considered efficacious for analgesia (3 mcg kg−1 h−1)32. Moreover, the intraoperative fentanyl requirement of the NaCl group was quite low (0.5 mcg kg−1), and further studies are needed to clarify the analgesic impact of dexmedetomidine at the dose used for TTA surgery without a locoregional block.
In our study, the DEX group had a lower HR and required significantly less vasopressor administration than did the NaCl group. The effects of dexmedetomidine on the cardiovascular system are well known, with bradycardia, increased peripheral resistance and decreased cardiac output being the most important33. The dexmedetomidine CRI was started 10 min prior to induction, as opposed to administering a 1 mcg kg−1 bolus, to reduce the cardiovascular impact of dexmedetomidine. The reason why a low dexmedetomidine concentration is associated with a lower requirement for vasopressors is not fully understood, and several mechanisms have been proposed to explain how alpha-2 agonists improve vasopressor responsiveness. Pichot et al. suggested that dexmedetomidine can reduce the downregulation of alpha-1 receptors and/or produce gradual desensitization of alpha-1 adrenergic receptors by reducing the sympathetic outflow and release of endogenous catecholamines observed in patients with sepsis34,35. It has also been proposed that alpha-2 agonists may act on vascular smooth muscle cells via local mechanisms, and the presynaptic action of alpha-2 agonists could lead to the upregulation of postsynaptic alpha-1 receptors, by reducing the release of endogenous norepinephrine35,36,37. In the study of Sarotti et al., the group that received dexmedetomidine infusion showed a lower incidence of hypotension (9%) in comparison to the control group (65%)25, demonstrating a role on hemodynamic maintenance. The potential benefit of dexmedetomidine in decreasing vasopressor administration has been studied in several human clinical settings35,38 but its application as a vasopressor sparing agent in the veterinary clinical setting is uncommon. That said, the effects of the dexmedetomidine CRI found in the current study agree with the cardiovascular effects reported in two experimental studies in dogs undergoing anesthesia39,40.
Our study has some limitations. The ideal CRI dosage of dexmedetomidine required to provide analgesia is not well known and varies within the veterinary literature. The most frequently reported doses range from 0.5 to 1 mcg kg−1 h−1 and are administered alone or in combination with other drugs as a CRI15,25,41. However, it has been suggested that a CRI dosage of 3 mcg kg−1 h−1 is necessary to achieve an analgesic effect in dogs and that 1 mcg kg−1 h−1 CRI doses provide only a sedative effect32. In the current study NSAIDs were only administered as rescue therapy, which may not reflect real-life settings where NSAIDS may be administered preoperatively, immediately following surgery and/or during anesthesia recovery. Two different operators performed the peripheral nerve blocks: this might have introduced bias regarding the success and duration of peripheral nerve blockade, even though the experience level of both operators was similar.
Conclusion
The current study confirmed that a CRI of dexmedetomidine 1 mcg kg−1 h−1 can prolong the analgesic effect of a peripheral nerve blockade performed with 0.5% ropivacaine in dogs undergoing TTA surgery. Dexmedetomidine infusion can also reduce the intraoperative need for supplemental analgesia and vasopressor administration. Further studies using different dexmedetomidine CRIs are recommended.
DataAvailability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Helb, J. R. et al. A preemptive multimodal pathway featuring peripheral nerve block improves perioperative outcomes after major orthopedic surgery. Reg. Anesth. Pain Med. 33, 510–517 (2008).
Axelsson, K. & Gupta, A. Local Anesthetics adjuvants: Neuraxial versus peripheral nerve block. Curr. Opin. Anaesth. 22, 649–654 (2009).
Adami, C. et al. Addition of magnesium sulphate to ropivacaine for spinal analgesia in dogs undergoing tibial plateau levelling osteotomy. Vet J. 209, 163–168 (2016).
Trein, T. A. et al. Effects of dexmedetomidine combined with ropivacaine on sciatic and femoral nerve blockade in dogs. Vet. Anaesth. Analg. 44, 144–153 (2017).
Acquafredda, C. et al. Clinical efficacy of dexmedetomidine combined with lidocaine for femoral and sciatic nerve blocks in dogs undergoing stifle surgery. Vet. Anaesth. Analg. 48, 962–971 (2021).
Kellihan, H. B. et al. Sedative and echocardiographic effects of dexmedetomidine combined with butorphanol in healthy dogs. J. Vet. Cardiol. 17, 282–292 (2015).
Pan, S. Y. et al. Efficacy and safety of dexmedetomidine premedication in balanced anesthesia: A systematic review and meta-analysis in dogs. Animals 11, 3254 (2021).
Kaygusuz, K. et al. Effects of adding dexmedetomidine to levobupivacaine in axillary brachial plexus block. Curr. Ther. Res. Clin. Exp. 73, 103–111 (2012).
Agarwal, S., Aggarwal, R. & Gupta, P. Dexmedetomidine prolongs the effect of bupivacaine in supraclavicular brachial plexus block. J. Anesth. Clin. Pharmacol. 30, 36–40 (2014).
Nema, N. et al. Effect of addition of dexmedetomidine to ropivacaine hydrochloride (0.75%) in brachial plexus block through supraclavicular route in upper limb surgeries: a clinical comparative study. J. Evol. Med. Dent. Sci. 3, 12612–12621 (2014).
Rao, S. & Rajan, N. Dexmedetomidine as an adjunct for regional anesthetic nerve blocks. Curr. Pain. Headache Rep. 25, 8 (2021).
Ghazaly, H. F. et al. Comparison of the efficacy of two doses of dexmedetomidine as an adjunct to levobupivacaine in infraclavicular brachial plexus block: Prospective double-blinded randomized controlled trial. BMC Anesth. 22, 338 (2022).
Marolf, V. et al. Effects of perineural administration of ropivacaine combined with perineural or intravenous administration of dexmedetomidine for sciatic and saphenous nerve blocks in dogs. Am. J. Vet. Res. 82, 449–458 (2021).
Marolf, V. et al. Effects of perineural dexmedetomidine combined with ropivacaine on postoperative methadone requirements in dogs after tibial plateau levelling osteotomy: A two-center study. Vet. Anaesth. Analg. 49, 313–322 (2022).
Stabile, M. et al. Evaluation of a constant rate intravenous infusion of dexmedetomidine on the duration of a femoral and sciatic nerve block using lidocaine in dogs. Front. Vet. Sci. 9, 1061605 (2023).
Ferrero, C., Borland, K. & Rioja, E. Retrospective comparison of three locoregional techniques for pelvic limb surgery in dogs. Vet. Anaesth. Analg. 48(4), 554–562 (2021).
Echeverry, D. F. et al. Ultrasound-guided block of the sciatic and femoral nerves in dogs: A descriptive study. Vet. J. 186, 210–215 (2010).
Costa-Farré, C., Blanch, X. S., Cruz, J. I. & Franch, J. Ultrasound guidance for the performance of sciatic and saphenous nerve blocks in dogs. Vet. J. 187, 221–224 (2011).
Di Franco, C. et al. Saphenous and sciatic nerve blockade with and without obturator nerve block for tibial plateau levelling osteotomy surgery in dogs: A randomized controlled trial. Animals 13(24), 3792 (2023).
Di Franco, C., Tayari, H., Nardi, S. & Briganti, A. Along or across the visual axis: A comparison of two ultrasound screen, needle and transducer orientation techniques. Vet. Anaesth. Analg. 48, 147–150 (2021).
Wenger, S. et al. Evaluation of the analgesic effect of lidocaine and bupivacaine used to provide a brachial plexus block for forelimb surgery in 10 dogs. Vet. Rec. 156, 639–642 (2005).
Portela, D. A. et al. Combined paravertebral plexus block and parasacral sciatic block in healthy dogs. Vet. Anaesth. Analg. 37, 531–541 (2010).
Brummett, C. M. et al. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization activated cation current. Anesthesiology. 115, 836–843 (2011).
Benito, J., Monteiro, B., Beaudry, F. & Steagall, P. Efficacy and pharmacokinetics of bupivacaine with epinephrine or dexmedetomidine after intraperitoneal administration in cats undergoing ovariohysterectomy. Can. J. Vet. Res. 82, 124–130 (2018).
Sarotti, D., Rabozzi, R. & Franci, P. Effects of intravenous dexmedetomidine infusion on local anesthetic block: T A spinal anesthesia clinical model in dogs undergoing hind limb surgery. Res. Vet. Sci. 124, 93–98 (2019).
Abdallah, F. W. et al. IV and perineural dexmedetomidine similarly, prolong the duration of analgesia after interscalene brachial plexus block: A randomized, three-arm, triple-masked. Placebo-controlled Trial. Anesthesiol. 124, 683–695 (2016).
Samar, P., Dhawale, T. A. & Pandya, S. Comparative study of intravenous dexmedetomidine sedation with perineural dexmedetomidine on supraclavicular approach brachial plexus block in upper limb orthopaedic surgery. Cureus 12(10), e10768 (2020).
Sehmbi, H. et al. Perineural and intravenous dexamethasone and dexmedetomidine: Network meta-analysis of adjunctive effects on supraclavicular brachial plexus block. Anaesthesia 76(7), 974–990 (2021).
Grape, S., Kirkham, K. R. & Frauenknecht, J. Albrecht, EIntra-operative analgesia with remifentanil vs. dexmedetomidine: a systematic review and meta-analysis with trial sequential analysis. Anesthesia 74, 793–800 (2019).
Di Franco, C., Evangelista, F. & Briganti, A. Multiple uses of dexmedetomidine in small animals: a mini review. Front. Vet. Sci. 5(10), 1135124 (2023).
Yu, D. H. et al. Application of dexmedetomidine as an opioid substitute in opioid-free anesthesia: A systematic review and meta-analysis. Pain Phys. 26, E635–E649 (2023).
Oostrom, H., Doornenbal, A., Schot, A., Stienen, P. J. & Hellebrekers, L. J. Neurophysiological assessment of the sedative and analgesic effects of a constant rate infusion of dexmedetomidine in the dog. Vet. J. 190, 338–344 (2011).
Kuusela, E. et al. Clinical effects and pharmacokinetics of medetomidine and its enantiomers in dogs. J. Vet. Pharmacol. Ther. 23, 15–20 (2000).
Pichot, C., Géloën, A., Ghignone, M. & Quintin, L. Alpha-2 agonists to reduce vasopressor requirements in septic shock?. Med. Hypotheses. 75, 652–656 (2010).
Cioccari, L. et al. ANZICS Clinical Trials Group and the SPICE III Investigators The effect of dexmedetomidine on vasopressor requirements in patients with septic shock: A subgroup analysis of the Sedation Practice in Intensive Care Evaluation [SPICE III]. Trial. Crit. Care. 24, 441 (2020).
Shimamura, K. et al. Clonidine induced endothelium-dependent tonic contraction in circular muscle of the rat hepatic portal vein. J. Smooth Muscle Res. 42, 63–74 (2006).
Geloen, A. et al. Clonidine and dexmedetomidine increase the pressor response to norepinephrine in experimental sepsis: A pilot study. Crit. Care. Med. 41, e431–e438 (2013).
Morelli, A. et al. The effect of propofol and dexmedetomidine sedation on norepinephrine requirements in septic shock patients: A crossover trial. Crit. Care. Med. 47, e89–e95 (2019).
Villela, N. R., do Junior Nascimento, P. & Carvalho, L. R. Cardiovascular effects of two dexmedetomidine doses: experimental study in dogs. Rev. Bras. Anestesiol. 53, 784–796 (2003).
Congdon, J. M., Marquez, M., Niyom, S. & Boscan, P. Cardiovascular, respiratory, electrolyte and acid-base balance during continuous dexmedetomidine infusion in anesthetized dogs. Vet. Anaesth. Analg. 40, 464–471 (2013).
Lovell, S. et al. Randomized clinical trial comparing outcomes after fentanyl or ketamine-dexmedetomidine analgesia in thoracolumbar spinal surgery in dogs. J. Vet. Intern. Med. 36, 1742–1751 (2022).
Author information
Authors and Affiliations
Contributions
C.D.F., A.B.: study design, data management, data interpretation, statistical analysis and preparation of manuscript. E.B., S.P. and E.A.: study design, data management, and preparation of manuscript. S.B.: study design, data interpretation, preparation and revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Di Franco, C., Batisti, E., Boysen, S. et al. Effect of dexmedetomidine constant rate infusion on the analgesic duration of peripheral nerve blocks in dogs: a randomized clinical study. Sci Rep 14, 17113 (2024). https://doi.org/10.1038/s41598-024-67894-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67894-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.