Abstract
Tin dioxide is regarded as an alternative anode material rather than graphite due to its high theoretical specific capacity. Modification with carbon is a typical strategy to mitigate the volume expansion effect of SnO2 during the charge process. Strengthening the interface bonding is crucial for improving the electrochemical performance of SnO2/C composites. Here, SnO2-embedded reduced graphene oxide (rGO) composite with a low graphene content of approximately 5 wt.% was in situ synthesized via a cetyltrimethylammonium bromide (CTAB)-assisted hydrothermal method. The structural integrity of the SnO2/rGO composite is significantly improved by optimizing the Sn–O–C electronic structure with CTAB, resulting a reversible capacity of 598 mAh g−1 after 200 cycles at a current density of 1 A g−1. CTAB-assisted synthesis enhances the rate performance and cyclic stability of tin dioxide/graphene composites, and boosts their application as the anode materials for the next-generation lithium-ion batteries.
Similar content being viewed by others
Introduction
There is an urgent need for the high-performance lithium-ion batteries (LIBs) to meet the rapid developments of electric vehicles and smart grids, because the conventional LIBs based on graphite anodes (372 mAh g−1) cannot satisfy the growing demand for the efficient energy storage1,2. As an alternative anode material, SnO2 has received considerable attention due to its low cost, high theoretical specific capacity (1494 mAh g−1), high energy density, and excellent safety3. However, the SnO2 anode undergoes an irreversible conversion reaction during the first charge and discharge process. As a result, some active lithium ions convert to inert substances (Li2O, Li2CO3, LiF etc.), thereby reducing the initial coulomb efficiency (ICE)4. When the cut-off voltage is set within the range of 0.01–3.0 V, the ICE ranges only from 43 to 69%5. Simultaneously, pulverization and exfoliation of the anode coating caused by the significant volume expansion (~ 260%) during alloying reaction lead to the poor cyclic stability6. These bottlenecks limit the commercial application of SnO2 anode material for the lithium-ion batteries.
In recent years, a series of strategies have been proposed for improving the electrochemical performance of tin-based anode materials, including nanoscaling7,8, modification with carbon materials9,10 and design of special structures11,12. Modification with graphene is a promising approach due to its inherent structural stability, excellent flexibility, and high electrical conductivity13. Firstly, the graphene acts as an exceptional conductor that enables rapid electron transfer, thus enhancing the overall electrical conductivity of the composite materials. Secondly, the cyclic stability of the anode can be improved due to the relieving effect of graphene on the volume expansion of SnO2 particles14. However, the hydrophobicity of graphene presents challenges in effectively immobilizing the SnO2 particles. Although graphene oxide with abundant oxygen-containing functional groups is usually employed to anchor the metal ions, the interfacial adhesion is subject to the agglomeration of SnO2 particles. The insufficient interface results in discontinuous conductive channels for the lithium ions and electrons, and weakens the buffering role of graphene as a substrate. Consequently, well-designed tin dioxide/graphene composites should possess uniform particle dispersion, high ionic/electronic conductivity, as well as strong interfacial bonding.
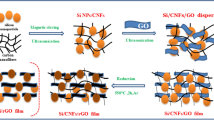
The tin dioxide/graphene composites in the relevant studies exhibited good cyclic stability owing to the establishment of robust interfacial bonding15,16,17. However, it is necessary to incorporate adequate graphene at least 20 wt.% in the composite. Excessive introduction of graphene not only reduces volumetric specific capacity but also escalates costs. Therefore, our objective is to decrease the graphene in order to optimize cost-effectiveness while preserving the electrochemical properties of the tin dioxide/graphene composite. In this study, SnO2-embedded graphene composite with a low graphene content of approximately 5 wt.% was in situ synthesized via a CTAB-assisted hydrothermal-hydrogen reduction route, as shown in Fig. 1. Furthermore, the impact of interfacial modification on the lithium storage performance of SnO2/rGO anode was thoroughly investigated.
Experimental
Preparation of SnO2/rGO materials
All reagents used in this study are of analytical purity grade. First, 0.75 g of sodium stannate (Na2SnO3·3H2O, Aladdin), 2 g of urea (Aladdin), 0.1 g of cetyltrimethylammonium bromide (CTAB, Macklin) and 5 mL of graphene oxide (GO) suspension (dispersed in H2O at a concentration of 2 mg/mL, Macklin) were mixed in 35 mL of deionized water, and an additional 40 mL of anhydrous ethanol (Aladdin) was added. The resulting solution was stirred using a magnetic stirrer for 1 h and then transferred into a Teflon-lined stainless-steel autoclave with a volume of 100 mL. Subsequently, the hydrothermal reaction lasted for 24 h at 150 °C in an air-blast drying oven. After naturally cooling to room temperature, the sediment was centrifugally separated and washed with the deionized water. After drying at 70 °C for 10 h in a vacuum oven, the SnO2/GO composite was heated at 400 °C in a tube furnace under a reduction atmosphere of hydrogen for 2 h. Finally, SnO2/rGO composite was obtained by cooling the samples to room temperature under an inert atmosphere of nitrogen. For comparison, SnO2 and SnO2/rGO materials without the assistance of CTAB were also synthesized.
Preparation of anodes
The slurry was prepared by co-grinding the active material, the conductive agent (acetylene black), and the binder (CMC) at a mass ratio of 8:1:1 in deionized water solvent. Subsequently, the slurry was uniformly coated onto the copper foil. Then, the coatings were placed in a vacuum oven at room temperature for 12 h, followed by drying at 110 °C for 3 h. The anode sheets were obtained by cutting the processed copper foil into circular pieces with a diameter of 12 mm.
Electrochemical measurements
CR2025 coin-type half-cell was assembled using the as-prepared anode in an argon-protected glove box, paring a lithium sheet (Φ16 × 0.4 mm, Trillion Metals) as the counter and reference electrodes. The polypropylene was used as the separator and 1 M LiPF6 (the solvent was ethylene carbonate and diethyl carbonate in a volume ratio of 1:1) was used as the electrolyte. Galvanostatic charge–discharge test was performed using a CT2001A battery test system (Land Electronics) at a current density of 1 A g−1 at room temperature in a voltage range from 0.01 V to 3.00 V (vs. Li/Li+) after activation at a low current density of 0.1 A g−1. The rate performance was also evaluated at various current densities (0.1–4 A g−1). Furthermore, the pristine cells were employed to carried out electrochemical measurements using a DH7001 electrochemical workstation (Donghua Test). Cyclic voltammetry (CV) test was performed at a scan rate of 0.1 mV/s. Electrochemical impedance spectroscopy (EIS) test was operated over a frequency range of 105 Hz to 10−2 Hz, with an applied AC amplitude of 5 mV.
Structural characterization
Crystal phase of the materials was characterized with a Cu Kα radiation source at a scanning rate of 5° min-1, over a 2θ angle range of 10° to 80°, using a Bruker D8 Advance X-ray diffraction (XRD) analyzer. The mass fraction of graphene in the composite was analyzed using a TGA 550 thermal analyzer (TA Instruments) with a heating rate of 10 ℃ min-1 from room temperature to 800 °C. The chemical states of the elements were determined using an X-ray photoelectron spectrometer (XPS, Thermo Fisher) with an Al Kα radiation source, and the XPS spectra were calibrated according to the C1s peak at 284.8 eV. The particle morphology was observed using a JSM-7900F scanning electron microscope (SEM, Nippon Electronics Co., Ltd), and the distribution of elements in the material was analyzed using its energy dispersive spectrometer (EDS). The crystal microstructure of the anode material was characterized using a Tecnai F30 transmission electron microscope (TEM, FEI).
Results and discussion
XRD patterns of SnO2, SnO2/rGO and SnO2/rGO-CTAB samples are shown in Fig. 2a. All of the samples exhibit characteristic peaks at 26.6°, 33.9°, and 51.8°, corresponding to the (110), (101), and (211) crystal planes of pure tetragonal SnO2 (PDF #99-0024). After the hydrogen reduction, it can be observed that SnO2 remains as the predominant component in both SnO2/rGO-CTAB and SnO2/rGO samples, exhibiting a sharper peak shape. This indicates that SnO2 in the sample has not been reduced by hydrogen18. However, it is difficult to confirm the presence of graphene in eitherSnO2/rGO-CTAB or SnO2/rGO sample due to the overlapping peaks around 2θ of 26.6° between the (002) crystal plane of graphene and the (110) crystal plane of SnO219. TG and DTG curves of SnO2/rGO-CTAB are illustrated in Fig. 2b. The minor endothermic peak at 50.4 °C suggests the potential evaporation of adsorbed water in the sample. The weight loss at approximately 422 °C is noteworthy, which can be attributed to the combustion and volatilization of graphene20. Thus, the SnO2/rGO-CTAB sample contains roughly 5 wt.% of graphene. The results confirm that the composite contains a significantly low weight ratio of graphene.
XPS spectra of SnO2, SnO2/rGO and SnO2/rGO-CTAB samples are presented in Fig. 3. C, O and Sn elements can be clearly observed from the survey spectra in Fig. 3a. Figure 3b shows the high-resolution Sn 3d spectra of SnO2, SnO2/rGO and SnO2/rGO-CTAB. In the case of the SnO2 sample, two distinct peaks at 494.9 eV and 486.5 eV are detected, corresponding to the binding energies of 3d3/2 and 3d5/2 orbitals for Sn4+ respectively. For the SnO2/rGO and SnO2/rGO-CTAB samples, a binding energy shift implies that the shielding effect on Sn 3d electrons is weakened due to the attraction of graphene to the electron cloud of SnO215. The micellar action of CTAB leads to an enhanced SnO2/rGO interface, thereby confirming the contribution of CTAB to the construction of Sn–O–C structure.
SEM images of the SnO2/rGO and SnO2/rGO-CTAB samples are displayed in Fig. 4. Figure 4a shows the agglomerated SnO2 particles those deposited on the surface of graphene sheets. In Fig. 4b, by contrast, monodispersed SnO2 particles are available due to the micellization of CTAB21. EDS mapping images (Fig. 4c–h) indicate the uniform elemental distribution of C, O, and Sn. It is reasonable to infer that the inserted SnO2 particles can suppress the stacking phenomenon of the graphene interlayers. On the other hand, the graphene can buffer the volume change of SnO2 during the cycling process.
TEM images in Fig. 5 offer further insight into the morphology and structure of the SnO2/rGO-CTAB sample. As shown in Fig. 5a, the SnO2 particles are composed of approximately 10 nm grains. Meanwhile, exposed graphene can be seen around the edges of the material. The (110), (101), and (211) crystal planes of SnO2, as well as the (002) crystal plane of graphene are calibrated in the pattern of selected area electron diffraction (SAED), which is consistent with the XRD results. From the HRTEM image in Fig. 5b, it can be seen that the sample has a lattice spacing of 0.335 nm, which corresponds to the (110) plane of the tin dioxide crystals, which is in agreement with the SAED results.
Cyclic voltammograms of SnO2, SnO2/rGO, and SnO2/rGO-CTAB materials during the first cycle in Fig. 6a reveal the lithiation and delithiation mechanisms. For the pristine SnO2, the reduction peak at 0.88 V is assigned to the transformation into Sn and Li2O. This peak shifts to 0.8 V for the SnO2/rGO and SnO2/rGO-CTAB samples because the forming process of solid electrolyte interphase (SEI) on the graphene is involved. Oxidation/reduction peaks appear at 0.55/0.3 V corresponding to the reversible conversion of LixSn (0≦x≦4.4) alloy. In addition, the reduction peak around 0.1 V is related to the further alloying reaction between Sn metal and Li+. Meanwhile, the more obvious reduction peaks of the SnO2/rGO and SnO2/rGO-CTAB materials at around 0.1 V are associated with the lithium intercalation into the graphene22. These peak potentials are in accordance with the charge and discharge plateaux of the first cycle in Fig. 6b. The ICEs of the SnO2, SnO2/rGO and SnO2/rGO-CTAB materials are determined to be 59.4%, 65.2% and 64.8% respectively. As well known, the ICE of graphene is much higher than that of SnO2. Consequently, the SnO2/rGO and SnO2/rGO-CTAB samples exhibit significantly higher ICEs compared to pure SnO2.
Electrochemical performance of SnO2, SnO2/rGO, and SnO2/rGO-CTAB materials (a) CV curves of 1st cycle at 0.1 mV s−1, (b) Charge–discharge curves of 1st cycle at 0.1 A g−1, (c) Cycle performance curves at 1 A g−1, (d) Rate performance curves at 0.1-4 A g−1, (e) Nyquist plots and equivalent circuit model before cycling, and (f) fitting lines for \(Z^/\) versus ω−1/2 before cycling.
Figure 6c presents the cycle performance of the anode materials at a current density of 1 A g−1. The specific capacities of SnO2 and SnO2/rGO samples gradually decrease to 437 mAh g−1 and 393 mAh g−1 after 100 cycles, and then dramatically declines with only about 50 mAh g−1 remaining after 200 cycles. The support of graphene does not effectively improve the structural stability of tin dioxide due to the insufficient adhesive force. In contrast, the interlayer embedded structure formed by the connection of CTAB can buffer the strain induced by volume change and inhibit the agglomeration of SnO2 particles23. As a result, SnO2/rGO-CTAB sample exhibits impressive cyclic stability with a reversible specific capacity of 598 mAh g−1 and a capacity retention rate of 63.8% after 200 cycles. Figure 6d shows the rate capabilities of the anode materials in a current density range of 0.1–4 A g−1. The capacities of the samples rapidly decrease in the early cycles due to the instable SEI, causing an inevitable consumption of active lithium. At low current densities, the capacity of SnO2/rGO sample is comparable to that of SnO2/rGO-CTAB sample. However, at a high current density of 4 A g−1, SnO2/rGO-CTAB sample demonstrates a higher capacity of 231 mAh g−1 at 4 A g−1 due to its continuous transport channels for ions and electrons, while maintains a reversible capacity of 581 mAh g−1 even when restoring the current density to 1 A g−1. As shown in Table 1, the initial discharge capacity, cycle and rate performance at high current density of the SnO2/rGO-CTAB composite are comparable to state-of-the-art SnO2/graphene anode materials. Moreover, the relatively low graphene content of approximately 5 wt.% is in favor of cost reduction which is significant for the commercial application of SnO2-based anode materials.
Figure 6e,f present the Nyquist diagrams of SnO2, SnO2/rGO, and SnO2/rGO-CTAB materials before cycling. The impedance spectra were fitted according to the equivalent circuit (inset in Fig. 6e), using the ZView software. Table 2 shows the corresponding Rs (the bulk series resistance of the electrolyte) and Rct (the charge transfer resistance between electrolyte and electrode materials) for each of the three samples9. The Rct of SnO2, SnO2/rGO, and SnO2/rGO-CTAB are 61.52 Ω, 37.39 Ω and 34.54 Ω, respectively. The lowest Rct of SnO2/rGO-CTAB composite benefits from the interlayer embedded structure which results in the abundant active sites and transport pathways for the lithium ions. In addition, the highly dispersed particles are easily infiltrated by the electrolyte and thereby enhancing the interfacial charge transfer. Figure 6f shows the fitted lines for \(Z^{/}\) versus ω−1/2 according to Eq. (1):
where \(Z^/\) is the real part of the complex impedance, σ is the Warburg constant, and ω is the angular frequency. The lithium-ion diffusion coefficient of the electrode can be calculated from the σ according to Eq. (2):
where R represents the gas constant, T is the absolute temperature, A is the surface area of the cathode, n is the number of electrons involved in the reaction, F is the Faraday constant, C the concentration of lithium-ion, and σ the Warburg factor associated with ZW (Warburg impedance of lithium-ion diffusion)22. The mean DLi+ values of SnO2, SnO2/rGO, and SnO2/rGO-CTAB are 8.4 × 10–17 cm2 s−1, 6.5 × 10–16 cm2 s−1 and 6.7 × 10–16 cm2 s−1, respectively. The results show that the interlayer embedded structure can shorten the diffusion distance of lithium ions and achieve rapid electron transfer through the Sn–O–C bonds on the surface of the material.
Conclusion
In this study, SnO2/rGO composite is synthesized through a CTAB-assisted hydrothermal-hydrogen reduction route. The micellar action of CTAB facilitates the dispersion of SnO2 particles even at a low graphene content of approximately 5 wt.%, and significantly strengthens the bonding between SnO2 particles and grapheme matrix. The as-formed interlayer embedded structure not only promotes the transfer of charge carriers, but also buffers the volume change of SnO2. Therefore, the SnO2/rGO composite exhibits superior cyclic stability that a reversible capacity of 598 mAh g−1 and a capacity retention of 63.8% are obtained at a current density of 1 A g−1 after 200 cycles, showing a promising prospect as the anode material for high performance lithium-ion batteries.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Zhu, C. et al. Confined SnO2 quantum-dot clusters in graphene sheets as high-performance anodes for lithium-ion batteries. Sci. Rep. 6, 25829 (2016).
Zhu, C. et al. Construction of SnO2-graphene composite with half-supported cluster structure as anode toward superior lithium storage properties. Sci. Rep. 7, 3276 (2017).
Duan, Y.-K. et al. Stannate-based materials as anodes in lithium-ion and sodium-ion batteries: A review. Molecules 28, 5037 (2023).
Hu, C. et al. Optimizing SnO2−x/Fe2O3 hetero-nanocrystals toward rapid and highly reversible lithium storage. Small 17, 2103532 (2021).
Hu, R. et al. Stabilizing the nanostructure of SnO2 anodes by transition metals: A route to achieve high initial coulombic efficiency and stable capacities for lithium storage. Adv. Mater. 29, 1605006 (2017).
Xin, F. & Whittingham, M. S. Challenges and development of tin-based anode with high volumetric capacity for Li-ion batteries. Electrochem. Energy Rev. 3, 643–655 (2020).
Zoller, F., Böhm, D., Bein, T. & Fattakhova-Rohlfing, D. Tin oxide based nanomaterials and their application as anodes in lithium-ion batteries and beyond. ChemSusChem 12, 4140–4159 (2019).
Chang, C. C. et al. Nano-sized tin oxide-modified graphite composite as efficient anode material for lithium ion batteries. Int. J. Electrochem. Sci. 13, 11762–11776 (2018).
Gao, S. et al. A multi-wall Sn/SnO2@carbon hollow nanofiber anode material for high-rate and long-life lithium-ion batteries. Angew. Chem. Int. Ed. 59, 2465–2472 (2020).
Seong, C.-Y., Jin, X., Kim, D. K., Hwang, T. & Piao, Y. Hydrothermal synthesis of uniform tin oxide nanoparticles on reduced activated graphene oxide as anode material for lithium-ion batteries. J. Electroanal. Chem. 845, 6–12 (2019).
Zhao, X. et al. Monodisperse tin nanoparticles and hollow tin oxide nanospheres as anode materials for high performance lithium ion batteries. Inorg. Chem. Front. 6, 473–476 (2019).
Cheng, Y. et al. Large-scale fabrication of core–shell structured C/SnO2 hollow spheres as anode materials with improved lithium storage performance. Small 13, 1701993 (2017).
Liu, X. et al. Enhancing the lithium storage performance of graphene/SnO2 nanorods by a carbon-riveting strategy. ChemSusChem 11, 1321–1327 (2018).
Lan, X. et al. Insight into reversible conversion reactions in SnO2-based anodes for lithium storage: A review. Small 18, 2201110 (2022).
Tu, M. et al. Chitosan modulated engineer tin dioxide nanoparticles well dispersed by reduced graphene oxide for high and stable lithium-ion storage. J. Colloid Interface Sci. 635, 105–116 (2023).
Liu, Y. et al. A novel carbon microspheres@SnO2/reduced graphene composite as anode for lithium-ion batteries with superior cyclic stability. Ceram. Int. 48, 18625–18634 (2022).
Wang, M. et al. Tin dioxide-based nanomaterials as anodes for lithium-ion batteries. RSC Adv. 11, 1200–1221 (2021).
Gervillié, C., Boisard, A., Labbé, J., Berthon-Fabry, S. & Guérin, K. Influence upon cycling of oxygen amount in tin-based compound used as negative electrode in lithium-ion battery. Synth. Metals 267, 116477 (2020).
Li, B. et al. Engineering tin dioxide quantum dots in a hierarchical graphite and graphene oxide framework for lithium-ion storage. J. Colloid Interface Sci. 600, 649–659 (2021).
Yang, D. et al. Bi-functional nitrogen-doped carbon protective layer on three-dimensional rGO/SnO2 composites with enhanced electron transport and structural stability for high-performance lithium-ion batteries. J. Colloid Interface Sci. 542, 81–90 (2019).
Yang, S., Yue, W., Zhu, J., Ren, Y. & Yang, X. Graphene-based mesoporous SnO2 with enhanced electrochemical performance for lithium-ion batteries. Adv. Funct. Mater. 23, 3570–3576 (2013).
Zhang, Y. et al. A SnOx quantum dots embedded carbon nanocage network with ultrahigh Li storage capacity. ACS Nano 15, 7021–7031 (2021).
Wang, X. et al. Heterogeneous Li-alloy interphase enabling Li compensation during cycling for high energy density batteries. Energy Stor. Mater. 54, 615–622 (2023).
Zhang, Q. et al. Porous a-SnO2/rGO nanocomposite via annealing treatment with stable high-capacity as anode of lithium-ion battery. ChemistrySelect 3, 4303–4309 (2018).
Liu, D. et al. Spray-drying-induced assembly of skeleton-structured SnO2/Graphene composite spheres as superior anode materials for high-performance lithium-ion batteries. ACS Appl. Mater. Interfaces 10, 2515–2525 (2018).
Wu, Y. Z., Brahma, S., Weng, S. C., Chang, C. C. & Huang, J. L. Reduced graphene oxide (RGO)-SnOx (x=0,1,2) nanocomposite as high performance anode material for lithium-ion batteries. J. Alloys Compd. 818, 152889 (2020).
Liang, B. et al. Hybrid of Co-doped SnO2 and graphene sheets as anode material with enhanced lithium storage properties. Appl. Surf. Sci. 533, 147447 (2020).
Xu, Z. et al. Facile fabrication of mesoporous SnO2@N-rGO nanocomposites by electrostatic self-assembly as anode for high performance lithium-ion batteries. Int. J. Electrochem. Sci. 19, 100610 (2024).
Funding
This work was supported by National Natural Science Foundation of China (No. 52172063), Natural Science Foundation of Hunan Province (No. 2023JJ30017, 2023JJ30030), and Natural Science Foundation of Changsha (No. kq2208223).
Author information
Authors and Affiliations
Contributions
Xiaolu Li: Writing-original draft, Methodology, Investigation, Data collection and analysis. Zhongtao Zhao: Methodology, Data collection. Yufeng Deng: Data analysis. Dongsheng Ouyang: Investigation. Xianfeng Yang: Supervision, Funding acquisition. Shuguang Chen: Methodology, Funding acquisition. Peng Liu: Supervision, Writing-review & editing, Conceptualization, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, X., Zhao, Z., Deng, Y. et al. Interfacial engineering in SnO2-embedded graphene anode materials for high performance lithium-ion batteries. Sci Rep 14, 16751 (2024). https://doi.org/10.1038/s41598-024-67647-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67647-w
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.