Abstract
Diabetes mellitus (DM) is a rapidly prevailing disease throughout the world that poses boundless risk factors linked to several health problems. Vildagliptin is the standard dipeptidyl peptidase-4 (DPP-4) inhibitor type of medication that is used for the treatment of diabetes anti-hyperglycemic agent (anti-diabetic drug). The current study aimed to synthesize vildagliptin-loaded ZnO NPs for enhanced efficacy in terms of increased retention time minimizing side effects and increased hypoglycemic effects. Herein, Zinc Oxide (ZnO) nanoparticles (NPs) were constructed by precipitation method then the drug vildagliptin was loaded and drug loading efficiency was estimated by the HPLC method. X-ray diffraction analysis (XRD), UV–vis spectroscopy, FT-IR, scanning electron microscope (SEM), and EDX analysis were performed for the characterization of synthesized vildagliptin-loaded ZnO NPs. The UV–visible spectrum shows a distinct peak at 363 nm which confirms the creation of ZnO NPs and SEM showed mono-dispersed sphere-shaped NPs. EDX analysis shows the presence of desired elements along with the elemental composition. The physio-sorption studies, which used adsorption isotherms to assess adsorption capabilities, found that the Freundlich isotherm model explains the data very well and fits best. The maximum adsorption efficiency of 58.83% was obtained. Further, In vitro, anti-diabetic activity was evaluated by determining the α-amylase and DPP IV inhibition activity of the product formed. The formulation gave maximum inhibition of 82.06% and 94.73% of α-amylase and DPP IV respectively. While at 1000 µg/ml concentration with IC50 values of 24.11 μg/per ml and 42.94 μg/ml. The inhibition of α-amylase can be ascribed to the interactive effect of ZnO NPs and vildagliptin.
Similar content being viewed by others
Introduction
Hyperglycemia, a metabolic disease, is the cause of diabetes mellitus (DM)1. In adults, 8.8% of people are diagnosed with it yearly2. Hyperglycemia results due to inadequate insulin production, poor insulin action, or the combined action of all the two factors. Due to these abnormalities in metabolism, glucose is discharged into the urine3. There are three major types of diabetes mellitus i-e Type 1 Diabetes (T1DM)4,5, Type 2 Diabetes (T2DM)6,7, and Gestational Diabetes8. The purpose of managing diabetes is to regulate blood glucose levels as close to normal as feasible9. Nanomaterials have demonstrated a diverse chemical makeup that includes metal, metal oxide, polymers, silicates, and carbon. These nanomaterials also come in a variety of morphologies, including spheres, sheets, cylinders, or tubes. Size-dependent features include band gap, melting point, mechanical strength, electrical conductivity, optical qualities, magnetic, and catalytic properties10. There are three different ways in which these materials can be manufactured including physical11, chemical12 as well as biological13. NPs may arise from the reduction of bulk materials to nanoscale scale. Effective absorption is made possible by a decrease in particle size, which increases the surface-volume ratio. Because more active sites on the surface are made available for molecule-to-molecule interaction due to size reduction, the adsorption effectiveness of the NPs is higher than that of their bulk counterparts14.
Nowadays metal oxide NPs (MO NPs) are quite important due to their various applications in the field of nano-sensors, catalysis, drug delivery, nanomedicines, wastewater treatment, and electronics15,16. Metal oxide nanoparticles have demonstrated potential as anti-oxidative, anti-inflammatory, anti-diabetic, and anti-cancer medicines in the field of biomedicine. These nanoparticles' adaptability in the medical arena has also been demonstrated by their use in drug delivery and bio-imaging applications. Because of their special qualities, biocompatibility, and possible therapeutic uses, metal oxide nanoparticles have drawn the attention of scientists looking into cutting-edge methods in nanomedicine and healthcare. The narrow particle size of MO NPs is primarily necessary for successful applications. The generation of the precursor, nucleation, aging, and growth are the four critical processes in the regulated synthesis of MO NPs17,18. Numerous studies have shown the importance of metals and metal oxides in the metabolism of glucose and their link to insufficient amounts of diabetes. Metal oxide nanoparticles present a multitude of opportunities for diverse applications, ranging from industrial operations to biomedical breakthroughs, underscoring their significance in contemporary scientific investigations and technological innovations. Vanadium19, chromium20, magnesium21, silver22, and zinc23 are all mentioned in the literature as being related to diabetes treatment and blood sugar regulation.
ZnO NPs are well-recognized in countless fields for their astonishing properties and applications24. These metal oxide nano-particles showed important physical and chemical properties25. Greater antimicrobial, antibacterial26, and UV-blocking27 characteristics have been shown by ZnO NPs. Sol–gel30, chemical precipitation29, solid-state pyrolysis31, solution-free mechano-chemical processes28, and biosynthesis32 are among the techniques used to create ZnO NPs. ZnO NPs play a positive role in diabetes control and their low concentration is enough to enhance insulin secretion33. They regulate glucose metabolism, insulin storage, and glucose homeostasis34. These NPs also improve the disorders linked to diabetes such as cardiomyopathy and nephropathy35.
Vildagliptin is a drug which is used to treat type II diabetes. This is used with exercise and proper diet to obtain better results. It works by causing the pancreas to release more insulin and decrease the hormones that cause the sugar level of blood to rise36,37. Vildagliptin binds with the DPP-4 enzyme present in the human body and inhibits it. Vildagliptin binds covalently with DPP-4 inhibitor and by doing so they prevent the inactivation of GLP-1 and therefore there is increased plasma concentration of GLP-1. Hence in this way, it increases the levels of GLP-1 bother after intake of meals and fasting. At hypoglycemic levels, the counter-regulatory response of glucagon is increased relative to vildagliptin. The protruding limitation of the drug is having a short half-life which is 2.5 to 3 h. Therefore, to get the desired results high frequency of the drug is needed38.
This research aims to synthesize ZnO NPs loaded with Vildagliptin to test their enhanced anti-diabetic activity39. The present study is linked to the UN SDG 3 (Good Health and Well-Being). Zinc sulfate heptahydrate (ZnSO4.7H2O) is used as a precursor material because it may be used with a wide range of precipitation reagents, allowing for a flexible selection of precipitating agents based on the desired properties of the final product. When the novel drug is tailored with the nanomaterials the area of attachment of the drug is increased dramatically. The drug is impregnated to the cellular level due to its nano size. As well as the ZnO NPs also give the maximum protein attachment and synergistic effect against diabetes. By viewing all these aspects this study was aimed to achieve the maximum anti-diabetic activity. The key objective throughout this research work will be the fabrication of ZnO NPs. Adsorption of Vildagliptin on ZnO NPs, characterization of fabricated NPs loaded with vildagliptin by using different techniques, determination of the efficiency of Vildagliptin loaded ZnO NPs against the anti-diabetic activity.
Experimental work
Chemical and reagents
Since the compounds used in this investigation were analytical grade, no purification was required. ZnSO4.7H2O was used as a ZnO precursor and NaHCO3 used for precipitation was acquired from Merck. Methanol was obtained from Honeywell. Vildagliptin was the API drug purchased from Pharmagen Limited, Pakistan.
Fabrication of ZnO NPs
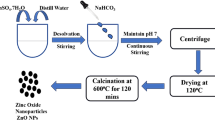
ZnO nanomaterials were fabricated by the chemical precipitation technique reported earlier40. 0.86 g of ZnSO4.7H2O was completely dissolved in 30 ml of distilled H2O with continuous stirring. 1 M NaHCO3 solution was inserted dropwise until pH 7 was obtained and precipitates were formed. After one hour of agitation, centrifugation, and a water wash, the product was dried for twelve hours at 120 °C in an oven. After drying, the substance was ground into a powder. Following that, the material was calcined for two hours at 600 °C and then stored for later use. The drying temperature and duration can have a significant impact on the final properties of ZnO nanoparticles. The drying temperature can affect the nanoparticles' crystallinity and phase composition. By pushing solvent molecules to evaporate more quickly, higher drying temperatures may hasten the nucleation and growth of ZnO nanoparticles. The phase purity and crystallinity of the nanoparticles may be affected by this. Phase shifts or crystal defects can also be the result of prolonged drying durations at high temperatures. Figure 1 represents the fabrication method of ZnO NPs. The various parameters that were noticed during the preparations of NPs are listed in Table 1.
Adsorption of vildagliptin on ZnO NPs
Stock solution 1 mg/ml was prepared in methanol by suspending 100 mg of the drug vildagliptin in 100 ml of methanol. Then sequential dilutions of several contents (0.1, 0.2, 0.3, 0.4, and 0.5 mg/ml) were constructed. For this 25, 20, 15, 10, and 5 ml of stock solutions were taken, and methanol was added to them until the final volume of 40 ml was obtained followed by the addition of 100 mg ZnO NPs. The solutions of different concentrations were continuously stirred at 500 rpm for 5 h by magnetic stirring at room temperature. All solutions were filtered using a vacuum pump with nylon filter paper of 0.45-micron meter. Figure 2 illustrates the adsorption method of the drug on prepared nanomaterials. The previously reported HPLC method36 was used to determine the percentage of unbound drugs. The drug removal percentage shows the amount of drug loaded and is denoted by P (%). Using the above formulas, the adsorption capacity at equilibrium, represented as qe (mg/g), is measured and calculated.
ZnO NPs: characterization
For the complete classification of compounds various characterization techniques were used like the Ultraviolet–visible Spectroscopy technique utilizing Shimadzu UV-1800 Japan. To identify the Crystallinity, Crystallite size, and orientation of the NPs X-ray diffraction spectroscopy was carried out with Maker Malvern Panalytical which operates at room temperature. Cu Kα radiation (1.54 Å) source was used to attain the XRD spectra in the range of 10°-80°41. Surface morphology and elemental composition were elucidated by SEM and EDX techniques. Both characterizations were assessed by operating Nova Nano SEM-450 “Field-Emission-Scanning-Electron-Microscope (FE-SEM)” with a TLD detector at 1 kV voltage. FTIR spectroscopy was carried out for the structural analysis of prepared NPs. It works by focusing on the various types of bonds and functional groups and is carried out by placing the KBr disk of the sample in front of the IR radiation source42. Using an IR-Prestige 21 Shimadzu spectrophotometer, the NPs' IR spectra in the 402–3998 cm–1 range were performed43.
Effect of various parameters on the features of ZnO NPs
The various parameters that greatly influenced the properties of ZnO nanoparticles include temperature, drying duration, pH control, and drying conditions. The phase composition and the crystallinity of the fabricated nanomaterials are greatly affected by drying temperature. Greater drying temperatures may accelerate the nucleation and growth of ZnO nanoparticles (ZnO NPs). Prolonged drying durations at high temperatures may result in crystal defects. Faster solvent evaporation and nucleation kinetics at higher temperatures may lead to a smaller particle size. Conversely, slower drying at lower temperatures could lead to larger particle sizes. Through the proper handling of these parameters, the desired results in a range of uses can be obtained, including improved crystallinity, controlled particle size and distribution, optimal shape, and enhanced surface area. The pH was adjusted to 7 during the precipitation step in the synthesis of ZnO nanoparticles using NaHCO3 solution helps to control the reaction kinetics, prevent unwanted side reactions, control particle size and morphology, and ensure the stability of the nanoparticles, and leads to the desired properties.
Anti-diabetic assay
The percentage inhibition activity of two enzymes named α-amylase and DPP-IV was estimated to check the potential of Vildagliptin-loaded ZnO NPs for the inhibition of diabetic diseases by using an already reported method44.
Role of α-amylase
The calcium metalloenzymes known as alpha-amylases are inactive in the absence of calcium. Humans contain a variety of digestive enzymes, the most significant of which is pancreatic alpha-amylase. It catalyzes a reaction that breaks down the alpha-1,4 glycosidic bonds that hold starch, amylopectin, amylose, glycogen, and several maltodextrins together and is thus responsible for the digestion of starch. Alpha-glucosidase, also known as maltase, is another significant enzyme that acts on 1,4-alpha bonds to catalyze the last stage of the digestion of carbohydrates, primarily starch, and produces glucose as a result. Alpha-amylase cleaves large starch molecules to get over the blood–brain barrier, which prevents large molecules like starch from doing so since glucose needs to get to the brain. Excess starch conversion to sugars raises blood sugar levels. Insulin plays a role in this situation by instructing cells to digest the extra sugar molecules and store them as glycogen, which is used as an energy source. When someone is healthy, this cycle never ends. However, there are instances where high blood glucose levels occur as a result of amylase enzyme overactivity, insulin insufficiency, or insulin resistance, which can lead to hyperglycemia45.
α-Amylase inhibition activity
Serial dilutions (750, 500, 250 100, and 50 µg/ml) of Vildagliptin-loaded ZnO NPs were prepared. Buffer Solution of pH 7 was prepared and 0.004 g of α- Amylase was dispersed in 100 ml buffer solution to make 2 units of solution37. Starch and Dinitro salicylic acid solution was prepared. The sample vials were taken and labeled for different concentrations i.e. Blank, 50,100, 250, 500, and 700 μg/ml. In each vial, 50 μL of solution was taken from respective dilution by using a micro-pipette. 50 μL of alpha-amylase solution was added in each vial. Further total volume of solution in each vial was made to 200 μL by adding about 100 μL of buffer solution (pH 6.9). All these vials were stored in an incubator for 15 min at 37 °C. 50 μL of starch solution was added in each vial containing sample solutions and deposited in an incubator for 15 min at 37 °C. 500 μL of 3,5-Dinitrosalicylic acid (DNS) solution was inserted in all dilutions followed by drying in an oven at 60 °C for 20 min and then allowing it to cool at room temperature (Fig. 3). Nothing was added to the control while it was being prepared. The sample solutions were diluted fivefold by adding about 4 ml DMSO. For all dilutions, the UV absorption spectrum was recorded at 540 nm. The following formula was used to calculate the percentage of inhibition of α-amylase.
Inhibition of DPP IV
0.0052 g of Gly-Pro-p-Nitroanilide was inserted in 10 ml of cooled distilled H2O to create a 1.6 mM solution. Five or six drops of acetic acid were added to maintain the pH at four after 8.2 g of sodium acetate was dispersed in 100 ml of H2O. The sample vials were taken, and labeled, and the solutions in each respective vial were for different concentrations i.e., 1000, 750, 500, 250, 100, and 50 μg/ml. In each vial, 25 μL of samples were taken and then, 50 μL of Gly-Pro-p-Nitroanilide (1.6 mM) solution was added. 1 mM buffer solution was added to the above solution to raise the volume to 200 μL. All the solutions were kept in an incubator for about 15 min at a temperature of 37 °C. 50 µL of DPP-IV solution was inserted again and the suspension was then again allowed to incubate at 38 °C for 90 min. Next, a 100 μL solution of sodium acetate was added to halt the process. Figure 4 shows the schematic diagram of DPP-IV Inhibition activity. The control was prepared without adding test samples. UV absorption was recorded for each sample at 405 nm. Using the following formula, the % inhibition of DPP-IV was estimated:
IC 50 calculation
The 50% threshold at which inhibition is half-maximized an inhibitory activity's half-maximum concentration is known as its IC50 value. It is represented visually by putting concentration on the x-axis and the % of inhibition along the y-axis. A slope equation can be utilized to calculate the IC50 value that emerged when the Trend line was chosen as an additive. The concentration that stopped protein denaturation was identified after IC50 values for the Sample and Standard were obtained.
where Y = Intercept; C = The point at which the line y = mx + c intersects the y-axis; m = The gradient of the line; x = An independent variable.
Results and discussion
Vildagliptin-loaded ZnO NPs: an overview
UV visible spectroscopy analysis
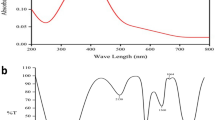
UV–visible spectroscopy is a technique that is commonly used to investigate the optical properties of zinc oxide (ZnO) nanoparticles (NPs). ZnO nanoparticles typically show a notable absorption peak in the UV region, at 350–380 nm, because they go through electrical transitions from the VB to the CB. UV–visible spectroscopy sheds light on ZnO nanoparticles' optical characteristics and band gap energy. By measuring the samples' absorption spectra in the visible and ultraviolet portions of the electromagnetic spectrum, this technique made it possible to determine the chemical makeup and electronic transitions of the produced nanoparticles46,47. ZnO NPs were scanned over the wavelength range of 300–400 nm. The maximum absorbance (λmax) was observed at 367 nm which is also shown in Fig. 5. This confirms the presence of ZnO NPs because this peak is due to the specific transition of an electron from the VB (O2p) to the CB (Zn3d) which is also reported in48.
FT-IR analysis
The 4000–400 cm-1 range was used to conduct the FT-IR measurements of vildagliptin-loaded ZnO nanoparticles, pure drug, and ZnO nanomaterials (Fig. 6). In the spectrum of ZnO NPs, the stretching at 435–450 cm-1 confirms the formation of ZnO NPs49. The stretching at 575 cm-1 implies the bond of metal (Zn)-oxygen50. The O–H extending because of adsorbed H2O molecules at the surface of ZnO NPs shows stretching in the range of 3200–3300 cm-151. In the spectra of Vildagliptin, the stretching at 3294 cm-1 refers to the overlapping of the NH and OH signals52. The two (N–O) bonds closest to the metal ion typically showed a symmetrical stretching frequency between 1006 and 1046 cm−1. The measured signal at 1046 cm−1 therefore indicated that the NO3- group was situated within the coordination sphere. The development of a low-rate distinct peak at 510 cm−1, which was absent from the spectra of the free medicine, indicated the coordination of the O atom of the ketone group and metal band, confirming the complex formation. The stretching vibration of metal–oxygen υ (M–O) was the source of this. The spectra of the free drug showed no υ (M–N) stretching vibrations, which validated the formation of a new peak at 429 cm−1, suggesting further coordination interaction between the metal and N atom of the secondary amino group53. In vildagliptin-loaded ZnO NPs, a smaller number of vildagliptin spectra were observed which explains that drug molecules were present inside the pores of ZnO NPs.
XRD analysis
XRD analysis was explored to identify the crystallized aspect of the vildagliptin ZnO NPs. The product’s strong and pointed diffraction peaks are seen in Fig. 7. This means the product is crystallized to a greater extent. Moreover, the average crystallite size was measured by using the Debye–Scherer formula which is
here D shows average crystallite size; K = Scherer’s constant of value 0.94; λ = wavelength of X-ray source which is 0.15406 nm; β refers to FWHM; θ is the diffraction angle.
SEM studies
The SEM analysis was enacted to conclude the size, shape, and morphology of the surface. The analysis of Vildagliptin-loaded ZnO NPs was conducted at a high voltage of 10 kV with a TLD detector as shown in Fig. 8. The analysis showed that ZnO NPs exhibit spherical morphology while ZnO NPs loaded with vildagliptin had a flaky texture. The maximum pores of the NPs were filled by the vildagliptin.
EDX analysis
To ascertain the sample's elemental makeup, EDX analysis was carried out. Vildagliptin-loaded ZnO NPs may usually be subjected to an EDX examination to verify the synthesis process and product purity. This confirms the presence and stoichiometric ratio of zinc, Nitrogen, and oxygen. The examination is simple and gives important information about the elemental makeup of the synthesized nanomaterial as well as any possible contaminants. The EDX spectra of vildagliptin ZnO NPs are shown in Fig. 9. Table 2 shows the elemental description of the sample which clearly shows that the product was made up of C, O, N, and Zn mainly.
Drug adsorption efficiency
Vildagliptin was subsequently loaded onto ZnO NPs using the batch adsorption technique, after their synthesis by precipitation. In this method, five concentrations of the drug (100, 300, 400, 200, and 500 mg/L) were constructed and the drug loading efficiency was determined by the HPLC method.
The results obtained by this method are summarized in Table 3. The maximum adsorption of the drug was seen in 100 mg/L which was 58.83% which dropped to 54.13% as concentration went to 200 mg/L. The minimum adsorption was 44.27% in 500 mg/L concentration which is also explained in Fig. 10 where a graph is plotted between the percentage of drug adsorbed and the concentration of the samples.
Adsorption isotherm studies
Two alternative isotherm models were compared to which model was optimal for design purposes. Table 4 indicates that the isotherm computations were finalized. The R2 values in Table 4 and Fig. 11 indicate that the isotherm that most closely matches the data is the Freundlich isotherm. The adsorbent may absorb many layers and have a heterogeneous surface, according to the Freundlich model. Certain important characteristics of the adsorption study may also be investigated based on the information provided by the different isothermal models that were used. These included the vildagliptin adsorption isotherm on the ZnO NP surface, which is shown by the following: Freundlich > Langmuir, with an R2 value of 0.904. By the Freundlich model, the adsorbent is heterogeneous on the surface and has the potential to adsorb in layers. And correlation coefficient R2 value must be less than 1 confirming the accuracy of the experimental data as the trendline is straightly following the trend and the adsorption process was in the same right direction there is no deviation in the trend nevertheless proceeding at different serial concentrations. As it is less than 1 also confirms that it was physio-sorption of the drug.
The preferred and required adsorption type was identified by the Freundlich model, as indicated by the n parameter, which was determined to be (3.84) between 1 and 10. The value of 1/n, at 0.541, was also found to be smaller than the unit, suggesting that heterogeneity occurs on the surface of ZnO NP during the adsorption procedure. The results indicate that, according to the Langmuir model, the experimental qe (200.0 mg/g) is higher than qL (160.4 mg/g). Further support for the positive adsorption process came from the RL, which was found to be 0.196 and was greater than 0 but less than unity. The observed value was 77.2; a value of less than 80 is preferred, and the obtained value was within the acceptable range. Its value of less than 80 suggests that the physisorption mode of adsorption was the most plausible theory.
Anti-diabetic assay
α-Amylase inhibition activity
In the present work, alpha-amylase and DPP-IV inhibition activity of Vildagliptin loaded ZnO NPs at various concentrations is studied and summarized in Table 5 which shows that maximum inhibition 82.06% was obtained at 1000 µg/ml concentration. A graph was plotted shown in Fig. 12 between percentage inhibition and concentration of samples which shows that minimum inhibition 49.74% was at 50 µg/ml. Therefore, it was discovered that the IC50 value for α-amylase activity was 5 µg/ml54. The IC50 of Vildagliptin ZnO nanoparticle concentrations for inhibition of α-amylase was about 24.11 µg/ml.
There is a reason why ZnO NPs are present and why alpha-amylase is suppressed. As has previously been shown, ZnO NPs themselves have biological roles connected to them55. They also exhibit anti-diabetic functions by inhibiting α-amylase. So maximum inhibition was observed by the synergistic effect of NPs and drugs.
Mechanism of action of DPP IV
Polypeptides with praline, alanine, or hydroxyproline residues at the penultimate N-terminal position, like GLP-1, are broken down by DPP IV. The incretin hormones are mostly hydrolyzed at the brush-border membranes of the kidney. The half-life of bioactive peptides, such as GLP-1, is extended by DPP IV inhibitors because they prevent their degradation. Vildagliptin is known to up to 90% suppress DPP IV action, increase endogenous GLP-1 plasma concentrations, prolong the half-life of exogenously administered GLP-1, and lessen postprandial glucose excursions in animal experiments56. MicroRNA-103 and microRNA-143 were significantly downregulated in the group of people without diabetes, indicating potential antidiabetic effects of ZnO NPs and the DPP-IV inhibitor vildagliptin, either alone or in combination. Numerous diabetes dysfunction indicators are improved by ZnO NPs, and insulin, including weight reduction, pancreatic SOD efficiency, fructosamine levels, and pancreatic histology. DPP-IV, however, even further improved these indices. Whereas ZnO NPs by themselves had strong antidiabetic effects, vildagliptin combined with ZnO NPs had a synergistic impact on the treatment of diabetes57. ZnO NPs and DPP-IV inhibitor (vildagliptin) significantly decreased the expression of microRNA-103 and microRNA-143 in comparison to the diabetic group, either alone or in combination, indicating potential antidiabetic effects. ZnO NPs were shown to improve several diabetes dysfunction markers, including glucose tolerance, pancreatic SOD activity, weight loss, fructosamine levels, insulin levels, and pancreas histology. Additionally, the addition of DPP-IV significantly improved these indices. ZnO NPs by themselves showed significant antidiabetic advantages; however, Vildagliptin added to the mix had a synergistic effect when administered as part of the diabetic treatment regimen.
Inhibition of DPP IV
Further, the ability of vildagliptin-loaded ZnO NPs to inhibit DPP-IV was determined at different concentrations which are 1000, 750, 500, 250, 100, 50 µg/ml. The percentage inhibition activity data is represented in Table 6.
Figure 13 represents the graph plotted between percentage inhibition activities against different concentrations of the sample which represents that Vildagliptin-loaded ZnO NPs exhibited better results in the inhibition of DPP-IV. About 94.73% inhibition of DPP-IV was done by Vildagliptin loaded ZnO NPs at 1000 µg/ml concentration with the IC50 of Vildagliptin loaded ZnO nanoparticle concentrations for DPP-IV inhibition was about 42.94 µg/ml. A minimum inhibition of 52.60% was recorded at a sample content of 50 µg/ml. The inhibitory capability of the enzyme decreased with the lowering of the content of the sample. The rapid release of drugs from nanomaterials can be the reason for the increase in the activity of formulated NPs to inhibit enzyme activity58. Quick release allows the drug to be absorbed into the bloodstream more rapidly, resulting in higher concentrations and faster onset of action. It can lead to higher bioavailability, meaning more of the drug is available for the body to use, enhancing its effectiveness. It can improve solubility, making the drug more easily dissolvable and absorbable, leading to increased activity. Rapid release can facilitate targeted delivery to specific sites or tissues, increasing local concentrations and activity. The faster release can promote quicker diffusion into tissues or cells, enhancing drug activity by allowing it to reach its site of action more rapidly. Due to these reasons as discussed above the rapid release of the drug from the formulated Nanoparticles increases the enzyme’s inhibition activity to control hyperglycemia. Table 7 depicts a comparative study of drug adsorption effectiveness.
Conclusion
In this study, ZnO NPs were constructed by precipitation technique and then an anti-diabetic drug Vildagliptin was loaded on them to enhance the efficacy of the drug. The fabrication of ZnO nanomaterials was established by UV Visible study as λmax of 363 nm was obtained. Vildagliptin was loaded on these ZnO NPs by batch adsorption method and maximum adsorption was observed at 100 mg/ml as determined by HPLC method. The results of SEM explained that drug-loaded nanomaterials exhibit a flaky texture. The Frendulich isotherm was found to be more appropriate for adsorption. In vitro, anti-diabetic analysis was performed, and the percentage of α-amylase inhibition was determined at different contents (50, 500, 100, 250, 750, and 1000 µg/ml). Vildagliptin is an inhibitor of DPP IV but in this work, the drug-loaded ZnO nanomaterials exhibited the inhibition of alpha-amylase too because of the synergistic effect of both ZnO NPs and Vildagliptin. According to the best of our knowledge, the synthesized material has not been previously reported for anti-diabetic activity. The novelty of the work lies in the fabrication of zinc oxide nanomaterials tailored with active drugs to enhance the efficacy of the drugs, particularly in anti-diabetic applications. This represents the increase in receptor sites for the formulation because ZnO NPs themselves have α-amylase inhibition potential and in the end the increase in efficacy of the formulation. The purpose of the study was to investigate how well these drug loaded nanoparticles treat diabetes. The maximum inhibition of 82.06% was seen at 1000 µg/ml. The DPP-IV inhibitory activity of vildagliptin-loaded ZnO NPs at various contents (1000, 500, 750, 100, 250, and 50 µg/ml) was determined which gave a maximum inhibition of 94.73% at 1000 µg/ml. The quick drug release during the incubation period can be the reason for this inhibitory function.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Li, J.-M. et al. Lipotoxicity-polarised macrophage-derived exosomes regulate mitochondrial fitness through Miro1-mediated mitophagy inhibition and contribute to type 2 diabetes development in mice. Diabetologia 66, 2368–2386 (2023).
Yang, Y.-Y. et al. Piperazine ferulate prevents high-glucose-induced filtration barrier injury of glomerular endothelial cells. Exp. Ther. Med. 22, 1–10 (2021).
Xu, Z. et al. Comparing SARC-CalF with SARC-F for screening sarcopenia in adults with type 2 diabetes mellitus. Front. Nutr. 9, 803924 (2022).
Tian, Z. et al. Gut microbiome dysbiosis contributes to abdominal aortic aneurysm by promoting neutrophil extracellular trap formation. Cell Host Microbe 30, 1450–1463 (2022).
Eisenbarth, G. S. Type I diabetes mellitus. N. Engl. J. Med. 314(21), 1360–1368 (1986).
Shen, W. et al. A polymeric hydrogel to eliminate programmed death-ligand 1 for enhanced tumor radio-immunotherapy. ACS Nano 17, 23998–24011 (2023).
Xiao, D. et al. The impacts of SLC22A1 rs594709 and SLC47A1 rs2289669 polymorphisms on metformin therapeutic efficacy in Chinese type 2 diabetes patients. Int. J. Endocrinol. 2016, 4350712 (2016).
Yadav, A., Pandey, S., Singh, A. & Gupta, A. A comprehensive review on diabetes mellitus: An overview. World J. Pharm. Res. 10(6), 1584–1596 (2021).
Zhou, Y., Chai, X., Yang, G., Sun, X. & Xing, Z. Changes in body mass index and waist circumference and heart failure in type 2 diabetes mellitus. Front. Endocrinol. 14, 1305839 (2023).
Imran Din, M. & Rani, A. Recent advances in the synthesis and stabilization of nickel and nickel oxide NPs: A green adeptness. Int. J. Anal. Chem. 2016, 3512145 (2016).
Hou, Y. et al. Pathogenesis and comprehensive treatment strategies of sarcopenia in elderly patients with type 2 diabetes mellitus. Front. Endocrinol. 14, 1263650 (2024).
Cushing, B. L., Kolesnichenko, V. L. & O’connor, C. J. Recent advances in the liquid-phase syntheses of inorganic NPs. Chem. Rev. 104(9), 3893–3946 (2004).
Zhao, X., Zhang, Y., Yang, Y. & Pan, J. Diabetes-related avoidable hospitalisations and its relationship with primary healthcare resourcing in China: A cross-sectional study from Sichuan Province. Health Soc. Care Community 30, e1143–e1156 (2022).
Afzal, S. et al. A facile approach for the bio-fabrication of monometallic manganese oxide nano-catalyst for enhanced photocatalytic degradation of dyes and drug. Inorg. Chem. Commun. 158, 111715 (2023).
Shahid, S. et al. The anti-inflammatory and free radical scavenging activities of bio-inspired nano magnesium oxide. Front. Mater. 9, 875163 (2022).
Mansoor, S. et al. Green synthesis of a MnO-GO-Ag nanocomposite using leaf extract of Fagonia arabica and its antioxidant and anti-inflammatory performance. Nano-Struct. Nano-Objects 29, 100835 (2022).
Liu, W. et al. Coupling protein scaffold and biosilicification: A sustainable and recyclable approach for d-mannitol production via one-step purification and immobilization of multienzymes. Int. J. Biol. Macromol. 269, 132196 (2024).
Bahadur, A. et al. Biocompatible waterborne polyurethane-urea elastomer as intelligent anticancer drug release matrix: A sustained drug release study. React. Funct. Polym. 119, 57–63 (2017).
Shoaib, M. et al. Sustained drug delivery of doxorubicin as a function of pH, releasing media, and NCO contents in polyurethane urea elastomers. J. Drug Deliv. Sci. Technol. 39, 277–282 (2017).
Guo, W. et al. Gut microbiota induces DNA methylation via SCFAs predisposing obesity-prone individuals to diabetes. Pharmacol. Res. 182, 106355 (2022).
Bahadur, A. et al. Regulating the anticancer drug release rate by controlling the composition of waterborne polyurethane. React. Funct. Polym. 131, 134–141 (2018).
Yao, H. et al. Comparative effectiveness of GLP-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes: Systematic review and network meta-analysis. bmj 384, e076410 (2024).
Alkaladi, A., Abdelazim, A. M. & Afifi, M. Antidiabetic activity of ZnO and silver NPs on streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 15(2), 2015–2023 (2014).
Iqbal, M. J. & Iqbal, S. Synthesis of stable and highly luminescent beryllium and magnesium doped ZnS quantum dots suitable for design of photonic and sensor material. J. Lumin. 134, 739–746 (2013).
Ruszkiewicz, J. A. et al. Neurotoxic effect of active ingredients in sunscreen products, a contemporary review. Toxicol. Rep. 4, 245–259 (2017).
Rasmussen, J. W., Martinez, E., Louka, P. & Wingett, D. G. ZnO NPs for selective destruction of tumor cells and potential for drug delivery applications. Expert. Opin. Drug. Deliv. 7(9), 1063–1077 (2010).
Kołodziejczak-Radzimska, A. & Jesionowski, T. ZnO from synthesis to application: A review. Materials 7(4), 2833–2881 (2014).
López-Cuenca, S. et al. Spheroidal ZnO NPs synthesized by semicontinuous precipitation method at low temperatures. Rev. Mex. Ing. Quim. 18(3), 1179–1187 (2019).
Sa-nguanprang, S., Phuruangrat, A., Karthik, K., Thongtem, S. & Thongtem, T. Tartaric acid-assisted precipitation of visible light-driven Ce-doped ZnO NPs used for photodegradation of methylene blue. Aust. Ceram. Soc. 56, 1029–1041 (2020).
Arif, M., Sanger, A., Vilarinho, P. M. & Singh, A. Effect of annealing temperature on structural and optical properties of sol–gel-derived ZnO thin films. J. Electron. Mater. 47, 3678–3684 (2018).
Jiang, J., Pi, J. & Cai, J. The advancing of ZnO NPs for biomedical applications. Bioinorg. Chem. Appl. 2018, 1062562 (2018).
Divya, M. et al. Biopolymer gelatin-coated ZnO NPs showed high antibacterial, antibiofilm and anti-angiogenic activity. J. Photochem. Photobiol. B Biol. 178, 211–218 (2018).
Matter, R. M. et al. Zinc supplementation improves glucose homeostasis in patients with β-thalassemia major complicated with diabetes mellitus: A randomized controlled trial. Nutrition 73, 110702 (2020).
Umrani, R. D. & Paknikar, K. M. ZnO NPs show antidiabetic activity in streptozotocin-induced Type 1 and 2 diabetic rats. Nanomedicine 9(1), 89–104 (2014).
Li, B. et al. The role of zinc in the prevention of diabetic cardiomyopathy and nephropathy. Toxicol. Mech. Methods 23(1), 27–33 (2013).
Khatun, R. & Mirazunnabi, M. A validated reversed-phase HPLC method for the determination of vildagliptin from tablet dosage form. Int. J. Pharm. Life Sci. 2(3), 90–98 (2013).
Shoaib, A. et al. Tailoring of an anti-diabetic drug empagliflozin onto ZnO NPs: Characterization and in vitro evaluation of anti-hyperglycemic potential. Sci. Rep. 14(1), 2499 (2024).
Nagaraja, S. H. et al. Novel preparation and effective delivery of mucoadeshive NPs containing anti-diabetic drug. Indian J. Pharm. Educ. Res. 53(2), S43–S49 (2019).
Abd El-Megeed, S. S., El-Sayed, W. Y. & Khamis, T. Antidiabetic efficacy of zinc oxide nanoparticles and empagliflozin combinations. Adv. Anim. Vet. Sci 10(11), 2421–2430 (2022).
Saddik, M. S. et al. Tailoring of novel azithromycin-loaded ZnO NPs for wound healing. Pharmaceutics 14(1), 1–23 (2022).
Kumar, D. V. & Rao, J. S. A new validated stability indicating RP-HPLC method for simultaneous estimation of metformin hydrochloride and empagliflozin in tablet dosage forms. IRJPMS 1, 16–22 (2018).
Srivastava, V., Gusain, D. & Sharma, Y. C. Synthesis, characterization and application of ZnO NPs (n-ZnO). Ceram. Int. 39(8), 9803–9808 (2013).
Lu, C. H. & Yeh, C. H. Influence of hydrothermal conditions on the morphology and particle size of ZnO powder. Ceram. Int. 26(4), 351–357 (2000).
Sathiyaseelan, A., Saravanakumar, K., Mariadoss, A. V. A. & Wang, M. H. Biocompatible fungal chitosan encapsulated phytogenic silver NPs enhanced antidiabetic, antioxidant, and antibacterial activity. Int. J. Biol. Macromol. 153, 63–71 (2020).
Agarwal, P. & Gupta, R. Alpha-amylase inhibition can treat diabetes mellitus. Res. Rev. J. Med. Health Sci 5(4), 1–8 (2016).
Hublikar, L. V., Ganachari, S. V. & Patil, V. B. Phytofabrication of silver nanoparticles using Averrhoa bilimbi leaf extract for anticancer activity. Nanoscale Adv. 5(16), 4149–4157 (2023).
Patil, S. B., Hublikar, L. V., Raghavendra, N., Shanbhog, C. & Kamble, A. Synthesis and exploration of anticancer activity of silver nanoparticles using Pandanus amaryllifolius Roxb. leaf extract: Promising approach against lung cancer and breast cancer cell lines. Biologia 76, 3533–3545 (2021).
Singh, D. K., Pandey, D. K., Yadav, R. R. & Singh, D. A study of nanosized ZnO and its nanofluid. Pramana J. Phys. 78(5), 759–766 (2012).
Khan, S. B., Faisal, M., Rahman, M. M. & Jamal, A. Low-temperature growth of ZnO NPs: Photocatalyst and acetone sensor. Talanta 85(2), 943–949 (2011).
Jay Chithra, M., Sathya, M. & Pushpanathan, K. J. A. M. S. Effect of pH on crystal size and photoluminescence property of ZnO NPs prepared by chemical precipitation method. Acta Metall. Sin. 28, 394–404 (2015).
Mesaros, A. et al. Synthesis, structural and morphological characteristics, magnetic and optical properties of Co-doped ZnO NPs. Ceram. Int. 40(2), 2835–2846 (2014).
Fayyaz, S. et al. Synthesis of vildagliptin conjugated metal NPs for type II diabetes control: Targeting the DPP-IV enzyme. New J. Chem. 44(47), 20853–20860 (2020).
Hassanein, A. M., Moharram, Y. I., Ebied, S. E., Sadek, M. E. & Khamis, A. A. Voltammetric assay of vildagliptin drug as vildagliptin-Cu2+ complex and its biological applications. J. Appl. Electrochem. 52(10), 1491–1511 (2022).
Tundis, R., Loizzo, M. R. & Menichini, F. Natural products as α-amylase and α-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: An update. Mini Rev. Med. Chem. 10(4), 315–331 (2010).
Dhobale, S. et al. ZnO NPs as novel alpha-amylase inhibitors. J. Appl. Phys. 104(9), 094907 (2008).
Kleppinger, E. L. & Helms, K. The role of vildagliptin in the management of type 2 diabetes mellitus. AOP. 41(5), 824–832 (2007).
El-Gharbawy, R. M., Emara, A. M. & Abu-Risha, S. E. S. Zinc oxide nanoparticles and a standard antidiabetic drug restore the function and structure of beta cells in Type-2 diabetes. Biomed. Pharmacother. 84, 810–820 (2016).
Govender, T., Stolnik, S., Garnett, M. C., Illum, L. & Davis, S. S. PLGA NPs prepared by nanoprecipitation: drug loading and release studies of a water-soluble drug. J. Control. Release 57(2), 171–185 (1999).
Saddik, M. S. et al. Formulation and evaluation of azithromycin-loaded silver nanoparticles for the treatment of infected wounds. Int. J. Pharm. X. 7, 100245 (2024).
Ahmadijokani, F. et al. Aluminum-based metal-organic frameworks for adsorptive removal of anti-cancer (methotrexate) drug from aqueous solutions. J. Environ. Manag. 277, 111448 (2021).
Ragab, M. A., Korany, M. A., Ibrahim, H. Z., Abdel-Kawi, M. A. & AbdElAal, A. A. Adsorption behavior of some metal ions on nanoparticles used in pharmaceutical matrices: Application to laboratory-made drug formulation. Bull. Fac. Pharm. Cairo Univ. 55(1), 155–162 (2017).
Acknowledgements
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work under Grant Number RGP2/144/45.
Author information
Authors and Affiliations
Contributions
The manuscript was written with the contributions of all authors. All authors have approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Samad, A., Shahid, S., Mansoor, S. et al. Fabrication of novel vildagliptin loaded ZnO nanoparticles for anti diabetic activity. Sci Rep 14, 17893 (2024). https://doi.org/10.1038/s41598-024-67420-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67420-z
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.