Abstract
The hormone receptor (HR) status plays a significant role in breast cancer, serving as the primary guide for treatment decisions and closely correlating with prognosis. This study aims to investigate the predictive value of radiomics analysis in long-axis and short-axis ultrasound planes for distinguishing between HR-positive and HR-negative breast cancers. A cohort of 505 patients from two hospitals was stratified into discovery (Institute 1, 416 patients) and validation (Institute 2, 89 patients) cohorts. A comprehensive set of 788 ultrasound radiomics features was extracted from both long-axis and short-axis ultrasound planes, respectively. Utilizing least absolute shrinkage and selection operator (LASSO) regression analysis, distinct models were constructed for the long-axis and short-axis data. Subsequently, radiomics scores (Rad-scores) were computed for each patient. Additionally, a combined model was formulated by integrating data from long-axis and short-axis Rad-scores along with clinical factors. The diagnostic efficacy of all models was evaluated by the area under the receiver operating characteristic (ROC) curve (AUC). The long-axis and short-axis models, consisting of 11 features and 15 features, respectively, were established, yielding AUCs of 0.743 and 0.751 in the discovery cohort, and 0.795 and 0.744 in the validation cohort. The calculated long-axis and short-axis Rad-scores exhibited significant differences between HR-positive and HR-negative groups across all cohorts (all p < 0.001). Univariate analysis identified ultrasound-reported tumor size as an independent predictor. The combined model, incorporating long-axis and short-axis Rad-scores along with tumor size, achieved superior AUCs of 0.788 and 0.822 in the discovery and validation cohorts, respectively. The combined model effectively distinguishes between HR-positive and HR-negative breast cancers based on ultrasound radiomics features and tumor size, which may offer a valuable tool to facilitate treatment decision making and prognostic assessment.
Similar content being viewed by others
Introduction
Breast cancer (BC) remains a significant global health challenge, affecting millions of women and accounting for a substantial number of cancer-related deaths1. Among its molecular sub-types, hormone receptor (HR) positive breast cancer represents a predominant form, characterized by the expression of estrogen receptor (ER) and/or progesterone receptor (PR)2. These receptors play a critical role in the tumor's pathogenesis and serve as important therapeutic targets for endocrine therapy3,4. Early and accurate prediction of HR positive breast cancer is vital for timely diagnosis, appropriate treatment selection, and improved patient outcomes.
The prevailing standard for diagnosing HR positive breast cancer involves histological examination and immunohistochemical analysis5. Nevertheless, this procedure involves tissue biopsy, which may elicit discomfort and pose potential risks, rendering it unsuitable for certain patients, particularly those unable to undergo tissue sampling in specific situations. Furthermore, breast cancer is characterized by its intricacy, with tumors demonstrating heterogeneity6. Then, relying solely on a single biopsy sample may not adequately represent the entirety of the tumor, thus increasing the risk of misjudging the HR status of the tumor7,8. Hence, there is an urgent need for a non-invasive and effective method to accurately identify the HR status of the tumor.
Medical imaging has emerged as a crucial tool in the diagnosis and management of breast cancer, enabling non-invasive evaluation of breast lesions. Among the imaging modalities, Ultrasound (US) has gained popularity due to its safety, affordability, and real-time imaging capabilities. Conventional B-mode US and Color Doppler US provide valuable insights into the characteristics of breast lesions, such as size, shape, margins, as well as blood flow velocity and direction. However, their diagnostic accuracy in distinguishing between HR positive and HR negative breast cancers is limited9,10,11, prompting the need to explore advanced imaging techniques that can improve prediction accuracy.
In recent years, US radiomics, an emerging field in medical imaging, has garnered significant attention for its potential in unlocking hidden information within medical images. US Radiomics involves the high-throughput extraction and analysis of quantitative imaging features12,13 representing the shape, intensity, and texture of tumors, which capture subtle patterns and heterogeneity within the tumor microenvironment14. These features provide valuable insights into tumor biology, treatment response, and patient prognosis, which can be analyzed by different machine learning methods. By leveraging US radiomics analysis, researchers have demonstrated promising results in various cancer types, including breast cancer, in predicting disease subtypes and treatment responses15,16,17,18.
Several studies have indicated that multiplane ultrasound imaging holds significant predictive value in various aspects of breast cancer. Specifically, it has demonstrated efficacy in the preoperative prediction of metastatic lymph node burden19, contributed to the reduction of unnecessary biopsies for breast lesions20, and exhibited favorable predictive value for the sonographic phenotypes associated with molecular subtypes of invasive ductal cancer21. Long-axis US plane allows for a comprehensive visualization along the longest axis of the lesion, while short-axis plane captures cross-sectional views, facilitating the assessment of internal tissue characteristics. Hence, long-axis and short-axis US planes may offer comprehensive views of breast lesions, each providing unique information about their spatial distribution and internal architecture. The integration of radiomics analysis with long-axis and short-axis US planes may potentially enhance the diagnostic accuracy and predictive value for HR positive breast cancer.
Therefore, this study aims to investigate the predictive value of radiomics analysis in long-axis and short-axis US planes for HR positive breast cancer. We hypothesize that the integration of radiomics features from both planes will improve the discrimination between HR positive and HR negative breast cancers, providing clinicians with a valuable non-invasive tool for early detection and personalized treatment planning.
Methods
Patients
The multicenter study was carried out at two hospitals. The study received approval from the Institutional Review Board of Zhejiang Cancer Hospital (Institute 1) and the Institutional Review Board of Dongyang People's Hospital (Institute 2), which exempted the requirement for written informed consent due to the retrospective nature of the study. Patients who met the inclusion criteria and were admitted between June 2019 and January 2022 at Zhejiang Cancer Hospital were designated as the discovery cohort, while those admitted between August 2021 and May 2023 at Dongyang People's Hospital constituted the validation cohort. Our study was conducted in accordance with the Declaration of Helsinki.
The study's inclusion criteria comprised the following: (1) the postoperative histopathological examination confirmed the presence of invasive breast cancer; (2) clear and complete ultrasound images of the patients' breast tumors; and (3) availability of comprehensive medical records. Conversely, the exclusion criteria included: (1) patients who underwent preoperative chemotherapy or radiation therapy; (2) patients with breast cancer presented with multiple lesions; and (3) cases with non-mass lesions that were difficult to delineate. The flowchart illustrating patient selection and dataset construction is presented in Fig. 1.
Baseline information was systematically collected for each patient, comprising key details such as age, tumor location, US-reported tumor size, Breast Imaging Reporting and Data System (BIRADS) category, US-reported lymph node metastasis, and histopathological features [ER, PR, pathological type, and human epidermal growth factor receptor 2 (HER-2)]. These data were retrieved from clinical records, postoperative pathology reports, and immunohistochemistry analyses, ensuring a comprehensive evaluation and analysis of the patients included in this study.
Ultrasound imaging acquisition
Ultrasound scans were conducted using four different ultrasound instruments: the LOGIQ E9 system by GE Healthcare, headquartered in Chicago, Illinois, USA; the Siemens Acuson S2000 system by Siemens Healthineers, located in Erlangen, Germany; the Toshiba Aplio 500 system manufactured by Canon Medical Systems in Otawara, Japan; and the Philips EPIQ 5 system by Philips Healthcare, headquartered in Amsterdam, Netherlands. These instruments were equipped with probes of various frequencies, ranging from 5 to 18 MHz, to acquire the largest long-axis and short-axis ultrasound images of the breast lesions. Tumor size was determined as the maximum diameter measured in the long axis of the lesion.
During the scanning process, patients were positioned in a supine or lateral decubitus position, and a water-based gel was applied to the skin surface to improve acoustic coupling. The transducer was placed on the breast region of interest, and real-time B-mode imaging was utilized to visualize the lesion. For each patient, both the long-axis and short-axis planes of the lesion were scanned and captured to ensure comprehensive coverage of the tumor region. The obtained ultrasound images were stored in Digital Imaging and Communications in Medicine (DICOM) format to maintain data integrity and facilitate standardized image analysis across multiple centers. A centralized picture archiving and communication system was utilized to store and manage the DICOM images securely.
Region of interest identification and segmentation
Following ultrasound imaging acquisition, two experienced Sonographers reviewed the stored DICOM images to identify the regions of interest (ROIs) corresponding to the breast lesions. The long-axis and short-axis ROIs were manually delineated using ITK-SNAP software (Version 3.4.0), respectively, which allowed for precise and consistent segmentation of the tumor area. To ensure accuracy and reliability, each ROI segmentation was carefully reviewed and validated by a senior Sonographer to minimize inter-observer variability.
Radiomics feature extraction and selection
The original ultrasound images were processed in Python (Version 3.7) using the PyRadiomics package. Isotropic pixel points were ensured through image preprocessing. Image normalization (normalize Scale = 25) and resampling (Resample Pixel Spacing = [1, 1, 1]) were applied during this process. Then radiomics features were extracted from the segmented ROIs by using pyradiomics package, which provided a comprehensive suite of algorithms to quantify various image-based features. These features included first-order statistics, texture features, shape-based characteristics, and wavelet features, all of which contributed to the characterization of the tumor's spatial heterogeneity and texture patterns. To ensure the robustness of the selected features, the intra- and inter-observer agreement in ROI segmentation was assessed using the intraclass correlation coefficient (ICC). A high ICC value > 0.75 indicated strong agreement, enhancing the reliability and reproducibility of the subsequent radiomics analysis process.
Z-score normalization was carried out independently in the discovery and validation cohorts to mitigate the influence of outliers and facilitate the comparison of radiomics features with different magnitudes. To identify the most discriminative features for predicting HR status in the discovery cohort, a rigorous selection process was employed. Firstly, the intra- and inter-observer agreement among Sonographers in ROI segmentation was assessed using the ICC. Subsequently, the Mann–Whitney U test was applied to compare the radiomics features between HR positive and HR negative subgroups, aiming to identify features with significant differences. Lastly, the least absolute shrinkage and selection operator (LASSO) regression was utilized to further select the features that contributed significantly to the predictive model. LASSO regression is a powerful technique to penalize less informative features, effectively promoting the selection of the most discriminative features for building predictive models22. By employing LASSO regression, the final set of radiomics features was identified, which played a crucial role in the subsequent model construction for HR status prediction.
Model construction and validation
In order to identify risk factors associated with HR positive breast cancer, both univariate and multivariate regression analysis were used. In the univariate regression analysis, potential risk factors, including age, tumor location, US-reported tumor size, BIRADS category, and US-reported lymph node metastasis were examined independently. Variables that showed a significant difference (p < 0.05) with HR positive breast cancer in the univariate analysis were then included in the subsequent model construction.
Utilizing the selected radiomics features, logistic regression models were constructed for both the long-axis and short-axis ultrasound planes, and radiomics score (Rad-score) was calculated. Furthermore, a combined model using long-axis and short-axis Rad-scores from both planes and clinical risk factor was established. The logistic regression models were trained using the discovery cohort and validated in the validation cohort to assess their generalization performance. Model performance was evaluated based on various performance metrics, including sensitivity, specificity, accuracy, and the area under the receiver operating characteristic (ROC) curve (AUC).
Clinical application
To assess its performance and reliability, we first evaluated the model's calibration using a calibration curve. This curve visually compares the predicted probabilities from the model with the actual observed outcomes. A well-calibrated model would show the points on the calibration curve close to the 45-degree reference line, indicating a good agreement between predicted and observed outcomes. Furthermore, we examined the model's discriminative ability through a decision curve analysis (DCA). The DCA allows us to assess the clinical utility of the model by plotting the net benefit against different threshold probabilities. The decision curve provides insights into the model's value in clinical decision-making compared to a "treat-all" or "treat-none" strategy. The complete model architecture is depicted in Fig. 2.
Statistical analysis
Statistical analyses were conducted using R software (Version 4.1.2). For comparing continuous variables between the HR-positive and HR-negative subgroups, the Student’s t-test was employed for variables with a normal distribution, whereas the Mann–Whitney U test was applied for variables with an abnormal or unknown distribution. For categorical variables, including tumor pathological type, tumor location, and lymph node metastasis, association analyses were performed using either the Chi-square test or Fisher's exact test, depending on sample size and data distribution. A significance level of two-tailed p < 0.05 was deemed as statistically significant.
Ethics statement
The research involving human participants underwent thorough scrutiny and received official approval from the Institutional Review Board of Dongyang People's Hospital (Approval No. 2024-YX-154) and the Institutional Review Board of Zhejiang Cancer Hospital (Approval No. IRB-2022-548).
Results
Patient characteristics
Table 1 provides a comprehensive summary of the patient and tumor characteristics in both the discovery and validation cohorts. It is important to note that no statistically significant differences were observed between these two cohorts, with all p-values > 0.05. This uniformity between the discovery and validation cohorts ensures the reliability of our findings and their potential applicability to a broader patient population.
Table 2 offers a detailed view of the clinicopathological characteristics of patients, categorized into HR positive and HR negative groups. In the discovery cohort, several parameters exhibited notable differences between these groups. Specifically, ER PR, HER-2, and tumor size displayed significant variations. Similar trends were observed in the validation cohort, where ER, PR, pathological type, and tumor size remained statistically significant variations distinguishing between HR positive and HR negative patients.
Radiomics feature extraction and selection
Our radiomics analysis involved the extraction of a number of features, comprising 765 features from long-axis images and 754 features from short-axis images, which were carefully selected based on their ICCs > 0.75. This selection process ensured the inclusion of only the most stable and reliable radiomics features in our analysis. To further refine our feature selection, the Mann–Whitney U test was applied. This step led to the identification of 155 long-axis features and 165 short-axis features with p-values < 0.05. These features were subsequently selected for further investigation. Using the LASSO method (Fig. 3), we narrowed down our selection to 11 significant features derived from long-axis images and 15 features extracted from short-axis images. Detailed information on these selected features can be found in Table 3.
Radiomic feature selection using the least absolute shrinkage and selection operator (LASSO) logistic regression. The selection of tuning parameter (Lambda) in the LASSO model. (A,C) The area under the curve (AUC) was plotted versus log (Lambda). The dashed lines indicate the selected optimal log(Lambda) value and the location of one standard error. The optimal Lambda values of 0.015879 and 0.012864 with log (Lambda) of −4.142732 and −4.353298 were chosen according to tenfold cross-validation. (B,D) Graphs of variation of the radiomics characteristic coefficients with log(Lambda) for long-axis and short-axis ultrasound planes, respectively. AUC area under the curve.
Long-axis and short-axis models
Based on the selected features, we developed separate long-axis and short-axis models. These models were instrumental in computing Rad-scores for each patient within the discovery and validation cohorts. In the discovery cohort, it is noteworthy that the long-axis model exhibited a marginally lower AUC value compared to the short-axis model. However, statistical analysis using the Delong test revealed no significant difference between the two models (p = 0.821). Contrarily, in the validation cohort, the long-axis model demonstrated a higher AUC value compared to the short-axis model (Delong test, p = 0.528); however, this difference was not statistically significant.
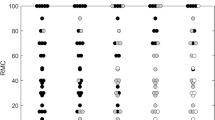
For a comprehensive visual comprehension, the distributions of the long-axis and short-axis Rad-scores, along with HR status, are presented in Fig. 4. Importantly, significant differences were observed in the distribution of Rad-scores for HR positive and HR negative patients in both the discovery and validation cohorts, with all p-values < 0.05, as highlighted in Table 4.
The distribution of radiomics scores (Rad-scores). Long-axis Rad-scores for each patient across both hormone receptor (HR) positive and HR negative breast cancers, as well as the distribution of short-axis Rad-scores, are assessed in both the discovery cohort (A,C) and the validation cohort (B,D), respectively. Rad-score radiomics score.
Clinical risk factors
Our univariate analysis found that US-reported tumor size held predictive potential as a clinical risk factor. The obtained result implies that tumor size may possess a more robust predictive capacity for HR status compared to other clinical factors. Based on the tumor size variable, we developed a regression prediction model. The results indicated that, for the discovery cohort, the model achieved an AUC of 0.592, with a sensitivity of 0.571 and a specificity of 0.598. For the validation cohort, the model achieved an AUC of 0.657, with a sensitivity of 0.701 and a specificity of 0.591.
Combined model development and assessment
To harness the full predictive power of our radiomics analysis and clinical risk factor, we constructed a combined model. This model integrated the long-axis Rad-score, short-axis Rad-score, and tumor size. The combined model was developed using the discovery cohort and subsequently validated using the validation cohort. The results were promising, with the combined model yielding AUC values of 0.788 and 0.822 in the discovery and validation cohorts, respectively. These outcomes were significantly better than those obtained from the model based solely on tumor size. These values surpassed those of the individual long-axis and short-axis models, suggesting the potential synergy of radiomics and clinical information.
Statistical analysis using Delong test revealed that the AUC value of the combined model demonstrated a statistically significant difference compared to the long-axis model (p = 0.010). However, there was no statistically significant difference when compared to the short-axis model in the discovery cohort (p = 0.073).
In the validation cohort, the combined model demonstrated no statistically significant differences compared to the long-axis and short-axis models (p = 0.515 and p = 0.399, respectively). Moreover, a comprehensive assessment of the precision, sensitivity, and specificity of the three models was performed, as detailed in Table 5.
To illustrate the discriminative efficacy of our models, ROC curves were generated, delineating the capacity to distinguish HR status in both the discovery and validation cohorts. These curves are depicted in Fig. 5, emphasizing the improved performance of the combined model.
Clinical application
In the practical application of our findings, we conducted calibration curves, as depicted in Fig. 6, for the long-axis, short-axis, and combined models. These curves ensure that our models are well-calibrated and suitable for clinical use. The Hosmer–Lemeshow test further confirmed the satisfactory fit of our models. Moreover, decision curve analyses were conducted to assess the clinical practicability of the three models, as shown in Fig. 7. These analyses demonstrate the net benefit of employing our models in clinical decision-making, further underscoring their utility.
Calibration curves of the three models. Calibration curves constructed for the long-axis model, short-axis model, and combined model within both the discovery (A) and validation (B) cohorts. These curves described a good fitness between predicted and observed outcomes for hormone receptor (HR) positive and HR negative breast cancers across all three models. The ideal prediction was depicted by the gray line. A closer fitness to the gray line represented a well-calibrated model.
In Fig. 8, we illustrate two examples showing the clinical application of our model. These nomograms serve as practical tools for estimating hormone receptor (HR) status in breast cancer patients. They incorporate factors such as the long-axis Rad-score, short-axis Rad-score, and tumor size, providing valuable insights for clinical decision-making.
Combined model nomograms. Nomograms were constructed by integrating the long-axis radiomics score (Rad-score), short-axis Rad-score, and tumor size to differentiate between hormone receptor (HR) positive and HR negative breast cancers. Patient 1 (A) was a 72-year-old female diagnosed with a 3.0-cm-sized breast lesion. Her long-axis and short-axis Rad-scores were 0.907 and 0.943, respectively. The total score calculated was 257 points, corresponding to a high probability of HR positivity (0.940). Pathological examination confirmed HR positive breast cancer in this patient. Patient 2 (B) was a 47-year-old female diagnosed with a 5.0-cm-sized breast lesion. Her long-axis and short-axis Rad-scores were 0.499 and 0.537, respectively. The total score obtained was 178 points, indicating a lower probability of HR positivity (0.357). Pathological analysis confirmed HR negative breast cancer in this case. L-Rad-score long-axis radiomics score, S-Rad-score short-axis radiomics score.
Discussion
This study aimed to explore the potential of US radiomics and clinical characteristics in predicting HR status in breast cancer patients. We selected radiomics features for stability and significance, and developed long-axis and short-axis models. We found that ultrasound-reported tumor size emerged as a clinical risk factor for HR status prediction. Furthermore, we integrated long-axis Rad-score, short-axis Rad-score, and tumor size into a combined model that outperformed individual models, achieving an AUC of 0.788 in the discovery cohort and 0.822 in the external validation cohort. Our models demonstrated well-calibrated performance suitable for clinical use, with decision curve analyses confirming their utility. We also presented a practical nomogram to aid clinicians in estimating HR status. These findings suggest that long-axis and short-axis radiomics features, when combined with tumor size, can enhance HR status prediction, potentially improving personalized treatment strategies for breast cancer patients.
Several studies have investigated the role of radiomics in breast cancer. Yu et al.23 established a radiomics model using machine learning to predict axillary lymph node metastasis through the analysis of ultrasound images, achieving a moderate AUC of 0.71. Romeo and colleagues24 constructed a prediction model based on radiomics features and machine learning applied to ultrasound imaging. This model exhibited the ability to discriminate between benign and malignant breast cancers, yielding a noteworthy AUC value of 0.82. However, in these investigations, there has been a notable emphasis on a singular imaging plane, particularly along the long axis. This raises the inquiry as to whether relying solely on a singular ultrasound plane inherently results in a reduced acquisition of radiomics information. In addressing this concern, our study systematically examined both long-axis and short-axis models, aiming to comprehensively evaluate potential disparities in imaging radiomics information linked to each orientation. We found that both orientations yielded comparable results, offering clinicians flexibility in their choice of imaging orientation for radiomics-based HR status prediction. However, the performance of single ultrasonic plane model is insufficient (AUC < 0.80), suggesting that combining the long-axis and short-axis features with clinical information might be necessary for achieving a higher predictive value.
Then, we developed a combined predictive model that demonstrated superior performance in predicting HR-positive breast cancer. The superior performance of our combined model is likely due to the integration of the long-axis and short-axis radiomics features, which show significant differences between HR-positive and HR-negative breast cancers. This suggests that capturing a comprehensive range of radiomics features from different planes can effectively predict the HR expression of breast cancer. This enhancement also may be attributed to the comprehensive insights into breast tumor morphology and structure provided by long-axis and short-axis perspectives, allowing for a more thorough understanding of the spatial distribution of biological features19,20,21. The comprehensive nature of information from both planes, along with the model's ability to capture diverse characteristics and variations, may contribute to increased robustness and adaptability, ultimately enhancing the overall predictive performance.
Previous literature reported that clinical features of breast cancer, such as tumor size and age, were associated with HR status25,26. In a study by Krizmanich-Conniff et al., it was demonstrated that triple receptor-negative cancer often appears as a hypoechoic or complex mass with an irregular shape and noncircumscribed margins on ultrasound and is more common in younger women with higher pathological grades27. In this study, we found significant difference between HR positive and HR negative groups in the tumor size of the breast cancer lesions using univariate logistic regression. This may be related to HR negative tumors, characterized by higher cell proliferation rates and possibly unique tissue structures, may contribute to larger apparent diameters26. Additionally, the propensity for HR negative tumors to exhibit lymph node involvement could further influence local tumor spread, impacting the overall tumor size.
We derived four distinct categories of radiomics features from ultrasound images, comprising shape-based, first-order statistical, texture, and wavelet features. The predominant elements integrated into the model were primarily texture and wavelet features. The utilization of wavelet features facilitates the computation of image signal resolution across various temporal, spatial, and frequency scale planes28,29. Texture analysis, a pivotal facet of this methodology, streamlines the extraction and quantification of intricate details, including regularity, roughness, and grey-level attributes of lesions, which might evade visual detection30,31. This approach affords a more comprehensive and nuanced portrayal of lesion characteristics. Consequently, our research demonstrates the critical role of texture and wavelet features in predicting HR positive breast cancer. Although the radiomics features extracted from the long-axis and short-axis planes in ultrasound images differ, they predominantly consist of texture and wavelet features. Moreover, the models established for the long-axis and short-axis planes exhibit comparable predictive performance, with no statistically significant differences noted in the validation set. By further integrating the radiomics features from the long-axis and short-axis planes, the combined model enhances predictive performance, demonstrating the comprehensive nature of these two imaging perspectives.
Huang et al.32 utilized dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) to establish a radiomics signature for distinguishing luminal and non-luminal molecular subtypes in invasive breast cancer. Their radiomics signature, derived from the second phase of DCE-MRI images in 135 patients, demonstrated strong discrimination in both training (AUC = 0.86) and testing sets (AUC = 0.80), without identifying clinical risk factors as independent predictors. Sheng et al.33 employed machine learning techniques integrating MRI radiomics features and clinical data to predict the HR positive subtype in invasive ductal breast cancer. The eXtreme gradient boosting method exhibited superior performance with an AUC of 0.828 in the validation cohort, which included 190 women with various molecular subtypes. However, limitations of above studies included a small sample size, potentially introducing selection bias, and highlighting shortages of MRI, such as high cost and limited suitability for preoperative evaluation. In comparison, our multicenter study, involving 505 patients and external validation, confirmed the robustness of our model with an AUC of 0.822. Uniquely leveraging ultrasound imaging, our study offered advantages like the absence of contrast agents, easy equipment accessibility, and user-friendly operation, addressing some of the limitations associated with MRI-based approaches. The inclusion of both long-axis and short-axis ultrasound images provided a comprehensive tumor assessment, potentially enhancing our model's predictive performance. This comprehensive model contributes to the increased clinical applicability and credibility of our predictive model.
Gong et al.'s research34 has illustrated the potential of two-dimensional ultrasound radiomics in predicting HR positive in breast cancer. Their US radiomics model achieved an AUC value of 0.817, indicating the capability of US radiomics in predicting HR positive. However, Gong et al. utilized a single ultrasound plane radiomics approach with much smaller sample size of 120 lesions, achieving an AUC of 0.817. In contrast, our study included a significantly larger sample size and underwent rigorous external validation, resulting in a slightly improved AUC of 0.822. Despite the similarity in predictive performance between the two studies, our strength lies in the comprehensive approach adopted. We integrated both long-axis and short-axis planes, along with tumor size, thereby enhancing the accuracy and reliability of the predictive model. As a result, our research provides a more robust and clinically valuable tool for patients diagnosed with breast cancer.
Despite the promising results, our study has several limitations. Firstly, our study primarily relied on retrospective data, which may introduce selection bias. Prospective studies could provide more robust evidence. Secondly, the exclusion of non-mass tumors from our study may introduce some bias in patient selection. However, it's important to note that the absence of a standardized segmentation method for non-mass tumors contributed to this limitation. Thirdly, we did not incorporate other potential biomarkers, such as genomics or proteomics data, which could further refine HR status prediction models. Lastly, the segmentation of the tumor's ROI was not automated, resulting in a time-consuming and labor-intensive process. This challenge could potentially be addressed in the future by leveraging an artificially intelligent system.
Conclusion
In conclusion, we have developed and validated a combined model for distinguishing between HR positive and HR negative breast cancers. This was achieved through the integration of key parameters, including the long-axis Rad-score, short-axis Rad-score, and tumor size. The superior performance exhibited by this combined model underscores its potential for tailoring individualized treatment for patients with invasive breast cancer. However, future research should prioritize the investigation of larger and more diverse cohorts and the validation of our model across multiple centers.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71(3), 209–249. https://doi.org/10.3322/caac.21660 (2021).
Ignatiadis, M. & Sotiriou, C. Luminal breast cancer: From biology to treatment. Nat. Rev. Clin. Oncol. 10(9), 494–506. https://doi.org/10.1038/nrclinonc.2013.124 (2013).
Alfarsi, L., Johnston, S., Liu, D. X., Rakha, E. & Green, A. R. Current issues with luminal subtype classification in terms of prediction of benefit from endocrine therapy in early breast cancer. Histopathology. 73(4), 545–558. https://doi.org/10.1111/his.13523 (2018).
De Marchi, T., Foekens, J. A., Umar, A. & Martens, J. W. Endocrine therapy resistance in estrogen receptor (ER)-positive breast cancer. Drug Discov. Today 21(7), 1181–1188. https://doi.org/10.1016/j.drudis.2016.05.012 (2016).
Hammond, M. E. et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 134(6), 907–922. https://doi.org/10.5858/134.6.907 (2010).
Turner, K. M., Yeo, S. K., Holm, T. M., Shaughnessy, E. & Guan, J. L. Heterogeneity within molecular subtypes of breast cancer. Am. J. Physiol. Cell Physiol. 321(2), C343–C354. https://doi.org/10.1152/ajpcell.00109.2021 (2021).
Pölcher, M. et al. Concordance of the molecular subtype classification between core needle biopsy and surgical specimen in primary breast cancer. Arch. Gynecol. Obstet. 304(3), 783–790. https://doi.org/10.1007/s00404-021-05996-x (2021).
Chen, J. et al. Comparison of core needle biopsy and excision specimens for the accurate evaluation of breast cancer molecular markers: A report of 1003 cases. Pathol. Oncol. Res. 23(4), 769–775. https://doi.org/10.1007/s12253-017-0187-5 (2017).
Rashmi, S. et al. Predicting the molecular subtype of breast cancer based on mammography and ultrasound findings. Indian J. Radiol. Imaging 28(3), 354–361. https://doi.org/10.4103/ijri.IJRI_78_18 (2018).
Irshad, A. et al. Assessing the role of ultrasound in predicting the biological behavior of breast cancer. AJR Am. J. Roentgenol. 200(2), 284–290. https://doi.org/10.2214/AJR.12.8781 (2013).
Cho, N. Molecular subtypes and imaging phenotypes of breast cancer. Ultrasonography 35(4), 281–288. https://doi.org/10.14366/usg.16030 (2016).
Lambin, P. et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 48(4), 441–446. https://doi.org/10.1016/j.ejca.2011.11.036 (2012).
Kumar, V. et al. Radiomics: The process and the challenges. Magn. Reson. Imaging 30(9), 1234–1248. https://doi.org/10.1016/j.mri.2012.06.010 (2012).
Wang, X. et al. Radiomics predicts the prognosis of patients with locally advanced breast cancer by reflecting the heterogeneity of tumor cells and the tumor microenvironment. Breast Cancer Res. 24(1), 20. https://doi.org/10.1186/s13058-022-01516-0 (2022).
Du, Y. et al. Ultrasound-based radiomics nomogram for differentiation of triple-negative breast cancer from fibroadenoma. Br. J. Radiol. 95(1133), 20210598. https://doi.org/10.1259/bjr.20210598 (2022).
Xu, Z., Wang, Y., Chen, M. & Zhang, Q. Multi-region radiomics for artificially intelligent diagnosis of breast cancer using multimodal ultrasound. Comput. Biol. Med. 149, 105920. https://doi.org/10.1016/j.compbiomed.2022.105920 (2022).
Jiang, M. et al. Ultrasound-based deep learning radiomics in the assessment of pathological complete response to neoadjuvant chemotherapy in locally advanced breast cancer. Eur. J. Cancer 147, 95–105. https://doi.org/10.1016/j.ejca.2021.01.028 (2021).
Zhang, M. Q. et al. Construction and validation of a personalized nomogram of ultrasound for pretreatment prediction of breast cancer patients sensitive to neoadjuvant chemotherapy. Br. J. Radiol. 95(1140), 20220626. https://doi.org/10.1259/bjr.20220626 (2022).
Zhao, F., Cai, C., Liu, M. & Xiao, J. Identification of the lymph node metastasis-related automated breast volume scanning features for predicting axillary lymph node tumor burden of invasive breast cancer via a clinical prediction model. Front. Endocrinol. (Lausanne) 5(13), 881761. https://doi.org/10.3389/fendo.2022.881761 (2022).
Wang, S. J. et al. Automated breast volume scanner (ABVS)-based radiomic nomogram: A potential tool for reducing unnecessary biopsies of BI-RADS 4 lesions. Diagnostics (Basel) 12(1), 172. https://doi.org/10.3390/diagnostics12010172 (2022).
van Zelst, J. C. M. et al. Sonographic phenotypes of molecular subtypes of invasive ductal cancer in automated 3-D breast ultrasound. Ultrasound Med. Biol. 43(9), 1820–1828. https://doi.org/10.1016/j.ultrasmedbio.2017.03.019 (2017).
Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. B 58, 267–288 (1996).
Yu, F. H. et al. Ultrasound-based radiomics nomogram: A potential biomarker to predict axillary lymph node metastasis in early-stage invasive breast cancer. Eur. J. Radiol. 119, 108658. https://doi.org/10.1016/j.ejrad.2019.108658 (2019).
Romeo, V. et al. Clinical value of radiomics and machine learning in breast ultrasound: A multicenter study for differential diagnosis of benign and malignant lesions. Eur. Radiol. 31(12), 9511–9519. https://doi.org/10.1007/s00330-021-08009-2 (2021).
Kadivar, M., Mafi, N., Joulaee, A., Shamshiri, A. & Hosseini, N. Breast cancer molecular subtypes and associations with clinicopathological characteristics in Iranian women, 2002–2011. Asian Pac. J. Cancer Prev. 13(5), 1881–1886. https://doi.org/10.7314/apjcp.2012.13.5.1881 (2012).
Fourati, A. et al. Descriptive analysis of molecular subtypes in Tunisian breast cancer. Asia Pac. J. Clin. Oncol. 10(2), e69-74. https://doi.org/10.1111/ajco.12034 (2014).
Krizmanich-Conniff, K. M. et al. Triple receptor-negative breast cancer: Imaging and clinical characteristics. AJR Am. J. Roentgenol. 199(2), 458–464. https://doi.org/10.2214/AJR.10.6096 (2012).
Jha, A. K. et al. Repeatability and reproducibility study of radiomic features on a phantom and human cohort. Sci. Rep. 11(1), 2055. https://doi.org/10.1038/s41598-021-81526-8 (2021).
Zhang, R., Shen, J., Wei, F., Li, X. & Sangaiah, A. K. Medical image classification based on multi-scale non-negative sparse coding. Artif. Intell. Med. 83, 44–51. https://doi.org/10.1016/j.artmed.2017.05.006 (2017).
Sivaramakrishna, R., Powell, K. A., Lieber, M. L., Chilcote, W. A. & Shekhar, R. Texture analysis of lesions in breast ultrasound images. Comput. Med. Imaging Graph. 26(5), 303–307. https://doi.org/10.1016/s0895-6111(02)00027-7 (2002).
Alvarenga, A. V., Pereira, W. C., Infantosi, A. F. & Azevedo, C. M. Complexity curve and grey level co-occurrence matrix in the texture evaluation of breast tumor on ultrasound images. Med. Phys. 34(2), 379–387. https://doi.org/10.1118/1.2401039 (2007).
Huang, T. et al. Application of DCE-MRI radiomics signature analysis in differentiating molecular subtypes of luminal and non-luminal breast cancer. Front. Med. (Lausanne) 25(10), 1140514. https://doi.org/10.3389/fmed.2023.1140514 (2023).
Sheng, W. et al. Invasive ductal breast cancer molecular subtype prediction by MRI radiomic and clinical features based on machine learning. Front. Oncol. 12(12), 964605. https://doi.org/10.3389/fonc.2022.964605 (2022).
Gong, X. et al. Conventional ultrasound and contrast-enhanced ultrasound radiomics in breast cancer and molecular subtype diagnosis. Front. Oncol. 23(13), 1158736. https://doi.org/10.3389/fonc.2023.1158736 (2023).
Funding
This study was founded by Research Programs of Jinhua Science and Technology Bureau Scientific Research Project (2023-4-225) and National Health Commission Capacity Building and Continuing Education Center (CSJRZC2021JJSJ001).
Author information
Authors and Affiliations
Contributions
Conception and design of study: Dong Xu and Zhengping Wang. Acquisition of data, analysis, and interpretation of data: Jiangfeng Wu , Yinghong Guo, and Lifang Ge. Drafting and revising the manuscript: Jincao Yao, Anli Zhao, and Lifang Ge. All authors approved the final manuscript version to be submitted.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, J., Ge, L., Guo, Y. et al. Predicting hormone receptor status in invasive breast cancer through radiomics analysis of long-axis and short-axis ultrasound planes. Sci Rep 14, 16503 (2024). https://doi.org/10.1038/s41598-024-67145-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67145-z
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.