Abstract
Colloidal quantum dots (QDs) are attracting research interest because of their unique optical properties that result from the quantum confinement effect. ZnSe QDs, which are II–VI semiconductors, offer a wide direct bandgap (2.7 eV), making them promising for applications such as light-emitting diodes, photodetectors, and biomedical labeling. In the present work, colloidal ZnSe (QDs) were synthesized by the hot-injection method with a Zn:Se ratio of 1:1. The optical properties of ZnSe QDs obtained at different reaction times were investigated by spectrophotometric UV–vis absorption and emission measurements. The as-synthesized ZnSe QDs exhibit blue excitonic emission, and no defect emission was detected. Transmission electron micrographs indicated that the QDs have a spherical morphology with dimensions ranging from 3.69 to 4.53 nm. In particular, the Brus model was applied to demonstrate a correlation between the QD sizes and the optical bandgaps obtained from Tauc plots.
Similar content being viewed by others
Introduction
Colloidal quantum dots (QDs) are nanoparticles with physical dimensions of a few nanometers. When QDs are smaller than the Bohr radius of the material, the quantum confinement effect occurs, leading to a blue-shift to higher energy1. The quantum size effect imparts colloidal QDs with a unique property: their optical and electrical properties can be tuned on the basis of their size. QDs have thus been intensively studied over the past three decades due to their unique optical and electrical properties and wide range of technological applications in catalysts, solar cells, biosensors, light-emitting diodes, and displays2.
Group II–VI semiconductor QDs, which are commonly Cd-based compounds, have been extensively studied for their fundamental properties and technological applications3,4. However, Cd is toxic, which limits the practical applications these QDs and raises concerns about their potential threats to the environment and human health3,5. Therefore, there is increasing demand for the development of Cd-free QDs6. ZnSe, an important II–VI semiconductor material with a wide bandgap of 2.7 eV and low toxicity, shows promise as a replacement for Cd-based QDs7. Because of their uncommon UV–blue luminescence properties, ZnSe QDs have strong potential for use in low-voltage electroluminescent devices and blue diode lasers8,9.
The wide bandgap of ZnSe QDs also enables them to be used as an inorganic passivation shell in various core/shell structure materials or doped with transition metal ions, enabling tunable emission spanning the range of visible wavelengths10,11. Since optical properties strongly depend on size, structure, and composition, it is crucial to understand synthesis conditions for the production of high-quality ZnSe QDs. So far, monodispersed ZnSe nanocrystals have been successfully prepared by various solution-based methods, including hot-injection and heating methods12. In the hot-injection method, a reagent is injected into a hot reaction solution at room temperature, causing burst homogeneous nucleation, followed by slow growth of the QDs6. Moreover, other studies have been reported that Zn/Se ratios that differ from 1:1 do not result in improved optical properties of ZnSe QDs13,14.
In the present study, the hot-injection technique was used to synthesize high-quality ZnSe QDs with a Zn/Se ratio of 1:1. The optical and morphological properties of the prepared ZnSe QDs were investigated by UV–vis absorption spectroscopy, photoluminescence (PL) spectroscopy, and transmission electron microscopy (TEM).
Results
Synthesis mechanism of colloidal ZnSe QDs
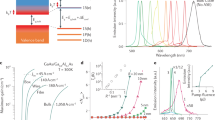
Figure 1 shows the synthesis scheme of the ZnSe QDs obtained by the hot-injection technique. This reaction scheme involves rapidly injecting a Se precursor into a hot Zn precursor, which leads to temporary separation nucleation and growth of ZnSe QDs14. A balance between nucleation and growth is necessary to control the QD size and size distribution15,16,17, which requires the synthesis to start with burst nucleation17 followed by slow growth to achieve precise size control18. Therefore, the reaction temperature and the reactivity of the monomers play important roles in the nucleation and growth of ZnSe QDs. High injection and growth temperatures increase the reaction rate. In addition, the selected precursor, zinc stearate (ZnSt2), is a long-alkyl-chain zinc carboxylate with a strong steric effect, which typically results in a low growth rate15,19. The ZnSt2 not only serves as a zinc source but also provides capping ligands18,20. However, to achieve the desired balance between nucleation and growth, we selected octadecylamine (ODA) as the reagent to activate the zinc carboxylate precursors18.
Absorption and photoluminescence analysis
UV–vis and PL spectroscopy are excellent tools for analyzing the optical properties of QDs, as shown in Fig. 2a, b. The absorption peaks of the prepared ZnSe QDs with reaction times of 0.5, 1, 5, 10, 30, and 60 min were observed at 393, 400, 408, 410, 412, and 414 nm, respectively. These peaks exhibit a sharp shape and well-defined excitonic features, indicating that ZnSe QDs with a narrow size distribution were formed21,22. The PL spectra show that the prepared ZnSe QDs fluoresce in the blue-light region, corresponding to the wavelength of their band-edge emission. No substantial defect emission is observed, although aqueous ZnSe QDs usually suffer from a broad PL band from 400 to 600 nm attributed to trapped states23. These results are evident in the PL intensity in Fig. 2c. The defect states, including Zn or Se vacancies, crystal defects, as well as surface states, commonly cause the light blue luminescence of ZnSe QDs13,23,24,25.
The absorption and PL peak positions of the ZnSe QDs with different reaction times are shown in Fig. 3a. The absorption and PL peaks were observed at higher energies than the bulk ZnSe bandgap (~ 2.7 eV) due to the quantum confinement effect9. With increasing reaction time, the size of the ZnSe QDs increased, resulting in a red-shift of both the absorption and PL peaks. Figure 3b shows that the difference between the absorption and PL energies of the ZnSe QDs—the so-called Stokes shift—decreases from 138 to 94 meV with increasing size of the ZnSe QDs. This result indicates that the Stokes shift of the ZnSe QDs is size dependent, as observed in previous studies26,27.
The size distribution of the QDs has also been mentioned as a factor influencing the Stokes shift28,29,30. The bandwidth of the PL peaks is well known to be related to the size distribution of ZnSe QDs in solution31,32. Figure 3c shows the FWHMs of the PL peaks of the ZnSe QDs, plotted as a function of the reaction time; the FWHMs rapidly decrease within a reaction time of 5 min, indicating that the growth stages started without the formation of new nuclei. As mentioned above, burst nucleation is necessary to achieve balance between nucleation and growth; however, if either too many or too few nuclei are formed, the balance between nucleation and growth will be disrupted15. After the initial formation of QDs, the size distribution broadens and the sizes of the QDs in the solution tend to be larger than the critical value15,16,17, which leads to smaller QDs growing faster than the larger ones, referred to as the focusing distribution phase33. Both the Stokes shift and the FWHM exhibit a similar trend, showing that the correlation between the size distribution and the Stokes shift can be strong. These results from the optical properties indicate that the high quality of the prepared ZnSe QDs is attributable to the favorable balance between nucleation and growth, which results in a narrow size distribution and good surface passivation of the ZnSe QDs.
Morphology and crystal structure analysis
The morphology and crystal structure of the prepared ZnSe QDs were examined by TEM image shown in Fig. 4a–c. The samples of ZnSe QDs exhibit a spherical shape; however, the limited contrast in the TEM images poses a challenge in characterizing their dispersion. High-magnification TEM images of a particular QD clearly show lattice fringes throughout the whole QD, indicating that ZnSe QDs with highly crystalline structures were obtained34,35. To identify the structure type of the ZnSe QDs, the line profiles were analyzed using the Gatan Digital Micrograph software36 by following the line directions in the high-magnification TEM images. The interplanar spacing measurement (Fig. 4d–f) indicated an average value of 0.32 nm, which corresponds to the d-spacing of the (111) lattice plane of the zinc blende crystal structure37,38. The size of the ZnSe QDs was investigated based on TEM images using the ImageJ software39. The histograms showing the size distribution of the ZnSe QDs are presented in Fig. 4g–i. We determined the average QD sizes by fitting a Weibull distribution, which resulted in values of 3.69, 4.06, and 4.53 nm corresponding to growth times of 1, 10, and 60 min, respectively. These QD sizes are substantially smaller than the ZnSe Bohr diameter (~ 7.6 nm)40,41, which we expected because their absorption and PL peak positions are shifted to higher energies than the bulk bandgap as a result of the quantum confinement effect.
Discussion
The optical bandgap energy (Eopt) of the ZnSe QDs can be obtained from the absorption spectra and Tauc’s expression41,42, which is given by Eq. (1):
where α is the absorption coefficient, h is Planck’s constant, ν is the photon frequency, A is a constant, and n is an index that takes the value of 2 for direct transition and 1/2 for indirect transition41,43. Therefore, the ZnSe QDs are a direct-bandgap material44. Equation (1) then becomes
Figure 5a shows the plot of \(\left( {\alpha h\nu } \right)^{2}\) as a function of the photon energy (\(hv\)) for the prepared ZnSe QDs. The optical bandgap energies were determined by extrapolating the linear part of the plot (dashed line) to the intersection with the x-axis. The obtained Eopt values are 3.05, 3.02, 2.96, 2.95, 2.93, and 2.92 eV for reaction times of 0.5, 1, 5, 10, 30, and 60 min, respectively. The optical bandgap energies decrease with increasing QD size, consistent with the quantum size effect.
Numerous theoretical models have been proposed to quantitatively correlate the expected relationship between bandgap energy (Eg) and QD size41. One of the most widely used theoretical models that enables the derivation of a relatively simple analytical formula for the dependence of Eg on the QDs’ size is the Brus model45. According to this model, the bandgap of a semiconducting nanocrystal assumed to be a sphere with diameter D is given by
where Ebulk is the bulk crystal bandgap value, h is Planck’s constant, and \( m_{{\text{e}}}^{*}\) and \(m_{{\text{h}}}^{*}\) are the electron and hole relative effective masses, respectively46. Figure 5b shows a plot of the optical bandgap energies against the corresponding size estimated by TEM analysis and the fitting curve (dashed line) based on Eq. (3). The fitting line agrees well with the analysis data of the prepared ZnSe QDs. Moreover, the Ebulk value obtained from the fitting procedure is ~ 2.71 eV, which is similar to the bandgap of bulk ZnSe. Thus, the theoretical Brus model considered here is in agreement with the experimental observation of the size dependence of ZnSe QDs on the confinement energy.
In summary, high-quality ZnSe QDs with a 1:1 ratio were successfully prepared by the hot-injection method. The resultant samples emitted blue PL with peak wavelengths ranging from 410 to 427 nm and a narrow FWHM. There was no evidence of defect emission, indicating effective surface passivation of the QDs. TEM images revealed that the synthesized ZnSe QDs had a zinc blende structure and spherical shape, with diameters ranging from 3.69 to 4.53 nm. The Brus model was used to establish a relationship between the ZnSe QD sizes and the optical bandgaps obtained from Tauc plots.
Methods
Materials
ZnSt2 (technical grade), Se powder (Se, 99.99%), 1-octadecene (ODE, technical grade, 90%), trioctylphosphine (TOP, technical grade, 90%), and ODA (≥ 99%) were purchased from Sigma-Aldrich. Chloroform (99.5%), and methanol (99.5%) were purchased from Daejung. All chemicals were used without further purification.
Synthesis of colloidal ZnSe QDs
The synthesis of ZnSe QDs was similar to the method reported previously10, with some modifications. In a typical synthesis, a three-neck flask connected to a Schlenk line was loaded with a mixture of 0.19 g (0.3 mmol) ZnSt2 and 10 mL ODE. The mixture was evacuated at room temperature for 10 min, then heated at 90 °C for 60 min under vacuum conditions. With the mixture under flowing N2 gas, the temperature was then raised to 318 °C. A Se solution containing 0.04 g (0.3 mmol) Se powder, 0.15 g ODA, 0.5 mL TOP, and 1 mL ODE was rapidly injected into the mixture at 318 °C, and the temperature was maintained at 300 °C for growth of the QDs. After the injection, the colorless solution gradually turned yellow and a small aliquot was extracted from the flask at various intervals. The as-synthesized QDs were mixed with an excess of methanol, followed by centrifugation. After the upper liquid was decanted, chloroform was added to dilute the precipitate.
Measurement techniques
The optical properties of each sample were recorded at room temperature using a FLAME-S spectrometer (Ocean Optics, Largo, FL, USA). The samples were analyzed using a TEM (JEM-ARM200F, JEOL) installed in the Center for University-wide Research Facilities at Jeonbuk National University. Samples for TEM were prepared by dipping carbon-coated TEM grids into the QD solutions and then allowing them dry under ambient air for several days. The size of the QDs was determined using ImageJ software (version 1.54g, https://imagej.net/ij/). The crystal profiles were provided using the Gatan Digital Micrograph software (version 3.6, https://www.gatan.com).
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Chang, J. & Waclawik, E. R. Colloidal semiconductor nanocrystals: Controlled synthesis and surface chemistry in organic media. RSC Adv. 4(45), 23505–23527 (2014).
Baum, F., Pretto, T., Köche, A. & Santos, M. J. L. Machine learning tools to predict hot injection syntheses outcomes for II-VI and IV-VI quantum dots. J. Phys. Chem. C 124(44), 24298–24305 (2020).
Aboulaich, A. et al. One-pot noninjection route to CdS quantum dots via hydrothermal synthesis. ACS Appl. Mater. Interfaces 4(5), 2561–2569 (2012).
Pradhan, N., Goorskey, D., Thessing, J. & Peng, X. An alternative of CdSe nanocrystal emitters: Pure and tunable impurity emissions in ZnSe nanocrystals. J. Am. Chem. Soc. 127(50), 17586–17587 (2005).
Moura, I. M. R. et al. Highly fluorescent positively charged ZnSe quantum dots for bioimaging. J. Lumin. 201, 284–289 (2018).
Byambasuren, N. et al. Phosphine-free-synthesized ZnSe/ZnS core/shell quantum dots for white light-emitting diodes. Appl. Sci. 11(21), 10060 (2021).
Molahossieni, E., Molaei, M., Karimipour, M. & Amirian, F. ZnSe:Cu/ZnS: Cu quantum dots (QDs): Synthesis via a simple UV-assisted approach and investigating optical properties. J. Mater. Sci. Mater. Electron. 31(1), 387–393 (2020).
Andrade, J. J., Brasil, A. G., Farias, P. M. A., Fontes, A. & Santos, B. S. Synthesis and characterization of blue emitting ZnSe quantum dots. Microelectron. J 40(3), 641–643 (2009).
Hines, M. A. & Guyot-Sionnest, P. Bright UV-blue luminescent colloidal ZnSe nanocrystals. J. Phys. Chem. B 102(19), 3655–3657 (1998).
Gul, S. et al. Synthesis, optical and structural properties, and charge carrier dynamics of Cu-doped ZnSe nanocrystals. J. Phys. Chem. C 115(43), 20864–20875 (2011).
Kim, D. J. & Koo, K. K. Synthesis of colloidal ZnSe nanospheres by ultrasonic-assisted aerosol spray pyrolysis. Crystal Growth Des. 9(2), 1153–1157 (2009).
Munro, A. M. Synthetic approaches for growing zinc sulfide and zinc selenide colloidal nanocrystals. J. Vac. Sci. Technol. A 38, 2 (2020).
Deshpande, A. C., Singh, S. B., Abyaneh, M. K., Pasricha, R. & Kulkarni, S. K. Low temperature synthesis of ZnSe nanoparticles. Mater. Lett. 62(23), 3803–3805 (2008).
Reiss, P. et al. Luminescent ZnSe nanocrystals of high color purity. Mater. Chem. Phys. 84(1), 10–13 (2004).
Chen, L. et al. Green chemical approaches to ZnSe quantum dots: Preparation, characterisation and formation mechanism. J. Exp. Nanosci. 5(2), 106–117 (2010).
Yu, W. W., Wang, Y. A. & Peng, X. Formation and stability of size-, shape-, and structure-controlled CdTe nanocrystals: Ligand effects on monomers and nanocrystals. Chem. Mater. 15(22), 4300–4308 (2003).
William, Y. W. & Peng, X. Formation of high-quality CdS and other II–VI semiconductor nanocrystals in noncoordinating solvents: Tunable reactivity of monomers**. Angew. Chem. Int. Ed. 41, 13 (2002).
Li, L. S., Pradhan, N., Wang, Y. & Peng, X. High quality ZnSe and ZnS nanocrystals formed by activating zinc carboxylate precursors. Nano Lett. 4(11), 2261–2264 (2004).
Zhang, L. J. et al. Controlled growth of ZnSe nanocrystals by tuning reactivity and amount of zinc precursor. J. Chem. 2013, 1–6 (2013).
Banski, M. et al. Special Role for zinc stearate and octadecene in the synthesis of luminescent ZnSe nanocrystals. Chem. Mater. 27(11), 3797–3800 (2015).
Zhang, Z. H., Chin, W. S. & Vittal, J. J. Water-soluble CdS quantum dots prepared from a refluxing single precursor in aqueous solution. J. Phys. Chem. B 108(48), 18569–18574 (2004).
Ramasamy, P., Kim, N., Kang, Y. S., Ramirez, O. & Lee, J. S. Tunable, bright, and narrow-band luminescence from colloidal indium phosphide quantum dots. Chem. Mater. 29(16), 6893–6899 (2017).
Wang, Y., Wang, C., Xu, S., Wang, Z. & Cui, Y. Cation-inverting-injection: A novel method for synthesis of aqueous ZnSe quantum dots with bright excitionic emission and suppressed trap emission. Nanotechnology 25(29), 295602 (2014).
Bailon-Ruiz, S., Perales-Perez, O., Su, Y. & Xin, Y. One-step synthesis of water-dispersible ZnSe(S)-alloy quantum dots in the presence of thiol species. Curr. Nanosci. 9(1), 117–121 (2013).
Yousefi, R., Azimi, H. R., Mahmoudian, M. R. & Basirun, W. J. The effect of defect emissions on enhancement photocatalytic performance of ZnSe QDs and ZnSe/rGO nanocomposites. Appl. Surf. Sci. 435, 886–893 (2018).
Efros, A. L. et al. Band-edge exciton in quantum dots of semiconductors with a degenerate valence band: Dark and bright exciton states. Phys. Rev. B 54(7), 4843 (1996).
Voznyy, O. et al. Origins of Stokes shift in PbS nanocrystals. Nano Lett. 17(12), 7191–7195 (2017).
Nikesh, V. V. & Mahamuni, S. Highly photoluminescent ZnSe/ZnS quantum dots. Semicond. Sci. Technol. 16(8), 687 (2001).
Puzder, A., Williamson, A. J., Grossman, J. C. & Galli, G. Computational studies of the optical emission of silicon nanocrystals. J. Am. Chem. Soc. 125(9), 2786–2791 (2003).
Cui, Y., Yin, P., Cui, X., Wu, D. & Yang, M. Stokes shifts of small ZnSe clusters from first-principles calculations. Mol. Phys. 115(24), 3192–3198 (2017).
Reynolds, R. A., Stramski, D., Wright, V. M. & Woźniak, S. B. Measurements and characterization of particle size distributions in coastal waters. J. Geophys. Res. Oceans 115, C08024 (2010).
Mutavdžić, D. et al. Determination of the size of quantum dots by fluorescence spectroscopy. Analyst 136(11), 2391–2396 (2011).
Kwon, S. G. & Hyeon, T. Formation mechanisms of uniform nanocrystals via hot-injection and heat-up methods. Small 7(19), 2685–2702 (2011).
Mirnajafizadeh, F. et al. Hydrothermal synthesis of highly luminescent blue-emitting ZnSe(S) quantum dots exhibiting low toxicity. Mater. Sci. Eng. C 64, 167–172 (2016).
Wang, A. et al. Bright, efficient, and color-stable violet ZnSe-based quantum dot light-emitting diodes. Nanoscale 7(7), 2951–2959 (2015).
Kim, S. H., Shin, T., Man, M. T. & Lee, H. S. Size-dependent energy spacing and surface defects of CdSe quantum dots in strong confinement regime. Appl. Nanosci. 12(11), 3297–3302 (2022).
Lan, G. Y., Lin, Y. W., Huang, Y. F. & Chang, H. T. Photo-assisted synthesis of highly fluorescent ZnSe(S) quantum dots in aqueous solution. J. Mater. Chem. 17(25), 2661–2666 (2007).
Seo, H. et al. Highly qualified InP based QDs through a temperature controlled ZnSe shell coating process and their DFT calculations. Nanoscale Adv. 2(12), 5615–5622 (2020).
Collins, T. J. ImageJ for microscopy. BioTechniques 43, 25–30 (2007).
Eilers, J., Van Hest, J., Meijerink, A. & Donega, C. D. M. Unravelling the size and temperature dependence of exciton lifetimes in colloidal ZnSe quantum dots. J. Phys. Chem. C 118(40), 23313–23319 (2014).
Zedan, I. T., Azab, A. A. & El-Menyawy, E. M. Structural, morphological and optical properties of ZnSe quantum dot thin films. Spectrochim. Acta A Mol. Biomol. Spectrosc. 154, 171–176 (2016).
Kim, J. S., Kim, S. H. & Lee, H. S. Energy spacing and sub-band modulation of Cu doped ZnSe quantum dots. J. Alloys Compd. 914, 165372 (2022).
Surana, K., Singh, P. K., Rhee, H. W. & Bhattacharya, B. Synthesis, characterization and application of CdSe quantum dots. J. Ind. Eng. Chem. 20(6), 4188–4193 (2014).
Senthilkumar, K., Kalaivani, T., Kanagesan, S. & Balasubramanian, V. Synthesis and characterization studies of ZnSe quantum dots. J. Mater. Sci. Mater. Electron. 23(11), 2048–2052 (2012).
Einevoll, G. T. Confinement of excitons in quantum dots. Phys. Rev. B 45(7), 3410 (1992).
Pejova, B. & Grozdanov, I. Three-dimensional confinement effects in semiconducting zinc selenide quantum dots deposited in thin-film form. Mater. Chem. Phys. 90(1), 35–46 (2005).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2021R1A2C1003074, 2022R1A4A1033358), the KIST Institutional Program (2E33323), and the research funds of Jeonbuk National University in 2022.
Author information
Authors and Affiliations
Contributions
H.S.L. conceived the experiments, D.H.N. and S.H.K. conducted the experiments, D.H.N., S.H.K., J.S.L., D.S.L. and H.S.L. analyzed the results. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nguyen, D.H., Kim, S.H., Lee, J.S. et al. Reaction-dependent optical behavior and theoretical perspectives of colloidal ZnSe quantum dots. Sci Rep 14, 13982 (2024). https://doi.org/10.1038/s41598-024-64995-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64995-5
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.