Abstract
This study aims to use a static-based solubility method for measuring the solubility of lumiracoxib at a temperature of 308–338 K and pressure of 120–400 bar for the first time. The obtained solubility data for lumiracoxib is between 4.74 × 10−5 and 3.46 × 10−4 (mole fraction) for the studied ranges of pressure and temperature. The solubility values reveal that the lumiracoxib experiences a crossover pressure of about 160 bar. Moreover, the measured solubility data of these two drugs are correlated with density-based semi-empirical correlations namely Bartle et al., Mendez-Santiago-Teja, Kumar and Johnstone, Chrastil and modified Chrastil models with an average absolute relative deviation of 10.7%, 9.5%, 9.8%, 7.8%, and 8.7% respectively for lumiracoxib. According to these findings, it is obvious that all of the examined models are rather accurate and there is no superiority between these models for both examined drugs although the Chrastil model is slightly better in the overall view.
Similar content being viewed by others
Introduction
It is well established that it is possible to increase the rate of dissolution for poorly soluble drugs by reducing the particle size (according to the dissolution equation). In other words, based on the dissolution equation derived from the film theory, the surface area, concentration gradient across the diffusion layer and the solubility of a compound are effective parameters on the dissolution rate. In general, micronizing the drug particles enhances the dissolution rate since the available surface area to the dissolving medium increases. Respecting this fact, extensive researches were performed on the micronization methods to find suitable methods without manipulating the physiochemical properties of the drug particles1,2,3,4,5,6,7. Among the possible micronization methods, supercritical fluid technologies (SFT) especially those that use supercritical carbon dioxide (SC-CO2) as the main solvent gain increasing attention for producing nano and micron-size particles with controlled morphology8.
The SC-CO2-based technologies are desired due to their undeniable advantages, such as being environmentally friendly, being nontoxic and producing low toxic wastes, low impact on the particle structure, high final quality, etc. In light of these unique advantages, supercritical fluid-based technologies are on the main radar of scientists for different applications of recycling valuable components from waste materials and streams such as batteries9,10, extraction of oil seeds such as portulaca oleracea and dracocephalum kotschyi11,12, measuring the solubility of drugs13,14,15,16, and producing nanoparticle13,17,18.
In the shadow of unique features, using SC-CO2 for micronization of drug particles in particular using SC-CO2 as the main solvent gained the attention of scientists to fabricate the nano and micron size particles using RESS which is rapid expansion of supercritical solution17,18,19,20,21,22,23, gas anti-solvent (GAS) which is gas anti-solvent, supercritical anti-solvent (SAS), etc24,25,26,27,28,29.
For more information and clarification, Sodeifian and Usefi13 provided a valuable review regarding the application of SC-CO2 for measuring the solubility of drugs, extracting the essential oil and seed oils besides the fabrication of nanoparticles using SC- CO2 as solvent and anti-solvent.
Although these methods are of great interest, one of the main common characteristics that dictate the successfulness of these methods is the substance solubility in SCF which in the case of pharmaceutical compounds, the desired fluid is carbon dioxide (CO2). So, it is highly important to determine the solubility of the model drug at different pressures and temperatures to conclude if the proposed micronization methods would be successful or not. On the other side, knowing the solubility of the compound in the SC-CO2 directly affects the size of the equipment which is highly required for economic issues. Respecting this importance, the solubility of severe al drugs and pharmaceuticals was measured at different temperatures and pressures with other researchers18,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49.
According to the measured and published solubility data, one can observe that concomitant with experimental solubility measurement, it is highly beneficial if one can use the modeling approaches to correlate/interpolate/extrapolate the solubility data. In this way, different approaches such as using equations of state (EoSs)50,51,52,53,54, artificial neural network-based methods55,56,57, and semi-empirical density-based correlations are proposed and used58,59,60,61,62,63.
Among these possible modeling approaches, EoSs suffer some critical shortcomings if they are used for solubility prediction and modeling purposes. In detail, these models are highly dependent on the parameters including sublimation pressure, critical pressure and, temperature of the solute which their experimental values are not available. So, the users must use the predicted and estimated values of these critical parameters to introduce some intrinsic deviation into the accuracy of the predicted solubility data. As a way to eliminate these shortcomings, researchers are widely concentrated on the application of new types of predictive approaches usually called semi-empirical density-based correlations58,59,60,61,62,63.
These methods are highly applicable since they use thermodynamic parameters (pressure and temperature) and solution density at the specific pressure and temperature which one can measure these parameters with high accuracy. For example, Pishnamazi et al.64 and Zabihi et al.65 measured the decitabine and temozolomide solubility at different pressures and temperatures. After that, they correlated the measured values with maximum AARD% of 9% using semi-empirical density-based correlations which is highly acceptable for such simple correlations although one of the examined correlations namely Garlapati and Madras led to the worst ARRD% of 15.20% for temozolomide solubility.
On the other hand, further examinations revealed that using semi-empirical density-based correlations provides the chance of predicting and estimating the solubility data out of the examined ranges of temperature and pressure if the linear pattern of the used models is justified during the self-consistency test. In other words, if an acceptable level of linearity is observed for the measured solubility data, it is possible to use these models for the thermodynamic conditions that are out of the examined ranges with proper care. Among the different possible families of drugs that can be used to reduce their particle size, nonsteroidal anti-inflammatory drugs (NSAIDs) are among the most studied families due to their wide applications in relief of pain66.
Unfortunately, although this family is highly prescribed as a medication around the world, they have their undesired side effects. In detail, NSAIDs are a family of drugs mainly prescribed to reduce the inflammation and even pain that comes with arthritis and other musculoskeletal disorders it is undeniable to face some undesired effects including raised liver enzymes, diarrhea, headache, dizziness, salt and fluid retention, high blood pressure if these medications being used especially in the high dosage form (increase the risk of heart stroke and attack even in the people with healthy condition).
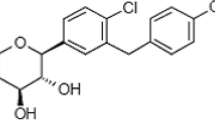
In this way, since there is no report for the solubility of lumiracoxib in the literature, the solubility of these drugs is measured in SC-CO2 at a temperature of 308–338 K and pressure of 120–400 bar for the first time. Lumiracoxib is a highly selective COX2 known as non-selective non-steroidal anti-inflammatory drugs (NSAIDs) which is more effective than placebo for pain relief in osteoarthritis (OA) and rheumatoid arthritis (RA), and acute pain with similar analgesic and anti-inflammatory impacts as non-selective NSAIDs and the selective COX2 inhibitor celecoxib. Besides, lumiracoxib has a lower incidence of upper gastrointestinal (GI) side effects in patients if they do not take aspirin, and a similar cardiovascular (CV) side effect similar to naproxen or ibuprofen. In this way, it seems highly effective if the size of the lumiracoxib particles is reduces for lower dosage usage for treating the diseases and relieving the pains.
Besides, the measured solubility data are modeled using four semi-empirical density based correlations namely Bartle et al., Mendez-Santiago-Teja (MST), Kumara and Johnstone (KJ), Chrastil and modified Chrastil models to find if they can accurately correlate the solubility data. Moreover, the self-consistency test is performed to know if they can extrapolate the solubility of these two drugs in SC- CO2 as a function of temperature and pressure.
Experimental procedure
Model drug (nimesulide and lumiracoxib with molecular masses of 308.31 and 293.72 g/mol) was purchased from Cayman Chemical, USA with purity better than 95% (HPLC analysis). The received drugs were further purified under pressure and temperature of 450 bar and 338 K, respectively for more than 2 h to ensure the elimination of impurities existed in the drug powder since there is this possibility that impurities being dissolved in SC-CO2 during the experimental measurements consequently leading to undesired inaccuracies. In detail, because of using the gravimetric-based method in this work, removing the impurities enhances the accuracy of the measurements. Also, CO2 with a purity better than 99% was provided from Dubai Industrial Gases, Sharjah, UAE (see Table 1).
Laboratory apparatus
A high-pressure visual chamber (volume of 400 cm3) (Apex Technologies Co. Arak, Iran) equipped with an internal magnetic mixer was used to measure the lumiracoxib solubility. The system also includes a high-pressure-low temperature liquefaction section (with a maximum working pressure of 200 bar and minimum temperature of − 20 °C) that allows the operator to change the gaseous CO2 into a liquid state before being pressurized to a desired value using an air-driven water-free non-lubricating reciprocating pump (Haskel, USA) (see Fig. 1).
The used apparatus14.
As aforementioned, the used PVT cell utilizes a magnetic mixer making it possible to reach the equilibrium condition inside the main measuring chamber. The PVT cell is generally designed and rated for maximum pressures of 600 bar (Keller pressure transmitter with accuracy = ± 35 kPa) and temperature of 426 K (with accuracy of ± 0.1 K). The used equipment is equipped with two needles and micro-metering vales for sudden depressurizing and fine controlling of the CO2inlet and outlet if required. The noteworthy point is that sudden depressurizing is required to prevent the deposition of drug particles in the main chamber. The point that must be mentioned is that the used PVT cell is an embedded pump-type visual PVT cell capable of the operator controlling the pressure without using the usual back pressure regulator. In other words, since the PVT cell is equipped with an automatic embedded pump, it is possible to control the pressure at a desired value with an acceptable level of accuracy without using a back pressure regulator to control the inside pressure of the main measuring chamber. In the first stage of the used procedure, the gaseous CO2 turns to a liquid state if it passes through a refrigeration system and is then pressurized to a desired pressure using a pneumatic high-pressure Haskel pump (USA). Before entering the pressurized CO2 into the main measuring chamber, the pressurized CO2 was delivered into a surge tank where the pressure fluctuations were dampened and the temperature was elevated.
Now the pressurized CO2 is ready to be transferred into the main measuring chamber which was filled with compacted drug (10 g) placed in the containment vial and glass wool. After reaching the desired pressure and temperature, the system was allowed for 3 h to reach the equilibrium while the magnetic mixer was slowly working to agitate the internal contents of the main PVT cell. This gentle agitation is required to reduce the dead volumes inside the main chamber and faster equilibrium. After 3 h, the system was suddenly depressurized and the wrapped sample was removed to be weighed. Since the weight of whole assembly (glass wool, tissue and, compacted drug) is known, it is the weight difference between the starting point and final point of the whole assembly can be considered as the dissolved amount of lumiracoxib during the measurement.
The accuracy and efficiency of the apparatus and experimental procedure were examined and validated by measuring the nimesulide solubility in a known range of temperature and different pressures as reported previously by Macnaughton et al.48 (see Table 2).
At this point, it is possible to calculate the solubility of drug using the following equation (Eq. 1) and using the density of CO2at different pressures and temperatures reported by Fat’hi et al.67.
The noteworthy point is that each reported solubility data was the average of at least three independent measurements due to the existence of uncertainties coming from the pressure and temperature fluctuations, working with high-pressure equipment, etc.
Genetic algorithm
Over the years, different computer-based methods have been proposed and examined to simulate and model various chemical phenomena68,69,70,71. Among these methods utilized for prediction, estimation, and correlation, genetic algorithms (GAs) proposed by John Holland in the 60s are among the most widely used techniques as a computational analogy of adaptive systems72. GAs that work with selection in the presence of variation-inducing operators such as mutation and recombination (crossover) are correlated to evolution via natural selection, and using individual populations. The main principle of GAs is exactly the way that mother nature uses to survive according to the principles proposed by Charles Darwin in the first attempts.
These unique features of GA put this method on the list of methods that are flexible and capable of correlating any system with considerable complexities including engineering fields that are dealing with complicated experimentations and measurements.
In detail, using GAs not only gives this chance to solve the problem with new insight, but it consistently outperforms other traditional methods in most of the problems linked especially the problems dealing with finding optimal parameters, which might introduce several difficulties to the conventional methods. The point is that this is its high capability and performance in optimization which put the GAs in the wrong way as an optimizer which is not fair enough to consider this high potential method only as an optimizer.
In general, the GA algorithm can be described as follow:
-
Random generation of initial population
-
Calculating and saving the obtained results using the initial values
-
Defining selection probabilities for each individual
-
Generating the next set of values by probabilistically selecting individuals to produce offspring via genetic operators.
-
Successive repeating of step 2 to reach the desired outcomes.
The point is that in the way of optimizing the fitting parameters, it is highly required to use some statistical parameters such as Average absolute relative deviation (AARD%), average relative deviation (ARD%), mean square error (MSE), and correlation coefficient (R2) to find the best sets of fitting parameters.
where N, yexpi, ycali, and y are the number of solubility data points, the ith experimental value of the solubility, the i th solubility data predicted with the GA model, and the average value of the experimental solubility data. In details, to optimize the fitting parameters for any correlation or equation, a trial and error approach must be used to reach the optimum fitting parameters leading to the best MSE, AARD%, etc. values.
For this purpose, the population size was randomly varied between 20 and 100 and the stopping criteria called generation was varied between 100 and 1000, while the ranges of the three fitting parameters which must be optimized were considered between some known values at the beginning of the optimization process. After this stage, several optimized values for each fitting parameter were achieved. Then, in the next stage, the population size and generation were again changed by trial and error approach while in this point the ranges of the fitting parameters were considered between the highest and the lowest values of each fitting parameter obtained in the previous stage. This kind of procedure was performed till a desired deviation was achieved for the objective functions including MSE and AARD%.
EoS modelling approach
In this study, the semi-empirical density-based correlations and EoS approach were used to model the measured solubility data. In this way, Esmaeilzadeh-Roshanfekr (ER) EoS along with the vdW2 mixing rule was used to measure and correlate the measured solubility data for lumiracoxib. This EoS which is developed by Esmaeilzadeh and Roshanfekr73 is a modified version of the cubic EoS models (Eq. 6) which its details are given elsewhere.
where R is the universal gas constant, “a” is a function of temperature and “b” and “c” are constants.
The used vdW2 mixing rule74 is as below:
The point must be mentioned is that the ER method parameters were optimized using the differential evolution (DE) method. This method is mainly based on Darwin’s theory of evolution and has been studied extensively to solve different areas of optimization and engineering applications. This method which was first proposed by Storn75 implements mutation, crossover, and selection as operators in its structure.
In detail, this method is a stochastic approach that simulates biological evolution. So, in the light of repeated iterations, those individuals that are adapted to the environment are preserved. However, compared with other evolutionary algorithms, DE retains the global search strategy based on population, adopts real number coding, simple mutation operation based on difference, and a one-to-one competitive survival strategy, which reduces the complexity of a genetic operation.
A close look into the optimization procedure of the DE algorithm shows that although a high similarity to the genetic algorithm (GA) approach with three main steps of mutation, crossover, and selection, the specific definitions of these operations are different from GA. In general, the DE algorithm utilizes a randomly generated initial group, uses the difference vector of two individuals randomly selected from the population as the source of the random change of the third individual, and weights the difference vector according to certain rules. After that, summing with a third individual creates a new individual which is generally called a mutation. Then, the mutant individual is mixed with a predetermined target individual to generate a test individually, and this process is called crossover. If the fitness value of the test individual is better than the fitness value of the target individual, the test individual will replace the target individual in the next generation, otherwise, the target individual will still be preserved, and this operation is called selection. In the evolution process of each generation, each vector is used as the target individual once, and the algorithm keeps good individuals and eliminates inferior individuals through continuous iterative calculation, and guides the search process to approach the global optimal solution76.
Results and discussions
In the present experimental study, the main target is set to obtain the NSAID drug solubility namely lumiracoxib using a simple gravimetric-based model using variable volume PVT equipment in the pressure range of 120–400 bar and temperature range of 308–338 K. In the first stage, the used method was validated using the measurement of nimesulide as the sample drug in a specific temperature and pressure reported by Macnaughton et al.48. The solubility values for nimesulide showed an acceptable level of consistency for the measured solubility data although comparing the results reported by Macnaughton et al.48 and those measured in the current investigation revealed a systematic higher measured solubility data with AARD% of 9.16% which is an acceptable level of deviation for the used solubility measurement method. After that, the solubility of lumiracoxib was measured from 120 to 400 bar and 308 to 338 K using the validated equipment. The measured data revealed the solubility of lumiracoxib between 4.74×10−5 and 3.46×10−4 based on the mole fraction (see Fig. 2). The measured solubility data reveal that the solubility of lumiracoxib experiences a crossover pressure of about 160 bar is the accuracy of the measurements77. A close look into the results tabulated in Table 3 which each reported data is the average of triplicate independent measurements (with a maximum relative standard deviation percent of 8.69 % coming from pressure and temperature fluctuations and the intrinsic nature of working with high-pressure high-temperature process) revealed that as the pressure was increased between 120 and 400 bar, the solubility experienced an increase for the entire range of temperature. However, with respect to the temperature influence, the situation was a bit complex because of two competing factors namely density reduction and sublimation pressure modification. The worth mentioning point is that the main limitation of the current method is directly correlated to its principals which is weight difference. In detail, since the weight difference is directly used for calculating the solubility of the drugs, it is impossible to use this method for thermodynamic conditions with low solubility probabilities such as pressures lower than 120 bar or temperatures higher than 65 °C as the solvating power of the SC-CO2 reduces. The other limitation of the used method is directly correlated to the solubility of the drugs which is mostly related to the molecular weight of the drugs. In detail, the current method is not suitable to be used for the drugs with high molecular weight since they intrinsically have low solubility in SC-CO2 in the absence of co-solvent making it impossible to some extent to be used for solubility measurements.
In detail, as the temperature increases, two independent variables change which can act in different ways with different effects on the substance solubility in the SC-CO2. In detail, increasing the temperature enhances the molecular movement and consequently causes more free movement of the molecules leading to lower density which is directly correlated to the solvating power of any solvent. In this way, as the density reduces, the solvating power experiences a reduction which means a lower amount of substance would be dissolved in the solvent. Besides, sublimation pressure modification due to temperature enhancement led to higher dissolution of drugs in the SC-CO2. So this is the net effect of these two factors dictates the solubility enhances or reduces and the point where this is the cumulative effect of these two competing factors changes called crossover pressure. According to these facts, it seems that there is a crossover pressure of about 160 bar for the lumiracoxib where the solubility-reducing effect of density reduction can be compensated with the sublimation pressure change causing better dissolution of substance in the SC-CO2.
Solubility modelling using semi-empirical density based correlations
The lumiracoxib solubility data were correlated with density-based correlations namely Chrastil, Bartle et al., KJ, and MST models which are well-known because of their acceptable level of accuracy and simplicity needing only simple multiple linear regression (see Table 4). As it is obvious in Table 4, all of the used correlations were rather the same considering the calculated AARD% and there is no superiority between these models for the examined drugs although the Chrastil model is slightly better in an overall view. The noteworthy point is that although the level of accuracy was rather the same, each correlation has its advantages regarding the calculation of specific characteristics of the binary mixture.
In detail, the first used model is Chrastil model which comprised of three fitting parameters a, b and c can be calculated using multiple-linear regression (see Eq. 13).
On the other side, there are known and specific parameters including s and ρ which are the solubility and the density of CO2 at the experimental absolute T and p. The point is that the theoretical background of this correlation is based on a well-known theory called association theory. This theory deals with the concept that each solute molecule is surrounded by c molecules of solvent in a mixture. According to its basics and concepts, it is possible to estimate two important parameters namely the enthalpies of vaporization and solvation using the fitting parameters of a which is equal to ΔHtotal/R, where ΔHtotal is the sum of enthalpies of vaporization and solvation of the solute and R is the universal ideal gas constant. According to this information, the measured solubility data and performed modeling using the Chrastil model revealed that the total and solvation enthalpies for lumiracoxib are 26.31 and 210.3 kJ/mol, respectively (see Fig. 3).
In the next phase of this section, the modified Chrastil method78 was used to find the potential of the Chrastil model compared with the modified Chrastil model. In contrast to the other used correlations in the current investigation which required a multiple linear regression approach to obtain the fitting parameters, the modified Chrastil model fitting parameters were r calculated and optimized using the genetic algorithm approach previously described and discussed.
where f is a known reference state and it may be chosen as unity or critical pressure of the SF or any other known value, R is the universal gas constant, T is the temperature in Kelvin, D is the density, y2 is the solubility, and the c, a, and b are the fitting parameters must be optimized using the optimization approaches which the genetic algorithm was used in the current investigation. Using the optimized fitting parameters which are c = 4.61, a = − 30.174, and b = − 2000 leading to solubility calculation with AARD% of 8.7% which is slightly higher than the Chrastil model 7.8%. In other words, there is no superiority between the modified Chrastil model and the Chrastil model for the lumiracoxib solubility measured in the current investigation. In other words, although calculating the fitting parameters of the modified Chrastil model required a more complicated optimization procedure than the Chrastil model only required two linear regressions, the method was not capable of providing better AARD% during the solubility prediction stage.
The next examined correlation is the one proposed by Méndez-Santiago60 in 1999 and is among the most widely used and accurate correlations using only three fitting parameters. This model is basically based on a model that needs sublimation pressure which is impossible in most cases to measure experimentally. So, the general form was modified with the assistance of Clausius − Clapeyron expression to the following form which requires no sublimation pressure information.
In the above equation, pref refers to the standard pressure of 0.1 MPa, y refers to the solubility and a, b, and c are the fitting parameters cab neb calculated using multiple linear regressions (see Fig. 4).
The worth mentioning point is that the performed self-consistency test using MST mode (see Fig. 5) revealed the correlative and extrapolative capabilities of the MST model through the 160–400 bar and 308–338 K for pressure and temperature, respectively, or out of these examined ranges. The reason behind this claim is that the performed self-consistency revealed that the measured solubility data form a linear pattern during the self-consistency test even for densities out of the examined range which means the extrapolative capability of the examined correlation.
The Bartle et al.63 model was the third examined correlative model used to predict/correlate the solubility of lumiracoxib in SC-CO2 in wide ranges of temperature and pressure similar to the previously used correlations has three fitting parameters must be calculated using multiple linear regression approach (see Eq. 16 and Fig. 6).
In the above equation, y, pref , p, ρ, and ρref refer to the solubility of lumiracoxib based on the mole fraction, the reference pressure of 0.1 MPa, the CO2 density, the reference density of 700 kg m−363.
One of the most important advantages of the current method is its lower sensitivity to the density variation as a function of pressure and temperature using ρref. Similar to the other examined correlations, it is required to use multiple linear regression steps to obtain the fitting parameters. In detail, for the first stage, the ln(y·p/Pref) values must be depicted vs density to find a straight line. At this point, this is the slope of the straight line must be used for the next stage of regression. In the ideal cases, the slope of all the examined isotherms must be the same. But, in the real situation, different slopes would be calculated for each isotherm. Respecting this fact, it is the average of the slopes of different lines (for different isotherms) must be used for the next regression step. So, after calculating the average of four slopes, a and b parameters can be obtained using the last linear data regression. Similar to the Chrastil model, the Bartle et al.63 model can be used to estimate the vaporization enthalpy, ΔHtotal, as follows: -Rb and then it the heat of solvation can be calculated based on Hess’s law.
The last used correlations is KJ59 (Eq. 10) model which can be utilized to correlate the solubility of substance using density of SC-CO2.
where y refers to the solubility of solute in SC-CO2, T refers to temperature, ρ refers to the density of supercritical CO2 at specific pressure and temperature while a, b and c are the fitting parameters can be calculated using multiple regression.
In the last stage of this investigation, the measured solubility data were compared with those reported in different literature to find if there is any correlation between the physiochemical properties of the drugs and their solubility in SC- CO2. In this way, the solubility of 9 drugs was tabulated in Table 5 and compared with the solubility data obtained for lumiracoxib in the pressure and temperature ranges of 120–400 bar and 308–338 K, respectively. At first glance, there is a direct relation between the drug solubility and the molecular weight. In detail, some of the measured solubility data revealed that as the molecular weight increases, the solubility of the drug in SC- CO2reduces by several orders. However, further investigation revealed that this trend is not a generalized relation for the molecular weight and solubility data. For example, the solubility for amiodarone hydrochloride with a molecular weight of 681.77 is higher than the solubility data measured for esomeprazole with a molecular weight of 345.42 g gmol−1. On the other sides, further examinations showed that the other effective parameter on the solubility of the drug is its structure and the number of rings that existed in its structure. In detail, the results revealed that as the number of the rings increases and the number of branches in the molecular structure reduces; the solubility of the drug reduces as is highly obvious for imatinib mesylate with a molecular weight of 589.71 g gmol−1 with the highest number of rings and the zero number of branches leading to the lowest solubility data of 4.8610−7 to 0.4.0610−6 based on the mole fraction. However, amiodarone hydrochloride with a molecular weight higher than imatinib mesylate but with branches in its structure provides better solubilization in the SC- CO2in the range of 2.510 × 10−5 to 1.012 × 10−3 based on the mole fraction.
According to these findings, it can be concluded that the simple gravimetric method for solubility measurement is not a good candidate for the solids and drugs with the low branches and high rings in its structure due to a very low solubility makes it impossible to measure their solubilities using gravimetric methods. Besides, it can be concluded that using SC-CO2-based technologies for micronizing and reducing the size of the drugs with structures with no branched sections in their structure and a high number of rings is inefficient due to probable low solubility limits even under high pressure of 300 bar which can be considered as one of the limitations of using SC- CO2 for drug particle size and morphology modifications.
EoS approach for solubility modelling
In the last stage of this investigation, the Esmaeilzadeh-Roshanfekr (ER) EoS approach was used to model the measured solubility data (see Table 6). For this purpose, it was necessary to predict the physio-chemical properties of the drug including critical temperature, critical pressure, and acentric factor using the Joback88 and Constantinou and Gani group contribution methods89.
The obtained results revealed that the used EoS was capable of correlating the solubility data with the assistance of the DE approach for optimization of the coefficient with minimum and maximum AARD of 7.86 and 14.28%, respectively. A glance into the results revealed that the used EoS method is as accurate as the used semi-empirical density-based correlations for temperatures of 308.15, 318.15, and 338.15 K, and the highest AARD% was obtained for temperatures of 328.15 K. The point is that the obtained deviations for EoS not only can be correlated to the fluctuations of working with high temperatures and pressures that appear during the measurement stage, but also the other source of deviations in the capability of the used ER method can be correlated to the using the estimated Tc, Pc, and acentric factor since the experimental values are not available. In other words, the accuracy of the EoS method including ER EoS can be improved if the experimental values of Tc, Pc, and ω were inserted into the EoS modeling approaches which are not unfortunately available at the current time.
To sum up, considering the obtained results using two approaches of semi-empirical density-based correlations and EoS, it can be concluded that the semi-empirical density-based correlations are more applicable since they use parameters that their experimental values exist and easily can be measured. However, the EoS approach not only uses estimated necessary physio-chemical properties, but it is also a complex method that requires some special optimization strategies to find the fitting parameters.
Conclusions
The present experimental work is concentrated on the measuring the solubility of lumiracoxib in SC-CO2 in temperatures of 308 to 338K, and pressures of 120 to 400 bar. The solubility data were measured based on a gravimetric method as the main core of the procedure coupled with the static method of solubility measurement using a variable volume PVT cell and gas-booster unit to maintain the desired pressure. In this way, before measuring the solubility of lumiracoxib, the solubility of nimesulide was measured using the proposed gravimetric-based method to ensure about the accuracy and validity of the used experimental procedure and apparatus, respectively.
The solubility data which were between 4.74 × 10−5 and 3.46 × 10−4 based on the mole fraction revealed that the pressure has a direct influence on the enhancement of solubility while the temperature effect is more complex than pressure. In detail, the measurements revealed a changing pressure point called crossover pressure around 160 bar which the effect of temperature turns to an increasing effect while for the pressures below this crossover point the temperature effect is decreasing point. The observed trend was correlated to the interactions between the density reduction and sublimation pressure modification and the cumulative effect of these two factors. After that, the measured solubility data were correlated using density-based correlations all of them use three adjustable parameters which can be calculated with the assistance of the multiple linear regression method. The performed regression approach and calculation the fitting parameters revealed that using those fitting parameters capable the operator to correlate the solubility data with AARD% of 10.7%, 9.5%, 9.8%, 7.8%, and 8.7% for Bartle et al., Mendez-Santiago-Teja (MST), Kumar and Johnstone (KJ), Chrastil, and modified Chrastil models, respectively which are an acceptable level of accuracy. Moreover, according to the self-consistency test performed for the MST model, it can be concluded that not only it is possible to correlate the solubility of lumiracoxib using these semi-empirical correlations but also it is possible to extrapolate the drug solubility for the temperatures and pressures beyond the examined range in the current investigation which is undeniably a significant capability for the examined models. To sum up, it seems that based on the measured solubility data, using SC-CO2-based particle formation technologies is an acceptable approach for producing micron or nano-size particles of lumiracoxib for better efficiency of this drug.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- a:
-
Fitting parameter
- AARD%:
-
Average absolute relative deviation percent
- ARD%:
-
Average relative deviation percent
- b:
-
Fitting parameter
- c:
-
Fitting parameter
- CV:
-
Cardiovascular
- D:
-
Density
- EoS:
-
Equations of state
- GA:
-
Genetic algorithm
- GI:
-
Gastrointestinal
- GAS:
-
Gas anti-solvent
- KJ:
-
Kumar and Johnstone
- MSE:
-
Mean square error
- MST:
-
Mendez-Santiago-Teja
- N:
-
Number of solubility data
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- OA:
-
Osteoarthritis
- P:
-
Pressure
- Pref :
-
Reference pressure, 0.1 MPa
- R:
-
Universal gas constant
- RA:
-
Rheumatoid arthritis
- R2:
-
Correlation coefficient
- s:
-
Solubility, Kg m−3
- SC-CO2 :
-
Supercritical carbon dioxide
- SFT:
-
Supercritical fluid technology
- SAS:
-
Supercritical anti-solvent
- T:
-
Temperature
- yexp i :
-
The ith experimental value of the solubility
- ycal i :
-
The ith solubility data predicted
- ρ:
-
Density, Kg M−3 or Kmol m−3
- ΔHtotal :
-
Sum of enthalpies of vaporization and solvation
- ρref :
-
Reference density equal 700 kg m−3
References
Beckman, E. J. Supercritical and near-critical CO2 in green chemical synthesis and processing. J. Supercrit. Fluids 28, 121–191 (2004).
Jessop, P. G. & Leitner, W. Chemical synthesis using supercritical fluids. (John Wiley & Sons, 2008).
Joshi, J. T. A review on micronization techniques. J. Pharm. Sci. Technol. 3, 651–681 (2011).
Ghamarpoor, R., Fallah, A. & Eghbali, T. Design of bifunctional sandwich-like Co@ Si/Ox-MXene nanocomposite to increase the supercapacitor properties and removal of pollutants from wastewater. J. Alloys Compounds 983, 173920 (2024).
Mansouri Zadeh, M., Amiri, F., Hosseni, S. & Ghamarpoor, R. Synthesis of colloidal silica nanofluid and assessment of its impact on interfacial tension (IFT) and wettability for enhanced oil recovery (EOR). Sci. Rep. 14, 325 (2024).
Ghamarpoor, R., Jamshidi, M. & Mohammadpour, M. Achieving outstanding mechanical/bonding performances by epoxy nanocomposite as concrete–steel rebar adhesive using silane modification of nano SiO2. Sci. Rep. 13, 9157 (2023).
Ghamarpoor, R., Jamshidi, M., Sayyadian, M. & Razavizadeh, M. Chemical/photochemical functionalization of polyethylene terephthalate fabric: Effects on mechanical properties and bonding to nitrile rubber. Sci. Rep. 13, 14533 (2023).
Ghamarpoor, R. & Ebrahimabadi, A. Optimum design of water-based drilling fluid in shale formations in Khangiran oilfields. Prog. Ind. Ecol. Int. J. 13, 42–62 (2019).
Zhang, J. & Azimi, G. Recycling of lithium, cobalt, nickel, and manganese from end-of-life lithium-ion battery of an electric vehicle using supercritical carbon dioxide. Resour. Conserv. Recycl. 187, 106628 (2022).
Zhang, J., Anawati, J. & Azimi, G. Urban mining of terbium, europium, and yttrium from real fluorescent lamp waste using supercritical fluid extraction: Process development and mechanistic investigation. Waste Manag. 139, 168–178 (2022).
Sodeifian, G., Sajadian, S. A. & Ardestani, N. S. Supercritical fluid extraction of omega-3 from Dracocephalum kotschyi seed oil: Process optimization and oil properties. J. Supercrit. Fluids 119, 139–149 (2017).
Sodeifian, G., Ardestani, N. S., Sajadian, S. A. & Moghadamian, K. Properties of Portulaca oleracea seed oil via supercritical fluid extraction: Experimental and optimization. J. Supercrit. Fluids 135, 34–44 (2018).
Sodeifian, G. & Usefi, M. M. B. Solubility, extraction, and nanoparticles production in supercritical carbon dioxide: A mini-review. ChemBioEng Rev. 10, 133–166 (2023).
Zabihi, S. et al. Measuring salsalate solubility in supercritical carbon dioxide: Experimental and thermodynamic modelling. J. Chem. Thermodyn. 152, 106271 (2021).
Pishnamazi, M. et al. Thermodynamic modelling and experimental validation of pharmaceutical solubility in supercritical solvent. J. Mol. Liquids 319, 114120 (2020).
Zabihi, S., Esmaeili-Faraj, S. H., Borousan, F., Hezave, A. Z. & Shirazian, S. Loxoprofen solubility in supercritical carbon dioxide: experimental and modeling approaches. J. Chem. Eng. Data 65, 4613–4620 (2020).
Hezave, A. Z. & Esmaeilzadeh, F. Fabrication of micron level particles of amoxicillin by rapid expansion of supercritical solution. J. Dispers. Sci. Technol. 33, 1419–1428 (2012).
Zeinolabedini Hezave, A. & Esmaeilzadeh, F. Precipitation of micronized piroxicam particles via RESS. J. Dispers. Sci. Technol. 33, 990–999 (2012).
Lee, J. & Teja, A. S. Synthesis of LiFePO4 micro and nanoparticles in supercritical water. Mater. Lett. 60, 2105–2109 (2006).
Zeinolabedini Hezave, A. & Esmaeilzadeh, F. Recrystallization of microparticles of fenoprofen using rapid expansion of supercritical solution. J. Dispers. Sci. Technol. 33, 1106–1115 (2012).
Sodeifian, G. & Sajadian, S. A. Solubility measurement and preparation of nanoparticles of an anticancer drug (Letrozole) using rapid expansion of supercritical solutions with solid cosolvent (RESS-SC). J. Supercrit. Fluids 133, 239–252 (2018).
Sodeifian, G., Sajadian, S. A. & Daneshyan, S. Preparation of Aprepitant nanoparticles (efficient drug for coping with the effects of cancer treatment) by rapid expansion of supercritical solution with solid cosolvent (RESS-SC). J. Supercrit. Fluids 140, 72–84 (2018).
Ghamarpoor, R., Fallah, A., Jamshidi, M. & Salehfekr, S. Using waste silver metal in synthesis of Z-scheme Ag@ WO3-CeO2 heterojunction to increase photodegradation and electrochemical performances. J. Ind. Eng. Chem. 128, 459–471 (2023).
Catchpole, O., Hochmann, S. & Anderson, S. In Process Technology Proceedings. 309–314 (Elsevier).
Dehghani, F. & Foster, N. Dense gas anti-solvent processes for pharmaceutical formulation. Curr. Opin. Solid State Mater. Sci. 7, 363–369 (2003).
Imchalee, R. & Charoenchaitrakool, M. Gas anti-solvent processing of a new sulfamethoxazole− l-malic acid cocrystal. J. Ind. Eng. Chem. 25, 12–15 (2015).
Kurniawansyah, F., Mammucari, R. & Foster, N. R. Polymorphism of curcumin from dense gas antisolvent precipitation. Powder Technol. 305, 748–756 (2017).
Foster, N. R., Kurniawansyah, F., Tandya, A., Delgado, C. & Mammucari, R. Particle processing by dense gas antisolvent precipitation: ARISE scale-up. Chem. Eng. J. 308, 535–543 (2017).
Palakodaty, S. & York, P. Phase behavioral effects on particle formation processes using supercritical fluids. Pharm. Res. 16, 976–985 (1999).
Sodeifian, G. & Sajadian, S. A. Experimental measurement of solubilities of sertraline hydrochloride in supercriticalcarbon dioxide with/without menthol: Data correlation. J. Supercrit. Fluids 149, 79–87 (2019).
Zúñiga-Moreno, A., Arenas-Quevedo, M. G. & Elizalde-Solis, O. Solubility of β-carotene and co-solutes in supercritical carbon dioxide for ternary systems: Experimental data and correlation. J. Supercrit. Fluids 148, 66–73 (2019).
Sodeifian, G., Razmimanesh, F. & Sajadian, S. A. Solubility measurement of a chemotherapeutic agent (Imatinib mesylate) in supercritical carbon dioxide: Assessment of new empirical model. J. Supercrit. Fluids 146, 89–99 (2019).
Shojaee, S. A., Rajaei, H., Hezave, A. Z., Lashkarbolooki, M. & Esmaeilzadeh, F. Experimental solubility measurement of cephalexin in supercritical carbon dioxide. Chem. Ind. Chem. Eng. Q. 20, 387–395 (2014).
Lashkarbolooki, M., Hezave, A. Z., Rahnama, Y., Rajaei, H. & Esmaeilzadeh, F. Solubility of chlorpheniramine maleate in supercritical carbon dioxide. J. Supercrit. Fluids 84, 29–35 (2013).
Lashkarbolooki, M. et al. Solubility of cyproheptadine in supercritical carbon dioxide; experimental and modeling approaches. J. Supercrit. Fluids 84, 13–19 (2013).
Hezave, A. Z., Shahnazar, S., Rajaei, H., Lashkarbolooki, M. & Esmaeilzadeh, F. Solubility of spironolactone in supercritical carbon dioxide: Experimental and modeling approaches. Fluid Phase Equilibria 355, 130–134 (2013).
Shojaee, S. A., Rajaei, H., Hezave, A. Z., Lashkarbolooki, M. & Esmaeilzadeh, F. Experimental investigation and modeling of the solubility of carvedilol in supercritical carbon dioxide. J. Supercrit. Fluids 81, 42–47 (2013).
Shojaee, S. A., Rajaei, H., Hezave, A. Z., Lashkarbolooki, M. & Esmaeilzadeh, F. Experimental measurement and correlation for solubility of piroxicam (a non-steroidal anti-inflammatory drugs (NSAIDs)) in supercritical carbon dioxide. J. Supercrit. Fluids 80, 38–43 (2013).
Shojaee, S. A., Hezave, A. Z., Aftab, S., Lashkarbolooki, M. & Esmaeilzadeh, F. Solubility of gabapentin in supercritical carbon dioxide. J. Supercrit. Fluids 78, 1–6 (2013).
Rajaei, H., Hezave, A. Z., Lashkarbolooki, M. & Esmaeilzadeh, F. Representing experimental solubility of phenylephrine hydrochloride in supercritical carbon dioxide and modeling solute solubility using semi-empirical correlations. J. Supercrit. Fluids 75, 181–186 (2013).
Hezave, A. Z., Rajaei, H., Lashkarbolooki, M. & Esmaeilzadeh, F. Analyzing the solubility of fluoxetine hydrochloride in supercritical carbon dioxide. J. Supercrit. Fluids 73, 57–62 (2013).
Ahmadi Sabegh, M., Rajaei, H., Zeinolabedini Hezave, A. & Esmaeilzadeh, F. Amoxicillin solubility and supercritical carbon dioxide. J. Chem. Eng. Data 57, 2750–2755 (2012).
Hezave, A. Z., Aftab, S. & Esmaeilzadeh, F. Solubility of sulindac in the supercritical carbon dioxide: experimental and modeling approach. J. Supercrit. Fluids 68, 39–44 (2012).
Zeinolabedini Hezave, A. & Esmaeilzadeh, F. Solubility measurement of diclofenac acid in the supercritical CO2. J. Chem. Eng. Data 57, 1659–1664 (2012).
Hezave, A. Z., Mowla, A. & Esmaeilzadeh, F. Cetirizine solubility in supercritical CO2 at different pressures and temperatures. J. Supercrit. Fluids 58, 198–203 (2011).
Gupta, R. B. & Shim, J.-J. Solubility in supercritical carbon dioxide (CRC Press, 2006).
Ashraf-Khorassani, M., Combs, M. T., Taylor, L. T., Schweighardt, F. K. & Mathias, P. S. Solubility study of sulfamethazine and sulfadimethoxine in supercritical carbon dioxide, fluoroform, and subcritical freon 134A. J. Chem. Eng. Data 42, 636–640 (1997).
Macnaughton, S. J. et al. Solubility of anti-inflammatory drugs in supercritical carbon dioxide. J. Chem. Eng. Data 41, 1083–1086 (1996).
Yamini, Y., Hassan, J. & Haghgo, S. Solubilities of some nitrogen-containing drugs in supercritical carbon dioxide. J. Chem. Eng. Data 46, 451–455 (2001).
Peng, D.-Y. & Robinson, D. B. A new two-constant equation of state. Ind. Eng. Chem. Fundam. 15, 59–64 (1976).
Soave, G. Improving the Treatment of Heavy Hydrocarbons by the SRK EOS. Fluid Phase Equilibria 84, 339–342 (1993).
Valderrama, J. O. A generalized Patel-Teja equation of state for polar and nonpolar fluids and their mixtures. J. Chem. Eng. Japan 23, 87–91 (1990).
Dashtizadeh, A., Pazuki, G., Taghikhani, V. & Ghotbi, C. A new two-parameter cubic equation of state for predicting phase behavior of pure compounds and mixtures. Fluid Phase Equilibria 242, 19–28 (2006).
Sayyadian, M., Jamshidi, M., Ghamarpoor, R. & Razavizadeh, M. Silanization of functionalized PET fabric to improve PET-nitrile rubber (NBR) adhesion; effects of functionalization type and silane concentration. Arabian J. Chem. 16, 105098 (2023).
Eslamimanesh, A., Gharagheizi, F., Mohammadi, A. H. & Richon, D. Artificial neural network modeling of solubility of supercritical carbon dioxide in 24 commonly used ionic liquids. Chem. Eng. Sci. 66, 3039–3044 (2011).
Gharagheizi, F., Eslamimanesh, A., Mohammadi, A. H. & Richon, D. Artificial neural network modeling of solubilities of 21 commonly used industrial solid compounds in supercritical carbon dioxide. Ind. Eng. Chem. Res. 50, 221–226 (2011).
Garnier, S., Neau, E., Alessi, P., Cortesi, A. & Kikic, I. Modelling solubility of solids in supercritical fluids using fusion properties. Fluid Phase Equilibria 158, 491–500 (1999).
Chrastil, J. Solubility of solids and liquids in supercritical gases. J. Phys. Chem. 86, 3016–3021 (1982).
Kumar, S. K. & Johnston, K. P. Modelling the solubility of solids in supercritical fluids with density as the independent variable. J. Supercrit. Fluids 1, 15–22 (1988).
Méndez-Santiago, J. & Teja, A. S. The solubility of solids in supercritical fluids. Fluid Phase Equilibria 158, 501–510 (1999).
Garlapati, C. & Madras, G. New empirical expressions to correlate solubilities of solids in supercritical carbon dioxide. Thermochimica Acta 500, 123–127 (2010).
Jouyban, A., Chan, H.-K. & Foster, N. R. Mathematical representation of solute solubility in supercritical carbon dioxide using empirical expressions. J. Supercrit. fluids 24, 19–35 (2002).
Bartle, K., Clifford, A., Jafar, S. & Shilstone, G. Solubilities of solids and liquids of low volatility in supercritical carbon dioxide. J. Phys. Chem. Ref. Data 20, 713–756 (1991).
Pishnamazi, M. et al. Experimental and thermodynamic modeling decitabine anti cancer drug solubility in supercritical carbon dioxide. Sci. Rep. 11, 1–8 (2021).
Zabihi, S. et al. Thermodynamic study on solubility of brain tumor drug in supercritical solvent: Temozolomide case study. J. Mol. Liquids 321, 114926 (2021).
Rostom, A. et al. Prevention of NSAID-induced gastroduodenal ulcers. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD002296 (2002).
Fat’hi, M. R., Yamini, Y., Sharghi, H. & Shamsipur, M. Solubilities of some recently synthesized 1, 8-dihydroxy-9, 10-anthraquinone derivatives in supercritical carbon dioxide. Talanta 48, 951–957 (1999).
Ghalami-Choobar, B., Ghiami-Shomami, A. & Nikparsa, P. Theoretical calculation of p K b values for anilines and sulfonamide drugs in aqueous solution. J. Theor. Comput. Chem. 11, 283–295 (2012).
Zhang, X., Han, X. & Xu, W. A computer simulation study on lewis acid–base interactions and cooperative C-H⋯ O weak hydrogen bonding in various CO2 complexes. J. Theor. Comput. Chem. 10, 483–508 (2011).
Lashkarbolooki, M., Shafipour, Z. S., Hezave, A. Z. & Farmani, H. Use of artificial neural networks for prediction of phase equilibria in the binary system containing carbon dioxide. J. Supercrit. Fluids 75, 144–151 (2013).
Lashkarbolooki, M., Hezave, A. Z. & Ayatollahi, S. Artificial neural network as an applicable tool to predict the binary heat capacity of mixtures containing ionic liquids. Fluid Phase Equilibria 324, 102–107 (2012).
Joni, I., Nulhakim, L., Vanitha, M. & Panatarani, C. in Journal of Physics: Conference Series. 012006 (IOP Publishing).
Esmaeilzadeh, F. & Roshanfekr, M. A new cubic equation of state for reservoir fluids. Fluid Phase Equilibria 239, 83–90 (2006).
Yazdizadeh, M., Eslamimanesh, A. & Esmaeilzadeh, F. Thermodynamic modeling of solubilities of various solid compounds in supercritical carbon dioxide: Effects of equations of state and mixing rules. J. Supercrit. Fluids 55, 861–875 (2011).
Storn, R. & Price, K. Differential evolution–a simple and efficient heuristic for global optimization over continuous spaces. J. Global Optim. 11, 341–359 (1997).
Lu, C., Yuan, H. & Zhang, N. in Intelligent Nanotechnology 71–111 (Elsevier, 2023).
Foster, N. R. et al. Significance of the crossover pressure in solid-supercritical fluid phase equilibria. Ind. Eng. Chem. Res. 30, 1955–1964 (1991).
Mahesh, R. V. G. & Garlapati, C. A new solvato-complex model for the calculations of solvation enthalpies of drugs-supercritical carbon dioxide systems. Austin Chemical Engineering 10 (2023).
Sodeifian, G., Alwi, R. S., Nooshabadi, M. A., Razmimanesh, F. & Roshanghias, A. Solubility measurement of Triamcinolone acetonide (steroid medication) in supercritical CO2: Experimental and thermodynamic modeling. The Journal of Supercritical Fluids, 106119 (2023).
Sodeifian, G., Sajadian, S. A. & Ardestani, N. S. Determination of solubility of Aprepitant (an antiemetic drug for chemotherapy) in supercritical carbon dioxide: Empirical and thermodynamic models. J. Supercrit. Fluids 128, 102–111 (2017).
Sodeifian, G., Sajadian, S. A. & Razmimanesh, F. Solubility of an antiarrhythmic drug (amiodarone hydrochloride) in supercritical carbon dioxide: Experimental and modeling. Fluid phase equilibria 450, 149–159 (2017).
Sodeifian, G., Razmimanesh, F., Sajadian, S. A. & Panah, H. S. Solubility measurement of an antihistamine drug (Loratadine) in supercritical carbon dioxide: Assessment of qCPA and PCP-SAFT equations of state. Fluid phase equilibria 472, 147–159 (2018).
Sodeifian, G., Ardestani, N. S., Sajadian, S. A. & Panah, H. S. Measurement, correlation and thermodynamic modeling of the solubility of Ketotifen fumarate (KTF) in supercritical carbon dioxide: Evaluation of PCP-SAFT equation of state. Fluid phase equilibria 458, 102–114 (2018).
Sodeifian, G., Detakhsheshpour, R. & Sajadian, S. A. Experimental study and thermodynamic modeling of Esomeprazole (proton-pump inhibitor drug for stomach acid reduction) solubility in supercritical carbon dioxide. J. Supercrit. Fluids 154, 104606 (2019).
Sodeifian, G., Razmimanesh, F., Ardestani, N. S. & Sajadian, S. A. Experimental data and thermodynamic modeling of solubility of Azathioprine, as an immunosuppressive and anti-cancer drug, in supercritical carbon dioxide. J. Mol. Liquids 299, 112179 (2020).
Sodeifian, G., Sajadian, S. A. & Derakhsheshpour, R. Experimental measurement and thermodynamic modeling of Lansoprazole solubility in supercritical carbon dioxide: Application of SAFT-VR EoS. Fluid phase Equilibria 507, 112422 (2020).
Sodeifian, G., Razmimanesh, F. & Sajadian, S. A. Prediction of solubility of sunitinib malate (an anti-cancer drug) in supercritical carbon dioxide (SC–CO2): Experimental correlations and thermodynamic modeling. J. Mol. Liquids 297, 111740 (2020).
Joback, K. G. & Reid, R. C. Estimation of pure-component properties from group-contributions. Chem. Eng. Commun. 57, 233–243 (1987).
Constantinou, L. & Gani, R. New group contribution method for estimating properties of pure compounds. AIChE J. 40, 1697–1710 (1994).
Author information
Authors and Affiliations
Contributions
F.H. Albadran: Conceptualization, Methodology, Validation and Resources, N.K.A.: Conceptualization, Methodology, Validation and Resources, M.A.M.: Conceptualization, Methodology, Validation and Resources, S.N H.: Supervisor, Conceptualization, Methodology, Validation and Resources, Data curation, editing the original and final manuscripts. M.S.A.: Methodology, Investigation, Data curation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Albadran, F.H., Abbood, N.K., Al-Mayyahi, M.A. et al. Solubility of lumiracoxib in supercritical carbon dioxide. Sci Rep 14, 13260 (2024). https://doi.org/10.1038/s41598-024-63416-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-63416-x
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.