Abstract
Calcium hydroxide (Ca(OH)2NPs), calcium titanate (CaTiO3NPs) and yttrium oxide (Y2O3NPs) nanoparticles are prevalent in many industries, including food and medicine, but their small size raises concerns about potential cellular damage and genotoxic effects. However, there are very limited studies available on their genotoxic effects. Hence, this was done to investigate the effects of multiple administration of Ca(OH)2NPs, CaTiO3NPs or/and Y2O3NPs on genomic DNA stability, mitochondrial membrane potential integrity and inflammation induction in mouse brain tissues. Mice were orally administered Ca(OH)2NPs, CaTiO3NPs or/and Y2O3NPs at a dose level of 50 mg/kg b.w three times a week for 2 weeks. Genomic DNA integrity was studied using Comet assay and the level of reactive oxygen species (ROS) within brain cells was analyzed using 2,7 dichlorofluorescein diacetate dye. The expression level of Presenilin-1, tumor necrosis factor-alpha (TNF-α) and Interleukin-6 (IL-6) genes and the integrity of the mitochondrial membrane potential were also detected. Oral administration of Ca(OH)2NPs caused the highest damage to genomic DNA and mitochondrial membrane potential, less genomic DNA and mitochondrial damage was induced by CaTiO3NPs administration while administration of Y2O3NPs did not cause any remarkable change in the integrity of genomic DNA and mitochondrial membrane potential. Highest ROS generation and upregulation of presenilin-1, TNF-α and IL-6 genes were also observed within the brain cells of mice administrated Ca(OH)2NPs but Y2O3NPs administration almost caused no changes in ROS generation and genes expression compared to the negative control. Administration of CaTiO3NPs alone slightly increased ROS generation and the expression level of TNF-α and IL-6 genes. Moreover, no remarkable changes in the integrity of genomic DNA and mitochondrial DNA potential, ROS level and the expression level of presenilin-1, TNF-α and IL-6 genes were noticed after simultaneous coadministration of Y2O3NPs with Ca(OH)2NPs and CaTiO3NPs. Coadministration of Y2O3NPs with Ca(OH)2NPs and CaTiO3NPs mitigated Ca(OH)2NPs and CaTiO3NPs induced ROS generation, genomic DNA damage and inflammation along with restoring the integrity of mitochondrial membrane potential through Y2O3NPs scavenging free radicals ability. Therefore, further studies are recommended to study the possibility of using Y2O3NPs to alleviate Ca(OH)2NPs and CaTiO3NPs induced genotoxic effects.
Similar content being viewed by others
Introduction
Nanotechnology is a cutting-edge field of science that deals with the engineering and study of technology at a nanoscale. The term “nano” refers to a unit of measurement that is one thousandth of a micro and demonstrates the minuscule size of these particles. Nanoparticles are incredibly tiny, measuring more than 8000 times smaller than a human hair1. This technology is incredibly versatile and has many applications, as it continues to grow as a field. It is crucial to study its effects on biological organisms to prevent any potential harm from the nanoparticles used in a wide range of industries, such as healthcare, food preservation, and cosmetics. Nanotechnology has a profound effect on every aspect of our daily lives, from enhancing security to advancing medicine2.
Sadly, advances in the production and applications of nanoparticles have led to a rise in human exposure to several manufactured nanoparticles such as Calcium hydroxide (Ca(OH)2NPs), Calcium titanate (CaTiO3NPs), and Yttrium oxide (Y2O3NPs) nanoparticles3. The very unique traits of Ca(OH)2NPs highly raise their uses in various nanotechnological and biotechnology applications: One of them is their ability to protect limestone's statues in their aqueous form and in this cane they are known as nanolime4. The initial studies on Ca(OH)2NPs as nanolime by scientists took place around 2000 at the University of Florence CSGI in Italy. The results of their research on its synthesis and use for preserving wall paintings were published in 20014. Moreover, CaTiO3NPs are also heavily used in fresh water and wastewater treatment to raise the water PH and remove particles from water in addition to their uses as a potent antimicrobial in intra-cranial medicine3,4.
Calcium titanite is a compound made up of calcium (Ca) and titanium (Ti). It can be produced through several techniques, including sol–gel, coprecipitation, hydrothermal, mechanochemical, solid-state, and co-precipitation. The sol–gel method is the most commonly used method for producing calcium titanite5. CaTiO3NPs are widely employed in a variety of applications, including electronic ceramics, energy storage, and biological imaging6. Despite the obvious advantages of CaTiO3NPs, they offer certain health and environmental risks. They have the potential to generate oxidative stress and DNA damage, which could result in toxicity in normal Human skin fibroblast Cells7. If released into the water system, they can also potentially be hazardous to aquatic life.
Nanoparticles of Yttrium oxide have a wide range of applications due to their unique properties. For example Y2O3NPs are used as a polarizer, laser host material, phosphor, bio-imaging, and biosensor, along with their uses in the treatment of cancer and Fulminant hepatic failure. Despite this, Y2O3NPs alone have not gained much attention as a nanomedicine8,9. Therefore, the present study was undertaken to estimate the effect of multiple oral administration with Ca(OH)2NPs, CaTiO3NPs or/and Y2O3NPs on the integrity of genomic and mitochondrial DNA in mice brain tissues. Alkaline Comet assay was done to assess genomic DNA integrity, while integrity of mitochondrial membrane potential was studied using 3-Rhodamine dye. The level of reactive oxygen species (ROS) generation within neural cells was studied using 2,7-dichlorofluorescein dye and expression level of presenilin-1 gene and inflammatory tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) genes was measured using quantitative Real time PCR.
Materials and methods
Chemicals
The Ca(OH)2NPs were procured from Nanotech Company (Giza, Egypt), while CaTiO3NPs were sourced from Sigma Aldrich Company (St. Louis, MO, USA) with the product code 633801. These particles are in the form of Nano powder with a particle size less than 100 nm and contain 99% trace metals. Y2O3NPs were also obtained from Sigma-Aldrich Company (St. Louis, MO, USA) with product number 544892 and in the form of nano-powders with a particle size of less than 50 nm and contain at least 99.9% trace metals.
Characterization of nanoparticles
The three tested nanoparticles: Ca(OH)2NPs, CaTiO3NPs and Y2O3NPs have been characterized well in our previous studies using transmission electron microscope (TEM), X-ray diffraction (XRD) and dynamic laser scattering (DLS)7,10,11.
Animals
This study involved using 45 male Swiss Webster mice, aged 10–12 weeks and weighing 20–25 g. The mice were purchased from the National Research Center and kept in normal conditions for 1 week prior starting the administration at the animal house of Zoology Department at Faculty of Science Cairo University. They were fed standard diet pellets and water. Mice were fed completely nutritional diet called Mazuri's vegetarian rat and mouse diet manufuctured by Land O' Lakes Inc Company (Arden Hills, Minnesota, USA).
Ethical approval
The design of this study was approved by the MSA University Research Ethics Committee. This study was reported according to ARRIVE guidelines and also Animal handling and experimentations were conducted in accordance with the Guidelines of the National Institutes of Health (NIH) regarding the care and use of animals for experimental procedures.
Determination of the nanoparticles' tested dose
An acute toxicity test was used to determine the appropriate utilized dose of the tested nanoparticles according to OECD standards 420 as follow: Twenty male mice were divided into four groups: an untreated control group and three treated groups, each with five mice. The three treated groups were orally given 2000 mg/kg of Ca(OH)2NPs, CaTiO3NPs or Y2O3NPs separately, while mice of the negative control group were orally given deionized distilled water. All mice of the four groups were monitored for any symptoms or morphological behavior of toxicity during the first 24 h of nanoparticles administration and up to 14 days of administration. Based on the mice's survival and the OECD standards 42012,13, the used dose of the tested nanoparticles was 2.5% of the safety tested dose determined from the OECD test.
Experimental design
In this study, 25 male mice were randomly separated into five groups with 5 mice each (Fig. S1). The first group served as the negative control, while the second group was taken 50 mg/kg of Ca(OH)2NPs through oral administration, the third group was given 50 mg/kg of CaTiO3NPs orally, the fourth group was given 50 mg/kg of Y2O3NPs, and the fifth group was given a combination of the tested nanoparticles orally at a dose level of 50 mg/kg each, three times a week for two consecutive weeks. After 24 h of the last administration, all mice of the five groups were put to death by cervical dislocation, dissected and their brains were extracted, frozen, and preserved at − 80 °C for further analysis.
Detection of reactive oxygen species generation
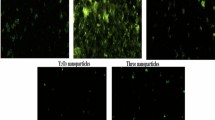
The generation of reactive oxygen species (ROS) within brain cells was detected using 2,7 dichlorofluorescein diacetate (DCFH-DA) according to14. This compound can penetrate cells and react with ROS to produce the fluorescent chemical dichlorofluorescein (DCF). The procedure involved homogenizing 50 mg of brain tissue in Phosphate buffered saline (PBS), then rinsing it twice with PBS. 50 μl of the cell suspension was mixed with 50 μl of DCFH-DA (20 mM) and left to incubate in the dark for 30 min. The mixture was then placed on slides and imaged under a fluorescent microscope (OLYMPUS CKX 41) at 20× magnification.
Estimation of DNA damage level
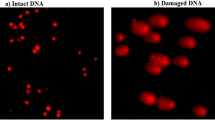
The level of DNA damage induction within brain cells was measured using the alkaline Comet test with a pH level higher than 1315. Slides were dipped in normal melting agarose (1%) and the samples were gently minced, mixed with low melting agarose (0.5%) and then placed on coated normal agarose slides. Sides were kept in the dark for 24 h at 4 °C in cold lysis buffer. After lysis, the slides were placed in staining jars containing alkaline electrophoresis buffer for 15 min, then electrophoresed for 30 min at 25 V and 300 mA in the same alkaline buffer. Slides were then neutralized, fixed with cold absolute ethanol and stained with ethidium bromide prior imaging. The slides were finally photographed under an epi-fluorescent microscope at 200× magnification and TriTek Comet ScoreTM Freeware v1.5 was used to assess the extent of DNA damage by measuring tail length, %DNA in tail, and tail moment.
Studying the mitochondrial membrane potential
The integrity of mitochondrial membrane potential was studied in brain tissues of the five groups using the method previously described by Zhang and his colleagues16. Briefly: a suspension of brain cells in PBS was combined with the fluorescent Rhodamine-123 dye and incubated in the dark for an hour at 37 °C. After incubation, the cells were washed twice with PBS. Then the fluorescence light emitted by Rhodamine-123 was captured and analyzed using an epifluorescence microscope at 200× magnification.
mRNA expression levels of presenilin-1 and inflammatory genes
For measuring the mRNA expression level of presenilin-1 gene and inflammatory tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) genes, quantitative RTPCR was conducted. The Gene JET RNA Purification Kit was used to extract RNA from frozen brain tissues in a 1.5 ml micro-centrifuge tube. About 30 mg of brain tissues were mixed with 300 µl of lysis buffer and β-mercaptoethanol and vortexed for 10 s, then 600 μl of diluted proteinase K was added, vortexed and incubated for 10 min at 25 °C. The tubes were centrifuged for 10 min at 12,000×g, and then the supernatant was transferred to a new RNase-free micro-centrifuge tube. 450 μl of 100% ethanol was added; the lysate solution was filtered through a GeneJet RNA Purification column and washed with washing buffer. Finally, 100 μl of nuclease-free water was introduced to the column, centrifuged for 60 s, and the RNA was eluted into a new micro-centrifuge tube, which was stored at − 80 °C. The RNA was then reverse transcribed into complementary DNA (cDNA) using the Revert Aid First Strand cDNA Synthesis Kit. For amplification, 1 μl of the cDNA of each sample for each gene was mixed with 0.5 µl of each forward and reverse primers listed in Table 117,18 for three genes (Presenilin, TNF-α and IL-6) along with 6 µl of SYBER green master mix and 4 μl of nuclease-free water to make a total of 12 μl. The RTPCR reaction was then run with an initial denaturation of 95 °C for 15 min followed by 35 cycles of denaturation at 95 °C for 15 s and annealing at for 30 s, and extension at 72 °C for 1 min. Then final extension was done at 72 °C for 10 min. The expression of the three studied genes was measured using β-actin gene as a housekeeping gene and the comparative ΔΔCt method was used for calculating the fold change in the gene expression.
Statistical analysis
The findings from this study were displayed as mean ± SD and evaluated using SPSS (version 20) at a significance level of < 0.05. One-way analysis of variance (ANOVA) was used to determine the impact of Y2O3NPs coadministration with Ca(OH)2NPs and CaTiO3NPs on induction of DNA damage and expression level of presenilin-1, TNF-α and IL-6 genes. Duncan's test was carried out to determine the similarities and differences between the control and four treated groups.
Results
Characterization of nanoparticles
Characterization of Ca(OH)2NPs, CaTiO3NPs and Y2O3NPs in our previous studies using XRD analysis, DLS and TEM confirmed the purity of purchased nanopowders along with stability and well distribution of suspended nanoparticles in deionized distilled water. Moreover, TEM imaging revealed the spherical morphology of Ca(OH)2NPs, CaTiO3NPs and Y2O3NPs with an average particles' size of 59.82, 88.79 and 14.00 nm, respectively7,10,11.
The nanoparticles' tested dose
Observation of mice administered orally with a single dose (2000 mg/kg b.w) of Ca(OH)2NPs, CaTiO3NPs or Y2O3NPs revealed that all mice were healthy and no signs of toxicity noticed during the first 48 h of Ca(OH)2NPs, CaTiO3NPs or Y2O3NPs administration until the end of the 14-days observation period. The half lethality dose (LD50) of Ca(OH)2NPs, CaTiO3NPs and Y2O3NPs above 2000 mg/kg was considered according to the OECD-420 guidelines, and the initial tested dose of Ca(OH)2NPs, CaTiO3NPs and Y2O3NPs in this study was calculated as 2½% (50 mg/kg body weight) of the LD50 Obtained from acute toxicity test.
Generation of intracellular ROS
Staining of brain cells with 2,7 DCFH-DA was highly informative and revealed that the highest generation of ROS was seen in the brain tissues of mice orally administered Ca(OH)2NPs alone compared to negative control and three treated groups (Groups III, IV and V) as depicted in Fig. 1. A slightly higher amount of ROS was observed in the brain tissue of mice orally ingested CaTiO3NPs alone compared to those noticed in the brain cells of negative control group. The last two groups administered Y2O3NPs alone or in combination with Ca(OH)2NPs and CaTiO3NPs exhibited the lowest amount of ROS generation in comparison to the other treated groups and almost identical to the ROS generated in the negative control brain cells, as displayed in Fig. 1.
Induction of DNA damage
Results of the Comet assay are shown in Table 2 and examples of the Comet nuclei scored with intact and damaged DNA are shown in Fig. 2. As depicted in Table 2, oral intake of Ca(OH)2NPs for six separate days over a 2-week period induced the highest statistical significant elevations in the DNA damage measured parameters: tail length, %DNA in tail, and tail moment compared to their values in the brain tissues of mice administered CaTiO3NPs or Y2O3NPs separately or together simultaneously with Ca(OH)2NPs (Table 2). Similarly, oral ingestion of CaTiO3NPs six times over a 2 weeks caused statistical significant increases in %DNA in tail and tail moment compared to the negative control values but remained significantly lower than the Ca(OH)2NPs administered group values (Table 2). On the other hand, oral administration of Y2O3NPs alone (Group IV) or simultaneously with Ca(OH)2NPs and CaTiO3NPs (Group V) did not cause any statistical changes in the tail length and tail moment compared to the negative control group values as displayed in Table 2.
Integrity of mitochondrial membrane potential
As illustrated in Fig. 3, oral administration of Ca(OH)2NPs alone caused a highest damage to the mitochondrial membrane potential as manifested by the remarkable decrease in the fluorescence intensity emitted by Rhodamine-123 stained brain cells compared to the negative control (Group I) and other three treated groups (Groups III, IV and V). Similarly, the oral administration of CaTiO3NPs led to a high decrease in the intensity of emitted fluorescent light compared to that emitted from the negative control brain cells, but still higher than that emitted from brain cells of mice administered Ca(OH)2NPs alone.
On the other hand, minimal damage to the mitochondrial membrane potential was noticed in the brain cells of mice orally administered Y2O3NPs alone (Group IV) or in combination with Ca(OH)2NPs and CaTiO3NPs (Group V) as slight decreases in the intensity of emitted light were observed in mice given Y2O3NPs alone (Group IV) and almost no changes were seen in mice orally given Y2O3NPs with Ca(OH)2NPs and CaTiO3NPs (Group V).
mRNA expression level
The quantitative RTPCR results are summarized in Fig. 4 and showed that the expression level of the three studied genes: presenilin-1, TNF-α, and IL-6 genes was statistically significantly elevated in the brain tissues of mice orally given Ca(OH)2NPs compared to their expression level in the negative control and other three groups CaTiO3NPs or Y2O3NPs separately or together with Ca(OH)2NPs. On the other hand, oral administration of administration of CaTiO3NPs significantly upregulated the expression level of TNF-α and IL-6 genes compared to the negative control expression level but significantly less than their expression level in Ca(OH)2NPs administered group as seen in Fig. 4. Meanwhile no remarkable changes were observed in the expression level of presenilin-1 gene after CaTiO3NPs administration compared to the negative control expression level (Fig. 4). On the contrary, the expression level of presenilin-1, TNF-α, and IL-genes did not change significantly and remained at the expression level of negative control after administration of Y2O3NPs alone (Group IV) or in simultaneously with Ca(OH)2NPs and CaTiO3NPs (Group V) as depicted in Fig. 4.
Expression level of Presenilin-1, TNF-α and IL-6 genes in in the brain tissues of the negative control group and groups orally administered Ca(OH)2NPs, CaTiO3NPs or/and Y2O3NPs. Results are expressed as mean ± SD and were analyzed using one-way analysis of variance followed by Duncan’s test to test the similarity between the control and three treated groups. Means with different superscript letters indicates statistical significant difference at p < 0.05 between the compared groups for the same gene.
Discussion
The study of the cytotoxic and genotoxic effects of nanomaterials and nanoparticles is crucial in the field of biotechnology as nanotechnology advances. Extensive uses of Ca(OH)2NPs and CaTiO3NPs in various industrial, medical, food and consumer products increase the incidence of human exposure to these nanoparticles. However, limited data are available on the effect of Ca(OH)2NPs and CaTiO3NPs on the integrity of genomic and mitochondrial DNA in vivo along with the recently discovered free radicals scavenging activity of Y2O3NPs. Therefore, the current study was undertaken to estimate the impact of Y2O3NPs coadministration with Ca(OH)2NPs and CaTiO3NPs on the integrity of genomic DNA and mitochondrial membrane potential in the mice brain tissues.
In this study tracking ROS generation demonstrated the highest generation of ROS within the brain cells of mice orally given Ca(OH)2NPs (Group II) compared to those generated by CaTiO3NPs (Group III) or Y2O3NPs (Group IV) separately or in combination with Ca(OH)2NPs (Group V) as shown in Fig. 1. These results are in consistent with the recent detection of excessive ROS generation after single oral administration of Ca(OH)2NPs in various mice tissues: brain, bone marrow, liver, heart, spleen and lung10,19. Moreover, our finding of excessive ROS generation after administration of CaTiO3NPs manifested the in vivo induction of oxidative stress by CaTiO3NPs and supported the recent discovery of ROS generation and oxidative stress induction by CaTiO3NPs in breast cancer MCF-7 cell line20.
Extra-ROS generation can cause single- and double-strand DNA breaks, which is a lethal form of DNA damage21,22. According to Mills study23, double stranded-DNA breaks play a key role in the initiation of proto-oncogenes and the pathogenesis of cancer, suggesting that increased intracellular ROS production can cause cancer since intensive ROS generation disrupts the balance between oxidants and antioxidants and attacks proteins, lipids, carbohydrates and DNA inducing lipid peroxidation, protein damage and destruction, oxidative DNA damage and alterations in DNA bases. Indeed, the alkaline Comet assay is a very sensitive technique in detecting both single- and double-stranded DNA breaks15. Consequently, the highest incidence of DNA damage induction demonstrated by Comet assay in the brain tissues of mice given Ca(OH)2NPs alone compared to the other three treated groups (Table 2 and Fig. 2) can be attributed to the aforementioned highest ROS generation by Ca(OH)2NPs attacking DNA and cause breakages of both single and double DNA strands. Similarly, a recent study by Mohamed10, manifested the induction of DNA breakages in the brain tissues of mice given orally Ca(OH)2NPs through increased generation of intracellular ROS.
Similarly, the detected remarkable DNA damage induction by CaTiO3NPs administration through significant increases in the percentage of damaged DNA in the tail and tail moment could be attributed to the increased ROS generation noticed within brain cells of mice orally given CaTiO3NPs alone compared to those generated in the negative control brain cells. High ROS consequently attacks genomic DNA and increases the amount of damaged DNA. However, non remarkable changes observed in the tail length value after CaTiO3NPs administration compared to the negative control value revealed the large size of fragmented DNA causing slow migration in the mini gel15,23.
On the other hand, oral administration of Y2O3NPs alone caused non remarkable changes in the integrity of genomic DNA as demonstrated by the observable non-significant changes in the tail length and tail moment compared to negative control levels. Similarly, simultaneous coadministration of Y2O3NPs with Ca(OH)2NPs and CaTiO3NPs caused a marked decrease in the incidence of DNA damage induction noticed after administration of Ca(OH)2NPs or CaTiO3NPs separately and became non-statistically different from the negative control levels (Table 2). This remarkable reduction in the DNA damage induction probably due to the recently discovered antioxidant properties of Y2O3NPs; Y2O3NPs act as a direct antioxidant, controlling and neutralizing the generated harmful ROS8,9,24. Our findings of minimal ROS generation within brain cells of mice that were given Y2O3NPs alone or in combination with Ca(OH)2NPs and CaTiO3NPs further confirmed the antioxidant and free radicals scavenging capabilities of Y2O3NPs. Consistent with our findings, Y2O3NPs showed a potent dose dependent antioxidant and neuroprotective effect against oxidative stress and apoptosis induction25,26,27,28.
For more understanding of the impact of Y2O3NPs coadministration with Ca(OH)2NPs and CaTiO3NPs on the integrity of genomic DNA, the mRNA expression of Presenilin-1, TNF-α, and IL-6 genes were measured. Presenilin-1 is responsible for cleaving amyloid protein that causes Alzheimer's disease and is overexpressed in Alzheimer's patients29,30,31. Results of RTPCR showed that Y2O3NPs coadministration with Ca(OH)2NPs and CaTiO3NPs highly declined presenilin-1 overexpression (Fig. 4) noticed after oral administration of Ca(OH)2NPs alone thus protecting brain cells from Alzheimer's risk caused by administration of Ca(OH)2NPs alone previously reported by the study of Li and his colleagues30. Moreover, a marked decreases in the expression of presenilin-1 genes after oral administration of Y2O3NPs indicating the potentiality of Y2O3NPs in protecting the brain cells from Alzheimer's disease.
Alzheimer's and other neurological diseases have been linked to elevated inflammatory cytokines expression and secretion. For example, the expression level of IL-6 and TNF-α genes is elevated in Alzheimer's disease30,31,32. Overexpression of inflammatory mediators including IL-6 and TNF-α genes also increases the expression level of Presenilin-1 and β-amyloid precursor protein genes causing aggregation and accumulation of β-amyloid peptides in the brain tissues and increasing the risk of Alzheimer's disease31,33. A marked overexpression of TNF-α and IL-6 genes observed in the brain tissue of mice orally exposed to Ca(OH)2NPs or CaTiO3NPs (Fig. 4), suggesting inflammation induction and immune stimulation19,28. However, no significant difference was found in the expression level of TNF-α and IL-6 genes after administration of Y2O3NPs alone revealing the antioxidant and protective properties of Y2O3NPs on brain cells. Therefore, our findings regarding the remarkable high decreases observed in the expression level IL-6 and TNF-α overexpressed genes by administration of Ca(OH)2NPs or CaTiO3NPs alone may explain the restored normal gene expression of presenilin-1 after Y2O3NPs coadministration with Ca(OH)2NPs and CaTiO3NPs (Fig. 4).
Variations in mitochondrial membrane potential can assess mitochondrial function and cell health using fluorescent dyes34. Mitochondria are critical to cell survival and any damage leads to cell death and disease. Therefore, the integrity of mitochondrial membrane potential has been studied in this study. Screening brain cells stained with Rhodamine-123 dye showed that administration of Ca(OH)2NPs caused a marked damage to mitochondria as manifested by the high reduction in the emitted fluorescent light (Fig. 3). This result supports findings of Mohamed study which demonstrated that Ca(OH)2NPs are genotoxic and can cause mitochondrial damage and even neurodegenerative diseases like Alzheimer's. Brain tissue exposed to CaTiO3NPs showed less damage, but still some harm to mitochondria10. Meanwhile, administration of Y2O3NPs alone or with Ca(OH)2NPs and CaTiO3NPs showed no remarkable decrease in emitted fluorescent light and appeared safe for mitochondria confirming the protective effect of Y2O3NPs (Fig. 3).
Conclusion
From above findings, it is concluded that administration of Ca(OH)2NPs alone induced the highest genomic DNA damage, ROS generation, disruption of mitochondrial membrane potential and inflammation, while administration of CaTiO3NPs alone had less toxic effects than Ca(OH)2NPs. Contrary, administration Y2O3NPs alone did not alter ROS generation, inflammatory genes expression and mitochondrial membrane potential. More interestingly, coadministration of Y2O3NPs with Ca(OH)2NPs and CaTiO3NPs alleviated the Ca(OH)2NPs and CaTiO3NPs induced genotoxicity, disruption of mitochondrial membrane potential and inflammation. However, single dose of nanoparticles, single tissue and limited techniques were used in this study, therefore further studies are necessary to fully understand the possibility of using Y2O3NPs to overcome Ca(OH)2NPs and CaTiO3NPs induced toxicity using different doses and more techniques in various organs.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Kumar, M. S. Nano Science and Nanotechnology: Journey from Past to Present and Prospect in Veterinary Science and Medicine. Nanoscience 2(1), 79–83 (2014).
Abiodun-Solanke, I., Ajayi, D. & Arigbede, A. Nanotechnology and its application in dentistry. Ann. Med. Health Sci. Res. 3, S171–S177 (2014).
Jacobsen, N. R. et al. Genotoxicity, cytotoxicity, and reactive oxygen species induced by single-walled carbon nanotubes and C(60) fullerenes in the FE1-Mutatrade markMouse lung epithelial cells. Environ. Mol. Mutagen. 49(6), 476–478 (2008).
Ambrosi, M., Dei, L., Giorgi, R., Neto, C. & Baglioni, P. Colloidal particles of Ca(OH)2: Properties and applications to restoration of frescoes. Langmuir 17(14), 4251–4255 (2001).
Maroneze, M., Zepka, L., Vieira, J., Queiroz, M. & JacobLopes, E. A tecnologia de remoção de fósforo: Gerenciamento do elemento em resíduos industriais. Ambiente e Agua 9, 445–558 (2014).
Křenek, T., Kovářík, J., Pola, T. & Stich, D. D. Nano and micro forms of calcium titanate: Synthesis, properties and application. Open Ceram. 8, 100177–100211 (2021).
Mohamed, H. R. H. et al. Estimation of calcium titanate or erbium oxide nanoparticles induced cytotoxicity and genotoxicity in normal HSF cells. Biol. Trace Elem. Res. https://doi.org/10.1007/s12011-022-03354-9 (2023).
Tang, K. S. Antioxidant and anti-inflammatory properties of yttrium oxide nanoparticles: New insights into alleviating diabetes. Curr. Diabetes Rev. 17(4), 496–502 (2021).
Rajakumar, G. et al. Yttrium oxide nanoparticle synthesis: An overview of methods of preparation and biomedical applications. Appl. Sci. 11(5), 2172–2179. https://doi.org/10.3390/app11052172 (2021).
Mohamed, H. R. H. Estimation of genomic instability and mitochondrial DNA damage induction by acute oral administration of calcium hydroxide normal- and nano-particles in mice. Toxicol. Lett. 304, 1–12 (2019).
Emad, B. et al. Yttrium oxide nanoparticles induce cytotoxicity, genotoxicity, apoptosis, and ferroptosis in the human triple-negative breast cancer MDA-MB-231 cells. BMC Cancer 23, 1151 (2023).
van den Heuvel, M. J. et al. The international validation of a fixed-dose procedure as an alternative to the classical LD50 test. Food Chem. Toxicol. 28(7), 469–482 (1990).
Whitehead, A. & Curnow, R. N. Statistical evaluation of the fixed-dose procedure. Food Chem. Toxicol. 30(4), 313–324 (1992).
Siddiqui, M. A. et al. Protective potential of trans-resveratrol against 4- hydroxynonenal induced damage in PC12 cells. Toxicol. In Vitro 24(6), 1592–1598 (2010).
Tice, R. R. et al. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 35, 206–221 (2000).
Zhang, Y. et al. Possible involvement of oxidative stress in potassium bromate-induced genotoxicity in human HepG2 cells. Chem. Biol. Int. 189, 186–191 (2011).
Gautheron, V., Auffret, A., Mattson, M. P., Mariani, J. & Vernet-der Garabedian, B. A new and simple approach for genotyping Alzheimer’s disease presenilin-1 mutant knock-in mice. J. Neurosci. Methods 181(2), 235–240 (2009).
Singh, U. P. et al. Resveratrol (trans-3,5,4′-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kappaB activation to abrogate dextran sulfate sodium-induced colitis. J. Pharmacol. Exp. Ther. 332(3), 829–839 (2010).
Mohamed, H. R. H. Induction of genotoxicity and differential alterations of p53 and inflammatory cytokines expression by acute oral exposure to bulk- or nano-calcium hydroxide particles in mice “Genotoxicity of normal- and nano-calcium hydroxide”. Toxicol. Mech. Methods 31(3), 169–181 (2021).
Mohamed, H. R. H., Ibrahim, M. M. H. & Diab, A. Induction of oxidative DNA damage, cell cycle arrest and p53 mediated apoptosis by calcium titanate nanoparticles in MCF-7 breast cancer cells. Cancer Cell Int. 22(1), 355–365 (2022).
Safwat, G., Soliman, E. S. M. & Mohamed, H. R. H. Induction of ROS mediated genomic instability, apoptosis and G0/G1 cell cycle arrest by erbium oxide nanoparticles in human hepatic Hep-G2 cancer cells. Sci. Rep. 12(1), 16333–16343. https://doi.org/10.1038/s41598-022-20830-3 (2022).
Sallmyr, A., Fan, J. & Rassool, F. V. Genomic instability in myeloid malignancies: Increased reactive oxygen species (ROS), DNA double strand breaks (DSBs) and error-prone repair. Cancer Lett. 270(1), 1–9 (2008).
Mills, K. D., Ferguson, D. O. & Alt, F. W. The role of DNA breaks in genomic instability and tumorigenesis. Immunol. Rev. 194, 77–95. https://doi.org/10.1034/j.1600-065X.2003.00060.x (2003).
Kassem, S., Arafa, M. M., Yehya, M. M. & Soliman, M. A. M. In vivo study of dose-dependent antioxidant efficacy of functionalized core-shell yttrium oxide nanoparticles. Naunyn Schmiedebergs Arch. Pharmacol. 395(5), 593–606 (2022).
Selvaraj, V. et al. Cytotoxicity and genotoxicity caused by yttrium oxide nanoparticles in HEK293 cells. Int. J. Nanomed. 9(1), 1379–1391 (2014).
Schubert, D., Dargusch, R., Raitano, J. & Siu-Wai, C. Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem. Biophys. Res. Commun. 342(1), 86–91 (2006).
Mohamed, H. R. H. et al. Alleviation of calcium hydroxide nanoparticles induced genotoxicity and gastritis by coadministration of calcium titanate and yttrium oxide nanoparticles in mice. Sci. Rep. 13(1), 22011. https://doi.org/10.1038/s41598-023-49303-x (2023).
Mohamed, H. R. H. et al. Genotoxicity and oxidative stress induction by calcium hydroxide, calcium titanate or/and yttrium oxide nanoparticles in mice. Sci. Rep. 13, 19633. https://doi.org/10.1038/s41598-023-46522-0 (2023).
Bagaria, J., Bagyinszky, E. & An, S. S. A. Genetics, functions, and clinical impact of presenilin-1 (PSEN1) gene. Int. J. Mol. Sci. 23(18), 10970. https://doi.org/10.3390/ijms231810970 (2022).
Li, P. et al. The expression of presenilin 1 enhances carcinogenesis and metastasis in gastric cancer. Oncotarget 7(9), 10650–10662 (2016).
Yang, W. et al. Presenilin1 exerts antiproliferative effects by repressing the Wnt/β-catenin pathway in glioblastoma. Cell Commun. Signal. 18, 22–30 (2020).
Kummer, K. K., Zeidler, M., Kalpachidou, T. & Kress, M. Role of IL-6 in the regulation of neuronal development, survival and function. Cytokine 144, 155582–155594 (2021).
Bates, K. A., Fonte, J., Robertson, T. A., Martins, R. N. & Harvey, A. R. Chronic gliosis triggers Alzheimer’s disease-like processing of amyloid precursor protein. Neuroscience 113, 785–796 (2002).
Sakamuru, S., Attene-Ramos, M. S. & Xia, M. Mitochondrial Membrane Potential Assay 17–22 (Springer, 2016). https://doi.org/10.1007/978-1-4939-6346-1_2.
Acknowledgements
Many thanks and appreciation to the Department of Zoology, Faculty of Science, Cairo University, for providing chemicals and equipment required for conducting experiments.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The present work was partially funded by Faculty of Science Cairo University and Faculty of Biotechnology, October University for Modern Sciences and Arts (MSA) Egypt.
Author information
Authors and Affiliations
Contributions
Hanan RH Mohamed designed the study, conducted the molecular experiments, wrote manuscript, and performed statistical analysis. Ahmed H. Farouk, Salma H. Elbasiouni, and Kirolls A. Nasif performed experimentations and wrote manuscript. Gehan Safwat and other authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamed, H.R.H., Farouk, A.H., Elbasiouni, S.H. et al. Yttrium oxide nanoparticles ameliorates calcium hydroxide and calcium titanate nanoparticles induced genomic DNA and mitochondrial damage, ROS generation and inflammation. Sci Rep 14, 13015 (2024). https://doi.org/10.1038/s41598-024-62877-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62877-4
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.